To evaluate the association between intra-ventricular hemorrhage and habituation responses to external stimuli in preterm infants at 36–38 weeks post-conceptual age.
MethodsCross-sectional study of infants with gestational age <32 weeks. Intra-ventricular hemorrhage was identified by cranial ultrasonography and classified according to Papile et al. (1978). The luminous (flashlight), sound (rattle, bell), and tactile stimuli were presented, and the responses were scored according to Lester and Tronik (2004). Habituation response scores were compared between groups by Student's t-test. The association between IVH and habituation scores was evaluated by linear regression adjusted for GA, clinical severity score, post-conceptual age at habituation assessment, sepsis, and bronchopulmonary dysplasia.
ResultsSixty-five infants were studied, 20 with intra-ventricular hemorrhage (16 grades I/II; four grades III/IV) and 45 without intra-ventricular hemorrhage. Infants with intra-ventricular hemorrhage had lower gestational age (28.2±2.2 vs. 29.7±1.7 weeks) and birth weight (990±305 vs. 1275±360g). Infants with intra-ventricular hemorrhage at 36–38 weeks post-conceptual age had lower habituation scores to light (4.21±2.23 vs. 6.09±2.44), rattle (3.84±2.12 vs. 6.18±2.27), and bell (3.58±1.74 vs. 5.20±2.47) after controlling for confounders. No differences were found for tactile stimulus.
ConclusionInfants with gestational age <32 weeks and intra-ventricular hemorrhage had poorer habituation responses to external stimuli than those without intra-ventricular hemorrhage at 36–38 weeks post-conceptual age.
Avaliar a associação entre hemorragia intraventricular e as respostas de habituação a estímulos externos em neonatos prematuros com idade pós-conceptual de 36–38 semanas.
MétodosEstudo transversal com neonatos com idade gestacional <32 semanas. A hemorragia intraventricular foi identificada por ultrassonografia craniana e classificada de acordo com Papile et al. (1978). Os estímulos luminosos (lanterna), sonoros (chocalho, sino) e táteis foram apresentados e as respostas foram pontuadas de acordo com Lester & Tronik(2004). Os escores das respostas de habituação foram comparadas entre os grupos pelo teste t de Student. A associação entre a hemorragia intraventricular e os escores de habituação foi avaliada por regressão linear ajustada para a idade gestacional, escore de gravidade clínica, idade pós-conceptual na avaliação da habituação, sepse e displasia broncopulmonar.
Resultados65 neonatos foram estudados, 20 com hemorragia intraventricular (16 graus I/II; 4 graus III/IV) e 45 sem hemorragia intraventricular. Os neonatos com hemorragia intraventricular apresentaram menor idade gestacional (28,2±2,2 vs. 29,7±1,7 semanas) e peso ao nascer (990±305 vs. 1275±360g). Os neonatos com hemorragia intraventricular na idade pós-conceptual de 36–38 semanas apresentaram escores de habituação menores a luz (4,21±2,23 vs. 6,09±2,44), chocalho (3,84±2,12 vs. 6,18±2,27) e campainha (3,58±1,74 vs. 5,20±2,47) após controle para variáveis de confusão. Nenhuma diferença foi encontrada para os estímulos táteis.
ConclusãoNeonatos com idade gestacional <32 semanas e hemorragia intraventricular apresentaram respostas de habituação piores a estímulos externos que os sem hemorragia intraventricular, na idade pós-conceptual de 36–38 semanas.
Survival of extremely preterm infants has increased, but the incidence of intra-ventricular hemorrhage (IVH) remains high.1 In a French cohort of 3495 infants with gestational age <32 weeks, the frequency of IVH classified according to Papile et al.2 was as follows: Grade I—17.0% (95% confidence interval 15.7–18.4), Grade II—12.1% (95% CI 11.0–13.3), Grade III—3.3% (95% CI 2.7–3.9), and Grade IV—3.8% (95% CI 3.2–4.5).3 In a multicenter prospective study of preterm infants with <32 weeks of gestational age born at 10 American hospitals, grade III/IV IVH was present in 5%.4
The association of IVH and moderate or severe neurodevelopmental impair (NDI: moderate or severe cerebral palsy, and/or moderate or severe cognitive delay, and/or severe visual impairment, and/or severe hearing impairment) at 18–24 months of corrected age of infants with gestational age <34 weeks was evaluated by meta-analysis. Among survivors and adjusted for confounders, NDI was higher in preterm infants with mild IVH (Grades I/II) vs. those without IVH (OR 1.34; 95% CI 1.09–1.77), in those with severe IVH (Grades III/IV) vs. those without IVH (OR 2.44; 95% CI 1.73–3.42), and in those with severe vs. mild IVH (OR 2.16; 95% CI 2.36–3.43).5
Recently, some studies have shown an association between less-optimal neurobehavior in preterm infants assessed at term corrected age by the NICU Neonatal Neurobehavioral Network Scale (NNNS)6 and development delay in infancy.7,8 It considers not only the organizational state of the patient, but also the limits of that organization, which can be tested through stressful situations. The integrity of the modulation systems allows the organization of behavioral responses to stressful situations.9 One of the domains of the neurobehavioral assessment is the habituation response, which tests perceptual and cognitive competences in the neonatal period.10 Habituation scores evaluate the ability of the newborn infant to diminish or eliminate the response to a stimulus when it is presented for a long period of time or for several repeated short periods; however, when a novel stimulus is presented, the infant's attention to the stimulus will subsequently increase. This pattern of response reflects information processing capacity. The delay in the habituation response to external stimuli means a lower ability to modulate the behavioral response to a stressful stimulus.11
Few studies have examined the habituation responses in preterm infants at term age, and the results are contradictory. Castillo et al. evaluated preterm infants with <32 weeks gestation age prospectively and, at term age, they presented similar habituation responses compared to term infants assessed in the first days of life.12 Pineda et al. found that preterm infants, at term age, had poorer habituation responses compared to term infants.13 The literature on the association between cerebral injury and neurobehavior of preterm infants at term corrected age using NNNS is scarce. In the existing studies, severe IVH was associated to poorer attention, more excitability, and greater number of non-optimal responses when reflexes are tested.13,14 Only Pineda et al. evaluated the association between cerebral injury and habituation responses, and they found no association.
In this context, the objective of this study was to evaluate the association between IVH and habituation responses to external stimuli in preterm infants with <32 weeks of gestational age, at 36–38 weeks of corrected gestational age.
Materials and methodsThis was a cross-sectional study of preterm infants born in a third-level public university hospital in São Paulo, Brazil, between June 2015 and March 2017. The study was approved by the Ethical Committee of the institution (CAAE 44415915.0.0000.5505). Newborn infants were enrolled after their parents signed the informed consent. The authors included consecutively born preterm infants with gestational age <32 weeks, according to the best obstetric estimate. Neonates with congenital malformations, genetic syndromes, congenital infections, muscular or other neurological disorders, or those who died during hospitalization were excluded.
Head ultrasounds were performed in the unit at least three times in the first month after birth: at days of life 3–4, 7–10, and 28–30, with the Acuson X300 Ultrasound System (Siemens Healthineers, Erlangen, Germany) and a 3.5MHz transducer. The anterior fontanelle window was used, with axial, coronal, and sagittal incidences. The exams were performed by radiologists of the Imaging Department of Escola Paulista de Medicina, Federal University of São Paulo, blind to infants’ behavioral assessment. The exams were always reviewed by the senior pediatric radiologist. Hemorrhage, if present in one or more exams, was classified according to Papile et al.2 The highest degree of hemorrhage observed in the exams was considered for each newborn, when present.
The habituation responses were evaluated with the infant dressed, in a warm and calm room, with low luminosity, in a regular crib, before hospital discharge, at 36–38 weeks of corrected gestational age. At the time of the examination, infants were in room-air, without venous access, and receiving oral feeding. All exams were performed by the first author, who was previously trained by a researcher certified to perform the NNNS at Rhode Island Hospital, Brown University, Rhode Island, according to the Maternal Lifestyle Study.15 The neurobehavioral exam started with the observation of the sleep–wake state, and evaluation of the habituation responses to external stimuli was initiated only when the patient was in the required sleep-wake state for the test.6
The external stimuli were always presented in the same order: light stimulus (a standard eight-inch flashlight was shined 30cm from the infant, directly into the eyes for 2s); auditory stimulus—rattle and bell (the rattle was briskly shaken 30cm from the infant twice, and after, the same action was performed with the bell); tactile stimulus (the heel was gently but firmly pressed with a plastic stick). The light and sound stimuli were presented up to 10 times, and the tactile stimulus was presented up to five times. The next stimulus was presented five seconds after the infant eliminated the response. To score the response, the stimulus to which the infant responded first was considered as the initial presentation. If, after two trials, there was still no response or if the infant's response to the stimulus lasted longer than 45s, the next habituation item was applied.
Habituation response was assessed based on the infant's ability to eliminate, diminish, or delay his/her responses to the repeated presentation of a stimulus. Response was considered present if the infant showed whole body or limb movement, writhing movements, or eye blinks with facial grimace. The responses were scored as follow: 1—no decrement in response over 10 stimuli; 2—no response decrement, but some delay in the responses over the 10 trials; 3—some response decrement over the 10 trials, but elimination was not complete; 4—some response decrement and delay in the responses over 10 trials, but elimination was not complete; 5—elimination of responses after nine stimuli; 6—elimination of responses after seven to eight stimuli; 7—elimination of responses after five to six stimuli; 8—elimination of responses after three to four stimuli; 9—elimination of responses after one to two stimuli. The scores from 5–9 indicate successful habituation, and scores of 4 or lower were considered as an incomplete response elimination.6
For tactile stimuli, the habituation responses were scored as follow: 1—response generalized to whole body and increased over trials; 2—both feet withdrew together with no decrement of response; 3—variable response to stimulus with response decrement, but not localized to stimulated leg; 4—response decrement after five trials localized to stimulated leg and no change in alertness state; 5—response decrement after five trials localized to stimulated foot and no change in alertness state; 6—response limited to stimulated foot after three to four trials and no change in alertness state; 7—response limited to stimulated foot or complete decrement of response after 1–2 trials and no change in alertness state; 8—response localized and minimal after two trials and change in alertness state; 9—complete response elimination and change in alertness state. For habituation to tactile stimuli, there is no definition of the score above which habituation is considered present.6
The following data were collected: maternal characteristics, neonatal demographic characteristics, the Score for Neonatal Acute Physiology—Perinatal Extension (SNAPPE-II),16 and morbidity during hospital stay. Therapeutic interventions such as mechanical ventilation (presence and number of days), exogenous surfactant, ibuprofen, analgesics, sedatives, and postnatal use of steroids were also collected. At discharge, the studied data included chronological age, post-conceptual age, weight, and head circumference.
The sample size required for the detection of an average difference of 1.0 point in the habituation scores between preterm infants with and without IVH, with a standard deviation of 1.0, an alpha error of 5%, and a power of 80%, was 16 newborns in each group.
Habituation responses’ scores were compared between groups by Student's t-test. Four multiple linear regressions models were constructed with habituation scores for light, rattle, bell, and tactile stimuli as the dependent variables and IVH as an independent variable, which was adjusted for the following confounders: gestational age, age at habituation assessment, clinical sepsis, bronchopulmonary dysplasia (oxygen dependence with 28 days), and SNAPPE-II score >15 (median score of the study population), assuming the minimum number of 10 patients for each factor included in the analysis. The analysis was performed with SPSS (IBM SPSS Statistics for Windows, v.20.0, Somers, NY, USA).
ResultsFrom June 2015 to March 2017, 130 preterm infants with gestational age <32 weeks were born in the studied hospital. Among these, 65 met one or more exclusion criteria: congenital malformations (27), congenital infections (5), death during hospitalization (23), refusal to participate in the study (6), and not assessed (4). Therefore, 65 babies were studied.
Preterm infants performed an average of 3.1±1.5 (range: 1–9) head ultrasounds during their hospital stay. The first exam was conducted on the 65 infants at a median of 5 (1–17) days of life; 59 (91%) had two exams at 13 (6–39) days of life; 38 (58%) had three exams at 26 (12–52) days of life; 21 (32%) had four exams at 32 (17–69) days of life; 9 (14%) had five exams at 41 (30–58) days of life; and 8 (12%) infants had more than five exams during hospital stay.
IVH was identified in 20 (30.8%) infants, being grade I in 12 (60.0%), grade II in 4 (20.0%), grade III in 2 (10.0%), and grade IV in 2 (10.0%) neonates. In 11 neonates (55%), the diagnosis of IVH was obtained within the first 10 days of life.
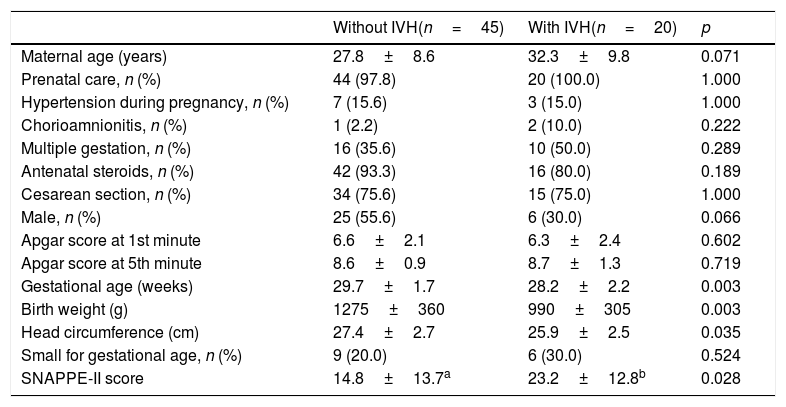
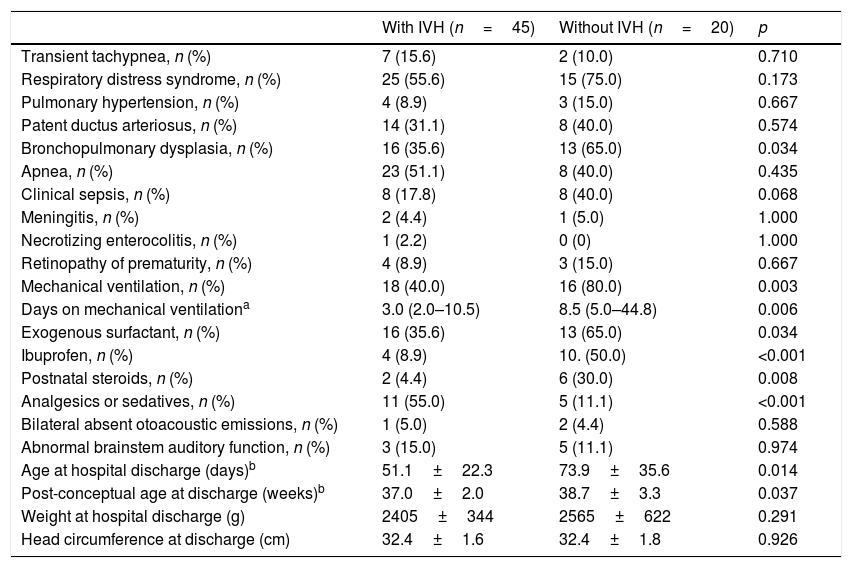
Demographic and clinical characteristics of the mothers and infants with and without IVH were similar, except that preterm infants with IVH, compared to those without IVH, had a lower gestational age, birth weight, and head circumference at birth, and a higher SNAPPE-II score (Table 1). Only one mother in each group used marijuana during gestation. Table 2 shows the morbidity and therapeutic interventions received during hospital stay. Infants with IVH more frequently used ibuprofen and sedatives and/or analgesics than those without IVH; however, they did not use any of these medications at the time of the neurobehavioral exam. Although three infants had bilaterally absent otoacoustic emissions and eight had abnormal auditory brainstem auditory function, their tonal threshold was between 40 and 50dB. As the sound intensity of the rattle and the bell is 90–100dB, the neonates were able to hear the rattle and the bell.
Maternal and neonatal characteristics, according to the presence of IVH.
| Without IVH(n=45) | With IVH(n=20) | p | |
|---|---|---|---|
| Maternal age (years) | 27.8±8.6 | 32.3±9.8 | 0.071 |
| Prenatal care, n (%) | 44 (97.8) | 20 (100.0) | 1.000 |
| Hypertension during pregnancy, n (%) | 7 (15.6) | 3 (15.0) | 1.000 |
| Chorioamnionitis, n (%) | 1 (2.2) | 2 (10.0) | 0.222 |
| Multiple gestation, n (%) | 16 (35.6) | 10 (50.0) | 0.289 |
| Antenatal steroids, n (%) | 42 (93.3) | 16 (80.0) | 0.189 |
| Cesarean section, n (%) | 34 (75.6) | 15 (75.0) | 1.000 |
| Male, n (%) | 25 (55.6) | 6 (30.0) | 0.066 |
| Apgar score at 1st minute | 6.6±2.1 | 6.3±2.4 | 0.602 |
| Apgar score at 5th minute | 8.6±0.9 | 8.7±1.3 | 0.719 |
| Gestational age (weeks) | 29.7±1.7 | 28.2±2.2 | 0.003 |
| Birth weight (g) | 1275±360 | 990±305 | 0.003 |
| Head circumference (cm) | 27.4±2.7 | 25.9±2.5 | 0.035 |
| Small for gestational age, n (%) | 9 (20.0) | 6 (30.0) | 0.524 |
| SNAPPE-II score | 14.8±13.7a | 23.2±12.8b | 0.028 |
IVH, peri/intraventricular hemorrhage; SNAPPE-II, Score for Neonatal Acute Physiology, Perinatal Extension, Version II.
Neonatal morbidity and therapeutic interventions, according to IVH.
| With IVH (n=45) | Without IVH (n=20) | p | |
|---|---|---|---|
| Transient tachypnea, n (%) | 7 (15.6) | 2 (10.0) | 0.710 |
| Respiratory distress syndrome, n (%) | 25 (55.6) | 15 (75.0) | 0.173 |
| Pulmonary hypertension, n (%) | 4 (8.9) | 3 (15.0) | 0.667 |
| Patent ductus arteriosus, n (%) | 14 (31.1) | 8 (40.0) | 0.574 |
| Bronchopulmonary dysplasia, n (%) | 16 (35.6) | 13 (65.0) | 0.034 |
| Apnea, n (%) | 23 (51.1) | 8 (40.0) | 0.435 |
| Clinical sepsis, n (%) | 8 (17.8) | 8 (40.0) | 0.068 |
| Meningitis, n (%) | 2 (4.4) | 1 (5.0) | 1.000 |
| Necrotizing enterocolitis, n (%) | 1 (2.2) | 0 (0) | 1.000 |
| Retinopathy of prematurity, n (%) | 4 (8.9) | 3 (15.0) | 0.667 |
| Mechanical ventilation, n (%) | 18 (40.0) | 16 (80.0) | 0.003 |
| Days on mechanical ventilationa | 3.0 (2.0–10.5) | 8.5 (5.0–44.8) | 0.006 |
| Exogenous surfactant, n (%) | 16 (35.6) | 13 (65.0) | 0.034 |
| Ibuprofen, n (%) | 4 (8.9) | 10. (50.0) | <0.001 |
| Postnatal steroids, n (%) | 2 (4.4) | 6 (30.0) | 0.008 |
| Analgesics or sedatives, n (%) | 11 (55.0) | 5 (11.1) | <0.001 |
| Bilateral absent otoacoustic emissions, n (%) | 1 (5.0) | 2 (4.4) | 0.588 |
| Abnormal brainstem auditory function, n (%) | 3 (15.0) | 5 (11.1) | 0.974 |
| Age at hospital discharge (days)b | 51.1±22.3 | 73.9±35.6 | 0.014 |
| Post-conceptual age at discharge (weeks)b | 37.0±2.0 | 38.7±3.3 | 0.037 |
| Weight at hospital discharge (g) | 2405±344 | 2565±622 | 0.291 |
| Head circumference at discharge (cm) | 32.4±1.6 | 32.4±1.8 | 0.926 |
IVH, peri/intraventricular hemorrhage.
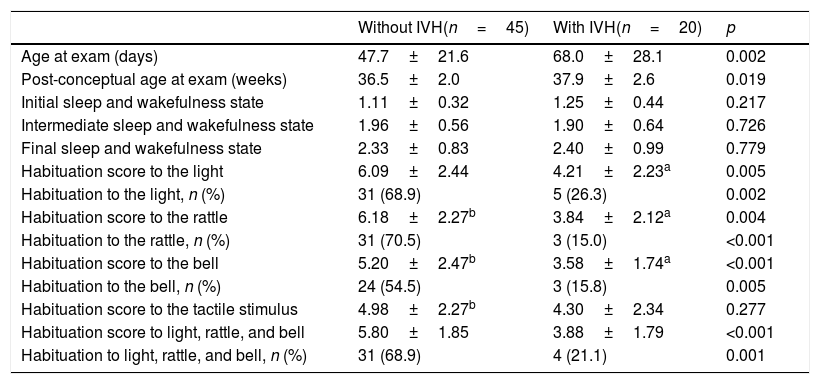
Table 3 shows the neonatal habituation responses to external stimuli. The evaluation was performed at a higher postnatal and post-conceptual age in preterm infants with IVH. No differences were found between infants with and without IVH in relation to time after last feeding at assessment (66±21 vs. 69±46min; p=0.701) or duration of the exam (13±4 vs. 13±5min; p=0.812). Preterm infants with IVH presented lower habituation scores to light and sound, but no differences in the tactile stimulus. Preterm neonates without IVH had a complete habituation response to light and sound after seven to eight stimuli, whereas those with IVH showed a decrease and a delay in the response to the stimulus, but were not fully habituated. When comparing the frequency of success in the habituation response to light and sound, this was less frequent in neonates with IVH compared to those without IVH. The association between the presence of IVH and the variation of the habituation responses to the different stimuli showed that IVH was associated with the reduction of habituation scores to light and sound, even after controlling for gestational age at birth, age at behavioral assessment, clinical sepsis, bronchopulmonary dysplasia and the SNAPPE-II severity score (Table 4). IVH was not associated with the tactile stimulus habituation score.
Evaluation of the habituation responses of the studied neonates, according to the presence of IVH.
| Without IVH(n=45) | With IVH(n=20) | p | |
|---|---|---|---|
| Age at exam (days) | 47.7±21.6 | 68.0±28.1 | 0.002 |
| Post-conceptual age at exam (weeks) | 36.5±2.0 | 37.9±2.6 | 0.019 |
| Initial sleep and wakefulness state | 1.11±0.32 | 1.25±0.44 | 0.217 |
| Intermediate sleep and wakefulness state | 1.96±0.56 | 1.90±0.64 | 0.726 |
| Final sleep and wakefulness state | 2.33±0.83 | 2.40±0.99 | 0.779 |
| Habituation score to the light | 6.09±2.44 | 4.21±2.23a | 0.005 |
| Habituation to the light, n (%) | 31 (68.9) | 5 (26.3) | 0.002 |
| Habituation score to the rattle | 6.18±2.27b | 3.84±2.12a | 0.004 |
| Habituation to the rattle, n (%) | 31 (70.5) | 3 (15.0) | <0.001 |
| Habituation score to the bell | 5.20±2.47b | 3.58±1.74a | <0.001 |
| Habituation to the bell, n (%) | 24 (54.5) | 3 (15.8) | 0.005 |
| Habituation score to the tactile stimulus | 4.98±2.27b | 4.30±2.34 | 0.277 |
| Habituation score to light, rattle, and bell | 5.80±1.85 | 3.88±1.79 | <0.001 |
| Habituation to light, rattle, and bell, n (%) | 31 (68.9) | 4 (21.1) | 0.001 |
IVH, peri/intraventricular hemorrhage.
Association between IVH and habituation scores by multiple linear regression analysis.
| B coefficient (95% CI) | p | |
|---|---|---|
| Habituation to the light: p=0.012; r2=0.116 | ||
| IVH | −1.465 (−2.893; −0.037) | 0.044 |
| Sepsis | −1.256 (−2.734; 0.222) | 0.094 |
| Habituation to the rattle: p=0.001; r2=0.176 | ||
| IVH | −2.291 (−3.584; −1.017) | 0.001 |
| Habituation to the bell: p=0.011; r2=0.095 | ||
| IVH | −1.697 (−2.996; −0.398) | 0.011 |
| Habituation to light, rattle, and bell: p=0.001; r2=0.167 | ||
| IVH | −1.891 (−2.965; −0.917) | 0.001 |
| Habituation to light, rattle, bell, and tactile stimulus: p=0.001; r2=0.159 | ||
| IVH | −1.600 (−2.526; −0.673) | 0.001 |
IVH, peri/intraventricular hemorrhage.
Preterm infants with <32 weeks gestational age with IVH, when examined before hospital discharge, have lower habituation responses to external light and sound stimuli compared to those without IVH. It should be noted that infants with IVH were assessed at a corrected age of 37.9 weeks and those without IVH, at 36.5 weeks. This happened because infants with IVH were hospitalized for longer periods, since they were more immature and sicker, and both groups were evaluated prior to hospital discharge. Therefore, the multiple analysis was adjusted for this potential confounders.
IVH was identified in 30.8% of the preterm infants, 80% being mild and 20% severe. The Vermont Oxford Trial Network Database Project reported a frequency of IVH of 26% among very low birth weight preterm infants admitted to 38 North-American neonatal units.17 More recently, the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network showed an incidence of severe IVH of 5% in infants with <28 weeks of gestational age.1 In 20 Brazilian public university hospitals, severe IVH was identified in 10% of preterm infants with gestational age from 23 to 33 weeks.18 A greater use of antenatal steroids and better practices in the neonatal care in Brazil are still needed to reduce the frequency of IVH in preterm infants.18
Studies have shown that IVH is associated with worse global development in childhood.7,8 In extreme preterm infants, those with grade III–IV IVH, compared to those without IVH, have higher rates of developmental delay (43.0% vs. 12.1%), cerebral palsy (30.1% vs. 6.5%), deafness (8.6% vs. 2.3%), and blindness (2.2% vs. 0.2%). Higher frequency of developmental impairment was also found in infants with grade I–II IVH compared to those without IVH: (22.0% vs. 12.1%), in addition to cerebral palsy (10.4% vs. 6.5%) and deafness (6.0% vs. 2.3%). Even after exclusion of other ultrasound abnormalities, including leukomalacia, porencephaly, and ventricular enlargement, isolated grade I–II IVH was associated with increased rates of moderate to severe neurosensory impairment (18.6 vs. 12.1%).19 In another study, infants with birth weight <1000g and grade I/II IVH had a higher frequency of MDI <70 and motor and neurosensory abnormalities compared to those without IVH.20 Another study also showed that even when the cerebral magnetic resonance was normal, extremely preterm infants have poorer cognitive and motor performance at 30 months of corrected age compared to term infants.21 Since behavioral changes in preterm infants assessed at term post-conceptual age are also associated with poorer development in childhood,7,8,22,23 it is worthwhile to identify neurobehavioral characteristics of preterm infants with brain lesions. The habituation responses are an important domain of neonatal neurobehavior, as they evaluate the perceptual and cognitive competences10 and reflect the capacity for information processing.11 Only one study evaluated the association between IVH and habituation responses to external stimuli in preterm infants, and the authors did not find an association between brain injury and habituation responses, although they found an association between cerebral lesions (cerebellar hemorrhage, severe IVH, or periventricular leukomalacia) and less self-regulation and greater excitability.13 The difference in relation to the present results may be due to a greater immaturity and a higher frequency of neonatal morbidities in the patients enrolled in the former study.
The central nervous system has a complex development and each function depends on the maturation of the corresponding anatomical neural substrate, which involves proliferation, migration, organization, and myelination of the neurons.24 In preterm infants between 23 and 32 weeks of gestational age, organization and myelination are rapidly developing and any brain injury at this stage may lead to changes in development and behavioral responses.25 Additionally, the environmental experience, such as the noxious nature of the NICU, may contribute to behavioral disorders.26 In the development of the habituation response, attention decreases as the newborn forms a mental representation of the stimulus and it becomes less interesting.27 The infant's attention toward a stimulus at first increases, as information about the stimulus is processed, and then decreases, as the stimulus processing is progressively completed.28 When a new stimulus is presented, the neonate compares it with the mental representation (memory), being able to discriminate the new stimuli.27 Preterm infants with IVH may have poorer organization and myelination of the neurons, interfering in the mental representation process and perhaps resulting in less habituation to external stimuli.
No difference was found between preterm infants with and without IVH in relation to habituation responses to tactile stimulus. Tactile and pain stimuli stimulate skin mechanical receptors and common afferent pathways to the central nervous system, and preterm infants may have a limited capacity to habituate to repetitive pain stimuli.29 Since these infants are exposed to repetitive pain procedures during their hospital stay, these aversive experiences may interfere in their capacity to habituate to the tactile stimulus presented in the present study.
The main limitation of this study is the sample size, which may have reduced the power to detect differences in the habituation response to the tactile stimulus between groups and limited the ability to control for other possible confounders. Also, it was not possible to evaluate the habituation responses of preterm infants with germinal matrix hemorrhage, intraventricular hemorrhage, post-hemorrhagic hydrocephalus, or hemorrhagic parenchymal infarction separately. The association between other brain lesions, such as periventricular leukomalacia, porencephaly, ventricular enlargement, or cerebellar hemorrhage13 and the infants’ behavior was not assessed, since magnetic resonance imaging was not obtained.
In conclusion, preterm infants with IVH showed a lower capacity to habituate to external light and sound stimuli at 36–38 weeks post-conceptual age compared to those without IVH, adjusted for confounders. Additional studies are needed to evaluate whether preterm infants with less habituation responses to external stimuli will have poorer neurodevelopment during childhood.
FundingThe principal investigator was funded by CNPQ: 128349/2015-8 and 119032/2016-3.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Bueno GG, Barros MC, Guinsburg R. Preterm infants with peri/intraventricular hemorrhage have poorer habituation responses to external stimuli. J Pediatr (Rio J). 2019:95:728–35.