To describe fidgety movements (FMs), i.e., the spontaneous movement pattern that typically occurs at 3–5 months after term age, and discuss its clinical relevance.
SourcesA comprehensive literature search was performed using the following databases: MEDLINE/PubMed, CINAHL, The Cochrane Library, Science Direct, PsycINFO, and EMBASE. The search strategy included the MeSH terms and search strings (‘fidgety movement*’) OR [(‘general movement*’) AND (‘three month*’) OR (‘3 month*’)], as well as studies published on the General Movements Trust website (www.general-movements-trust.info).
Summary of the dataVirtually all infants develop normally if FMs are present and normal, even if their brain ultrasound findings and/or clinical histories indicate a disposition to later neurological deficits. Conversely, almost all infants who never develop FMs have a high risk for neurological deficits such as cerebral palsy, and for genetic disorders with a late onset. If FMs are normal but concurrent postural patterns are not age-adequate or the overall movement character is monotonous, cognitive and/or language skills at school age will be suboptimal. Abnormal FMs are unspecific and have a low predictive power, but occur exceedingly in infants later diagnosed with autism.
ConclusionsAbnormal, absent, or sporadic FMs indicate an increased risk for later neurological dysfunction, whereas normal FMs are highly predictive of normal development, especially if they co-occur with other smooth and fluent movements. Early recognition of neurological signs facilitates early intervention. It is important to re-assure parents of infants with clinical risk factors that the neurological outcome will be adequate if FMs develop normally.
Descrever os movimentos irregulares (FMs), ou seja, o padrão de movimentos espontâneos que normalmente ocorrem entre 3 e 5 meses após o nascimento e discutir sua relevância clínica.
FontesUma pesquisa abrangente na literatura foi realizada nas seguintes bases de dados: MEDLINE/PubMed, CINAHL, The Cochrane Library, Science Direct, PsycINFO e EMBASE. A estratégia de busca incluiu os termos e cadeias de pesquisa do MeSH [(“fidgety movement*”) OU [(“general movement*”) E (“three month*”) OU (“3 month*”)], bem como estudos publicados no website da General Movements Trust (www.general-movements-trust.info).
Resumo dos dadosPraticamente todos os neonatos se desenvolveram normalmente se os FMs estiveram presentes e foram normais, mesmo se seus resultados do ultrassom do cérebro e/ou históricos clínicos indicassem tendência a déficits neurológicos posteriores. Por outro lado, quase todos os neonatos que nunca desenvolveram FMs apresentaram maior risco de déficits neurológicos, como paralisia cerebral, e doenças genéticas de início tardio. Caso os FMs fossem normais, porém simultâneos a padrões posturais não adequados para a idade, ou o caráter geral dos movimentos fosse monótono, as capacidades cognitivas e/ou de linguagem na idade escolar seriam abaixo do ideal. Os FMs anormais não são específicos e têm baixo poder preditivo, porém ocorrem em grande parte em neonatos posteriormente diagnosticados com autismo.
ConclusõesFMs anormais, ausentes ou esporádicos indicam um risco maior de disfunções neurológicas posteriores, ao passo que FMs normais são altamente preditivos de desenvolvimento normal, principalmente se forem simultâneos a outros movimentos suaves e fluentes. O reconhecimento precoce de sinais neurológicos facilita a intervenção antecipada. É importante garantir aos pais de neonatos com fatores de risco clínicos que o resultado neurológico será adequado se os FMs se desenvolverem normalmente.
Even without constant triggering by specific sensory input the fetal, neonatal, and young nervous system generates a variety of motor patterns.1 Present from 9 weeks postmenstrual age until 5 months after term, general movements (GMs) are part of this early spontaneous motor repertoire.1–3 From birth until the end of the 2nd month post term age, GMs have a writhing character; thereafter they occur as so-called fidgety movements (FMs).1–3
Since its introduction 25 years ago,4 the general movement assessment (GMA)5 has been increasingly used to predict motor dysfunction, especially cerebral palsy (CP).2,3,5–13 It is based on visual gestalt perception of normal vs. abnormal movements of the entire body. GMA is non-invasive, even non-intrusive, cost-efficient, and easy to learn within three to five days of training.3,5 Bosanquet et al.13 recently compared different structural and functional assessments used for early identification of CP risk and found that GMA had the best predictive power and accuracy. Summary estimates of the sensitivity and specificity of GMA were 98% and 91%, respectively.13 Apart from normal vs. abnormal (cramped-synchronized)6 writhing GMs, it is mainly FMs that contribute to excellent predictive values.6,9,12,13
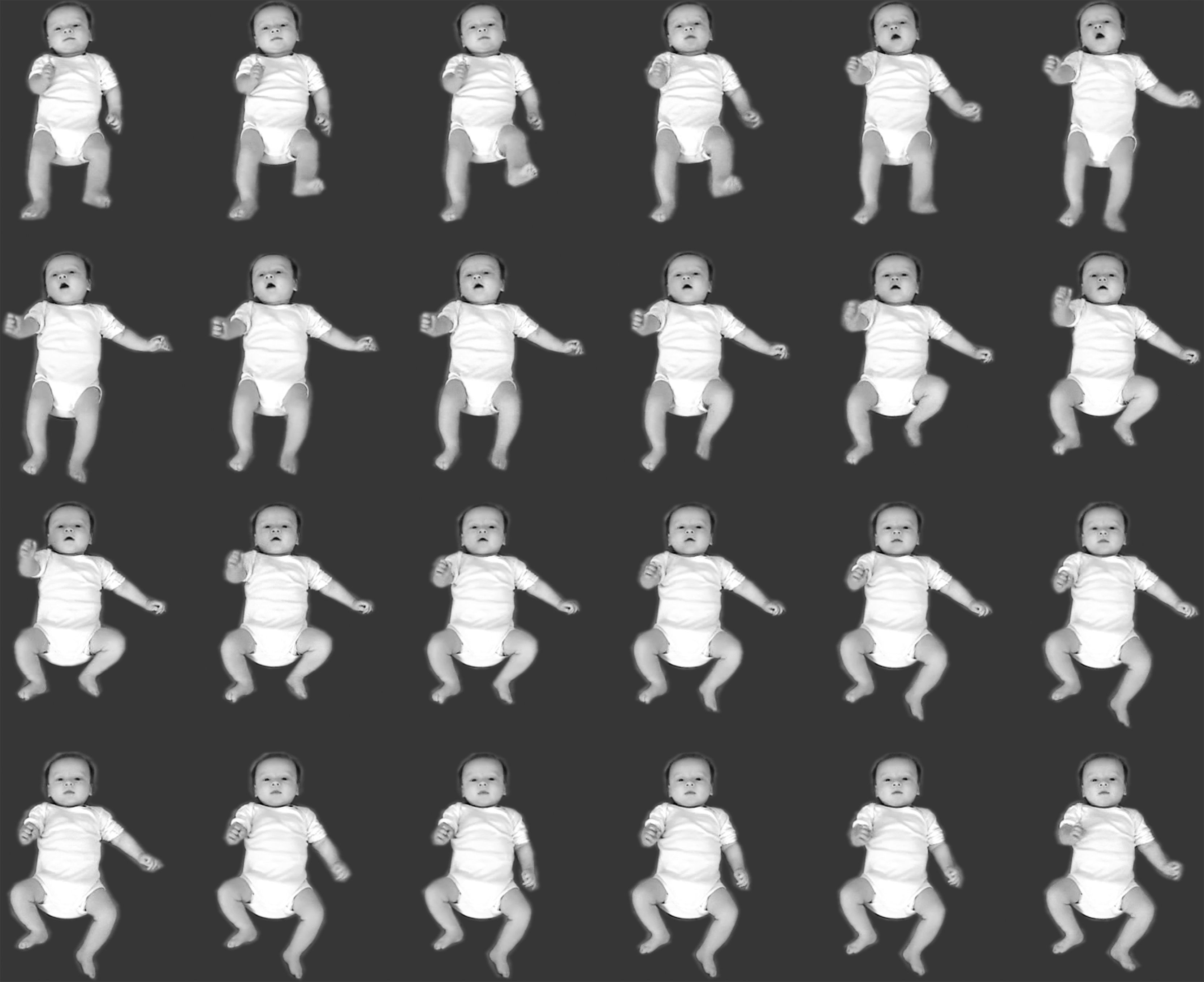
Normal FMsFMs are small movements of moderate speed with variable acceleration of the neck, trunk, and limbs in all directions (Fig. 1).5 They may appear as early as six weeks after term, but usually occur from around 9 weeks until 16–20 weeks, occasionally even a few weeks longer. They fade out when antigravity and intentional movements start to dominate.1,3,5,6
FMs occur regardless of the position of the infant, but can be best observed if the infant is in supine or in a semi-upright position in a relaxing chair. It is important to note that FMs are state-dependent. They are only present if the infant is awake; they disappear when the infant starts being fussy or cries, is drowsy or sleeps.3,5
The temporal organization of FMs varies with age. At first (i.e., at 6–8 weeks) they occur as isolated events; their frequency then increases, only to decrease again after 15–18 weeks.5,6 The temporal organization of FMs can be defined as follows:
Continual FMs (score: ++)Continual FMs are frequent, though interspersed with very short (1–2s) pauses. As they are by definition GMs, they involve the whole body, particularly the neck, shoulders, wrists, hips, and ankles. Depending on the actual body posture, especially the position of the head, FMs may occur asymmetrically. When infants focus on the environment, their FMs are mainly displayed in the hips and ankles, not so much in the shoulders and wrists.14,15
Intermittent FMs (score: +)Intermittent FMs occur in all body parts, though with longer pauses (up to 10s), which creates the impression that FMs are only present during half of the observation time.14,15
Sporadic FMs (score: +−)Isolated fidgety bursts of 1s to 3s are interspersed with long pauses of up to 1min. Sporadic FMs are age-adequate between 6 and 8 weeks post term age and during the 5th month when FMs fade out.14,15
After Prechtl had described FMs as an age-specific, distinct form of GMs, he speculated about the potential biological function of this transient movement pattern. One ontogenetic adaptive function of these tiny movements might be the postnatal calibration of the proprioceptive system.5,16 It takes an optimal re-calibration of this sensory domain to achieve proper control of the co-occurring visual hand regard, of intentional reaching and visually controlled manipulation of objects, and, eventually, fine motor activity. As a matter of fact, children and adolescents with fine motor dysfunction had less pronounced or even abnormal FMs during infancy.17,18
FMs may also enhance bonding. A recent study has demonstrated that mothers of infants with well-pronounced and continual FMs (as compared to less well-pronounced and intermittent FMs) are more affectionate when touching their infants and more cautious when putting them down. They hold them closer to their own body and cradle them so as to keep the infant's head, trunk, and limbs in midline. Furthermore, infants who display smooth and fluent movements engage more easily with their mother.19
To sum up, infants with normal FMs are very likely to show a neurologically normal development. This is irrespective of pre- or perinatal complications and is therefore vital for their parents’ comfort.3–6
Abnormal, absent, or abnormally sporadic FMs and their clinical significanceAbnormal FMs (score: AF)Abnormal FMs look like normal FMs, though with a greater amplitude, speed, and jerkiness.3,5,6 Abnormal FMs are rare; they occur more often in infants born preterm who show uncoordinated sucking.20 Abnormal FMs have been described in infants with trisomy 21 (Down syndrome)21,22 and infants intra-uterinely exposed to maternal opiate abuse and/or HIV.23 The predictive value of abnormal FMs is low. Infants with abnormal FMs may develop normally,6,18,24,25 but could also develop CP.6,15 Some studies documented an association between abnormal FMs and coordination difficulties and/or fine manipulative disabilities.17,18,25 Recently, an exceedingly high rate of abnormal FMs was described in infants who were later diagnosed with autism spectrum disorder.23,26–28
Absent FMs (score: F−)Whenever FMs are missing altogether from 9 to 20 weeks post term age, this abnormality is called “absent FMs.” Infants with absent FMs show other normal or abnormal movements.6 Absent FMs with a positive likelihood ratio (LR+>51) are highly predictive of later neurological deficits,3 particularly of CP.3–6,9–13,15,22,24–35 Further observation allows for determination of the eventual type of CP as well as the anatomical distribution and severity of the activity limitation. Quite apart from the lack of FMs, infants with an increased risk of non-spastic CP showed circular arm movements with or without spread fingers.12,36 Infants who went on to develop unilateral CP showed an asymmetry of distal segmental movements, which were reduced or absent on the contralateral side of the lesion.37–39 A cramped-synchronized movement character, repetitive opening and closing of the mouth, repetitive kicking, and abnormal finger postures characterized children who would later demonstrate poor self-mobility.30,34
Sporadic FMs (score: F+/−)FMs are confined to a few body parts and never last longer than 3s (median: 1s). There is no evidence that occasional isolated fidgety bursts (from 9 to 16 weeks post term age) indicate, for example, a milder type of CP. The functional mobility and activity limitation of 3–5-year-old children with CP was consistent, regardless of whether the child had had sporadic or no FMs as an infant.15
Observers are able to reliably differentiate between normal and abnormal/absent FMs (Kappa values between 0.75 and 0.92).6,11,40,41 A 3–4-day course proved sufficient for more than 700 trainees to correctly assess 87% of 3750 video clips of FMs.42 Yet in spite of its high objectivity and reliability, GMA remains prone to the observers’ fatigue and their failure to re-calibrate according to given standards of normal and abnormal patterns.43 Hence, a number of computer-based movement assessment tools have been developed for FM analysis, using optical flow meters44 or electromagnetic tracking systems.45 The so-called General Movement Toolbox by Adde et al.44 revealed that the variability in displacement of a spatial center of active pixels in the image had the highest sensitivity (81.5%) and specificity (70%) in classifying FMs. A recent study carried out by the same group showed that this kind of computer-based analysis can differentiate reliably between intermittent and continual FMs.46
Does sensory stimulation affect FMs?A series of experiments have been conducted to investigate the effects of visual, acoustic, social, and proprioceptive stimulation on FMs.14,47 Neither stimulation with a red ring nor unanimated acoustic stimulation (68, 77, 88dB) or interaction with the mother had any influence on the appearance or temporal organization of FMs.14 Only when presented with a red puppet with a white face rich in contrast (black eyes and mouth, red nose) did the infants show a significant level of focused attention with a decrease of FMs for a maximum of 20s, followed by a subsequent increase of FMs.14 It is frequently observed that FMs concentrate at the hips and ankles rather than the shoulders and wrists when infants focus their attention on something particular.15
To better understand the role vision plays in the development of movements and postures, Prechtl et al.48 studied the effects of early blindness by longitudinally assessing video recordings of 14 totally blind infants who showed no evidence of brain injury. Interestingly, all infants had exaggerated FMs. The authors speculated that these exaggerated movements might indicate some kind of compensation for the lack of visual integration and proprioception.48
As already mentioned, the authors regard FMs as an age-specific fine-tuning of the proprioceptive system.5,16 This raised the question whether FMs change during or after uni- or bilateral proprioceptive stimulation. A study was carried out in which, surprisingly, FMs remained identical even when the infant was hemi-loaded with up to 280 grams.47 Yet, in a more recent study on infants with obstetric brachial plexus lesion, a significant number of infants with severe lesions had abnormal GMs at 3 months.49
FMs in neurological examinations of infants born pretermChildren born preterm have higher rates of adverse neurodevelopmental outcomes.50 Identifying increased-risk infants is still a challenge today. In various preterm cohorts GMA has proved a reliable early predictor of the motor outcome, especially of CP.4,6–8,11–13,29–34,37,51,52 A significant relationship between white matter abnormalities on magnetic resonance imaging (MRI) and absent FMs in infants born at <30 weeks of gestation supports the idea that abnormal GMs reflect white matter injury.53 MRI at term equivalent age revealed reduced bifrontal, biparietal, and cerebellar transverse diameters, along with an increase in lateral ventricle sizes if the infant did not develop FMs. However, when controlling for white matter abnormality and grade III/IV intraventricular hemorrhage, only the cerebellar transverse diameter was predictive of absent FMs.54
A frequently asked question is whether preterm infants with normal FMs can also have an adverse developmental outcome. The answer is yes; in rare cases, FMs do not preclude an adverse outcome. Mild, usually unilateral CP6,15,32 and attention deficit hyperactivity disorder (ADHD)22,28 were reported in high-risk infants who had shown normal FMs. As a rule, however, normal FMs along with a smooth concurrent motor performance indicate a normal neurological outcome.22,55
A special case: normal FMs with abnormal concurrent movementsAmong high-risk children who developed FMs, abnormal concurrent movements – i.e., monotonous, jerky, and/or stiff gross movements at 3–4 months after term – predicted a poor motor outcome at 10 years.55 Children born with an extremely low birth weight who had normal FMs but abnormal monotonous, jerky, and/or stiff co-occurring gross movements had lower scores on the working memory and processing speed indices at age 10. They also had poorer balance and total motor skills on the Motor Assessment Battery for Children, and their parents reported more hyperactivity, inattention, and behavioral problems than those of infants with smooth and fluent concurrent movements.35 If, apart from normal FMs and abnormal concurrent movements, the asymmetric tonic neck response (ATNR) was still obligatory, the risk of developing complex minor neurological dysfunctions increased to 75% – as opposed to a mere 15% if the ATNR was no longer obligatory.56 An obligatory ATNR combined with monotonous finger movements and normal FMs was associated with a lower intelligence quotient at elementary school.57 All these studies35,55–57 included only infants born preterm.
FM in high-risk infants born at termAs early as 1993, Prechtl et al.58 reported on GMs in a sample of term-born infants affected by mild/moderate/severe hypoxic–ischemic encephalopathies (HIE). Longitudinal video recordings showed that hypokinesis occurred very frequently during the first days of life, followed by transient or prolonged abnormal GMs. Alterations in GMs, and especially the presence or absence of FMs, were good predictors of the neurological outcome. The predictive value of GMA was found to be similar to that of EEG and neuro-imaging, and better than that of neurological examinations.58 These results were confirmed by a recent study conducted in Iran on term-born infants with HIE: the assessment of FMs revealed a sensitivity of 80% and a specificity of 100%. The authors pointed out that the results of their study facilitated the decision as to who required early intervention in a country with limited health care resources.59
Basal ganglia and thalami damage associated with mild/moderate/severe white matter changes with or without cortical injury is usually associated with an adverse neurological outcome. These MRI findings correlate with absent FMs. If, however, an infant with such a brain injury has normal FMs, there is a fair chance of a normal neurological outcome.60
MRI is not available at all times and in all places. Hence, early identification of a high risk for hemiplegia in infants with cerebral infarction on the basis of MRI is not always feasible. Here, too, observation comes into play: absent FMs and the presence of asymmetrical wrist movements indicate a need for early rehabilitation.38
FMs associated with genetic disordersA case report of an infant with DiGeorge syndrome (del22q11.2) revealed that this infant had normal FMs.22 However, many infants with trisomy 21 (Down syndrome) show abnormal FMs.21,22
The fact that none of the 14 (published) individuals later diagnosed with Rett syndrome had had normal FMs was certainly surprising, as a normal early development had been considered as one of the criteria for typical Rett syndrome.61 FMs were either absent; abnormally jerky and too slow; or abnormally jerky, abrupt, and disorganized.27,62–64 FMs were also missing in a 4-month-old boy who was later diagnosed with Smith-Magenis syndrome.65 An absence of FMs associated with subtle dysmorphic features justifies referral for genetic evaluation, which may facilitate earlier diagnosis.
FMs in infants later diagnosed with autism spectrum disordersVarious authors have published on the assessment of FMs in, individuals later diagnosed with autism spectrum disorders.22,23,26–28 Ten individuals were reported to have normal FMs, 12 had abnormal FMs, and four showed no FMs at all. The rate of abnormal FMs was exceedingly high in infants later diagnosed with autism.27 The present authors endorse further studies on GMs in high-risk siblings to evaluate the predictive power of abnormal FMs that are otherwise rare, even in infants with brain injury.
Conclusion and perspectiveFMs are tiny in appearance but have frequently proved enormously valuable as a reliable predictor of neurodevelopment. New efforts are being made for automated detection of deviations in the early motor repertoire using state-of-the-art sensor technologies and machine learning. Nonetheless, gestalt perception is efficient and well-established, and therefore remains the classical GMA, with smartphone-based solutions currently under development to further disseminate it as a method.
FundingRobert Peharz was supported by BEE-PRI, Brain, Ears & Eyes – Pattern Recognition Initiative, funded by BioTechMed Graz. Research for a smartphone-based GMA tool, the GMApp (gmapp.idn-research.org), was funded by the Grand Challenges Explorations grant of the Bill and Melinda Gates Foundation (OPP112887).
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank Miha Tavcar (scriptophil) for copy editing the manuscript.
Please cite this article as: Einspieler C, Peharz R, Marschik PB. Fidgety movements – tiny in appearance, but huge in impact. J Pediatr (Rio J). 2016;92(3 Suppl 1):S64–70.