To evaluate the prevalence and risk factors associated with progression to recurrent wheezing in preterm infants.
MethodsThe cross-sectional study was carried out in 2014 and 2015 and analyzed preterm infants born between 2011 and 2012. The search for these children was performed in a university maternity hospital and a Special Immunobiological Reference Center. The evaluation was performed through a questionnaire applied during a telephone interview.
ResultsThe study included 445 children aged 39 (18–54) months. In the univariate analysis, the risk factors with the greatest chance of recurrent wheezing were birth weight <1000g, gestational age <28 weeks, living with two or more siblings, food allergy, and atopic dermatitis in the child, as well as food allergy and asthma in the parents. In the multivariate analysis, there was a significant association between recurrent wheezing and gestational age at birth <28 weeks, food allergy and atopic dermatitis in the child, and living with two or more children. Of the 445 analyzed subjects, 194 received passive immunization against the respiratory syncytial virus, and 251 preterm infants were not immunized. There was a difference between the gestational age of these subgroups (p<0.001). The overall prevalence of recurrent wheezing was 27.4% (95% CI: 23.42–31.70), whereas in the children who received passive immunization it was 36.1% (95% CI: 29.55–43.03).
ConclusionsPersonal history of atopy, lower gestational age, and living with two or more children had a significant association with recurrent wheezing. Children with lower gestational age who received passive immunization against the respiratory syncytial virus had a higher prevalence of recurrent wheezing than the group with higher gestational age.
Avaliar a prevalência e os fatores de risco associados à evolução para sibilância recorrente em prematuros.
MétodosO estudo transversal foi feito em 2014 e 2015 e analisou crianças prematuras nascidas entre 2011 e 2012. A busca dessas crianças foi feita em maternidade de hospital universitário e em um Centro de Referência para Imunobiológicos Especiais. A avaliação foi feita por questionário dirigido em entrevista telefônica.
ResultadosO estudo incluiu 445 crianças com 39 (18–54) meses de vida. Na análise univariada, os fatores de risco com maior chance de sibilância recorrente foram peso de nascimento menor do que 1.000g, idade gestacional menor do que 28 semanas, convivência com dois ou mais irmãos, alergia alimentar e dermatite atópica na criança e alergia alimentar e asma nos pais. Na análise multivariada houve associação significativa entre sibilância recorrente e idade gestacional ao nascer menor do que 28 semanas, alergia alimentar e dermatite atópica na criança e a convivência com duas ou mais crianças. Dos 445 sujeitos analisados, 194 receberam imunização passiva contra vírus sincicial respiratório e 251 eram prematuros não imunizados. Houve diferença entre a idade gestacional desses subgrupos (p<0,001). A prevalência geral de sibilância recorrente foi 27,4% (IC 95%: 23,42–31,70) e nas crianças que receberam a imunização passiva foi 36,1% (IC 95%: 29,55–43,03).
ConclusõesHistória pessoal de atopia, menor idade gestacional e convivência com duas ou mais crianças apresentaram associação significativa com sibilância recorrente. As crianças com menor idade gestacional, que receberam a imunização passiva contra o vírus sincicial respiratório, apresentaram maior prevalência de sibilância recorrente que o grupo de maior idade gestacional.
Recurrent wheezing (RW) is an important cause of morbidity and has a high prevalence in the first years of life.1 The International Study of Wheezing in Infants (EISL), which standardizes its investigation, defines RW as the occurrence of three or more episodes of wheezing crises in 1 year.1 The wheezing represents turbulent airflow due to the reduced caliber of the lower airways, caused by obstruction or constriction.2 Approximately 90% of wheezing episodes in children up to the third year of life are caused by viral respiratory infections, mainly by the respiratory syncytial virus (RSV).3 Infant RW is associated with viral infections and asthma, in addition to other pulmonary and extrapulmonary diseases.4 Cohort studies have allowed the identification of RW phenotypes with the objective of diagnosing asthma and establishing prevention and therapeutic strategies.5
In the literature, the risk factors associated with RW are correlated with genetic characteristics, lower airway caliber, and exposure to environmental agents.5
Technological advances have resulted in an increase in the preterm infant population, who are at higher risk of developing recurrent wheezing, which interferes with morbidity rates and cost increases for public health services.6
Severe RSV infection is an important risk factor associated with RW.7 It is yet to be defined whether the infection by this virus results in RW or if the severe infection and wheezing are markers of an underlying vulnerability, such as atopy.8 This virus activates the standard T-helper type 2 (Th2) immune response.3,8 Some authors affirm that the occurrence of infection in the early stages of life can permanently alter the immune response, promoting RW and asthma.9 On the other hand, severe RSV infection may be a marker of genetic predisposition to asthma.10
Since the late 1990s, the monoclonal antibody Palivizumab has been used as a passive immunization method to reduce the rates of severe RSV infection in individuals at higher risk, i.e. premature infants with bronchopulmonary dysplasia or congenital heart disease with hemodynamic repercussions.11 According to the guidelines of the American Academy of Pediatrics, Palivizumab has a limited effect on respiratory infections in children with no risk factors for severe disease as well as minimal effects on subsequent wheezing, and its use is not recommended for asthma prevention or to reduce episodes of wheezing.11
Some studies have shown evidence of a causal effect of Palivizumab use on the reduction of wheezing rates in preterm infants.12–14 However, Simões et al.15 verified that the use of passive immunization only resulted in RW protection in children with no family history of atopy. This protective effect was not observed in the group of atopic children.15 In a cohort of previously healthy preterm infants, protection against RSV did not change the risk of asthma or pulmonary function test results at 6 years of age.16
The objectives of this study were to evaluate the prevalence of RW in a group of preterm infants and to analyze the risk factors associated with it—among the risk factors, our objective was to verify whether lower risk of severe RSV infection provided by passive immunization against RSV or a higher gestational age protected preterm infants from the progression to subsequent RW.
MethodsThe cross-sectional study was carried out between June 2014 and August 2015.
The study group included preterm infants born in 2011 and 2012, with a GA <37 weeks, defined by gestational ultrasound or by the Capurro method, at the Centro de Atendimento Integral à Saúde da Mulher (CAISM) (Women's Comprehensive Health Care Center)—Unicamp; and preterm children born between 2010 and 2012 in the area of the Regional Health Division-DIR VII (Health Secretariat of the State of São Paulo), who were referred to passive immunization against RSV (Palivizumab) in 2012 at the Special Immunobiological Reference Center (CRIE) of Unicamp.
The research tool was a questionnaire directed at risk factors associated with the reduced version of the EISL17 questionnaire, which investigated demographic data and risk factors for RW such as gender, ethnicity, maternal schooling, birth weight, GA at birth, breastfeeding, daycare attendance, exposure to pets, number of children in the same household, maternal smoking, caregiver smoking, personal history of food allergy and atopic dermatitis, parental history of food allergy or asthma, and protection against severe RSV infection through the use of Palivizumab. This questionnaire was applied to the parents or guardians through a telephone interview. Children whose parents or guardians were not found as well as those who died were excluded. The definition of RW was three or more wheezing crises within 1 year in the first year of life or in the last year before the interview.
Data were processed with the SPSS software, version 16.0 (SPSS Inc., Chicago, IL, USA). Qualitative variables were shown as absolute and relative frequencies, and their association was assessed with the χ2-test. The mean, standard deviation, median, minimum, and maximum values of the quantitative variables were determined, and the comparison of their distribution was calculated using the Mann–Whitney test. The level of significance was set at 5%. The non-adjusted odds ratio values, 95% CI, and the p-value of RW were determined in relation to each predictor variable through univariate logistic regression (Enter method). Subsequently, the predictor variables with p-value <0.200 in the univariate analysis were selected to comprise the multivariate logistic model. The Forward Stepwise method (Wald) was used, with an inclusion p-value of 0.05 and an exclusion p-value of 0.10. The prevalence of RW and its confidence interval were defined using the OpenEpi software, version 3.03a.
The research project was approved by the CAISM Research Committee (CP 019/2014) and by the Research Ethics Committee of Unicamp (Opinion n. 142,928/2012 and Opinion n. 1,030,707/2015). The study was exempted from the need for the Free and Informed Consent Form due to the impossibility of performing face-to-face interviews.
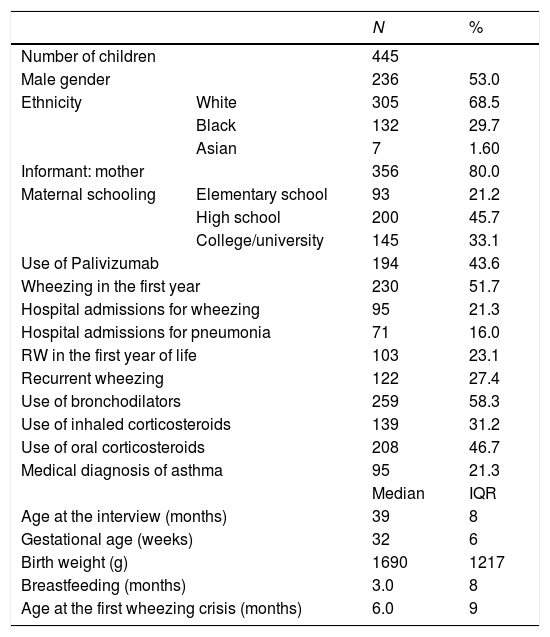
ResultsA total of 825 preterm infants were born in CAISM in 2011 and 2012. Telephone calls were made to all contact phones, and 261 children were located. Of the 10 that were excluded, five children had died and five were in the group that received Palivizumab. The parents or guardians of 251 children were interviewed. There were 425 children in the group that received Palivizumab in 2012. A total of 282 of these children were found, of which 84 were excluded for not being preterm, and four because of death. The parents or guardians of 194 children were interviewed. There were no refusals to the interview. In total, 445 preterm infants, aged 39 months (minimum: 18; maximum: 54; interquartile range: 8) and with gestational age of 32 weeks (minimum: 24; maximum: 36; interquartile range: 6) were assessed. The demographic and clinical characteristics of this group are shown in Table 1.
Demographic and clinical characteristics of the study group.
| N | % | ||
|---|---|---|---|
| Number of children | 445 | ||
| Male gender | 236 | 53.0 | |
| Ethnicity | White | 305 | 68.5 |
| Black | 132 | 29.7 | |
| Asian | 7 | 1.60 | |
| Informant: mother | 356 | 80.0 | |
| Maternal schooling | Elementary school | 93 | 21.2 |
| High school | 200 | 45.7 | |
| College/university | 145 | 33.1 | |
| Use of Palivizumab | 194 | 43.6 | |
| Wheezing in the first year | 230 | 51.7 | |
| Hospital admissions for wheezing | 95 | 21.3 | |
| Hospital admissions for pneumonia | 71 | 16.0 | |
| RW in the first year of life | 103 | 23.1 | |
| Recurrent wheezing | 122 | 27.4 | |
| Use of bronchodilators | 259 | 58.3 | |
| Use of inhaled corticosteroids | 139 | 31.2 | |
| Use of oral corticosteroids | 208 | 46.7 | |
| Medical diagnosis of asthma | 95 | 21.3 | |
| Median | IQR | ||
| Age at the interview (months) | 39 | 8 | |
| Gestational age (weeks) | 32 | 6 | |
| Birth weight (g) | 1690 | 1217 | |
| Breastfeeding (months) | 3.0 | 8 | |
| Age at the first wheezing crisis (months) | 6.0 | 9 | |
N, total number; %, percentage; RW, recurrent wheezing; IQR, interquartile range.
When the questionnaire was applied, the age of the children with RW was 39 months (minimum: 18; maximum: 50; interquartile range: 7). Among the children without RW, the age was 40 months (minimum: 22; maximum: 54; interquartile range: 9). Statistical analysis of the chronological ages did not show any significant difference (p=0.142).
The GA of the Palivizumab group was 28 weeks (minimum: 24; maximum: 36; interquartile range: 3.25). The GA of the non-immunized group was 34 weeks (minimum: 28; maximum: 36; interquartile range: 3). The evaluation of the association of GA in children who used Palivizumab and in those who did not showed a significant difference (p<0.001), and the group that received Palivizumab had a lower GA.
The overall prevalence of RW was 27.4% (95% CI: 23.42–31.70). In the group receiving Palivizumab, RW prevalence was 36.1% (95% CI: 29.55–43.03), and in the group without prophylaxis it was 20.7% (95% CI: 16.04–26.06). The group that received passive immunization had a higher RW prevalence (p<0.001).
Hospitalization due to wheezing crisis was more frequent in children with recurrent wheezing (p<0.001).
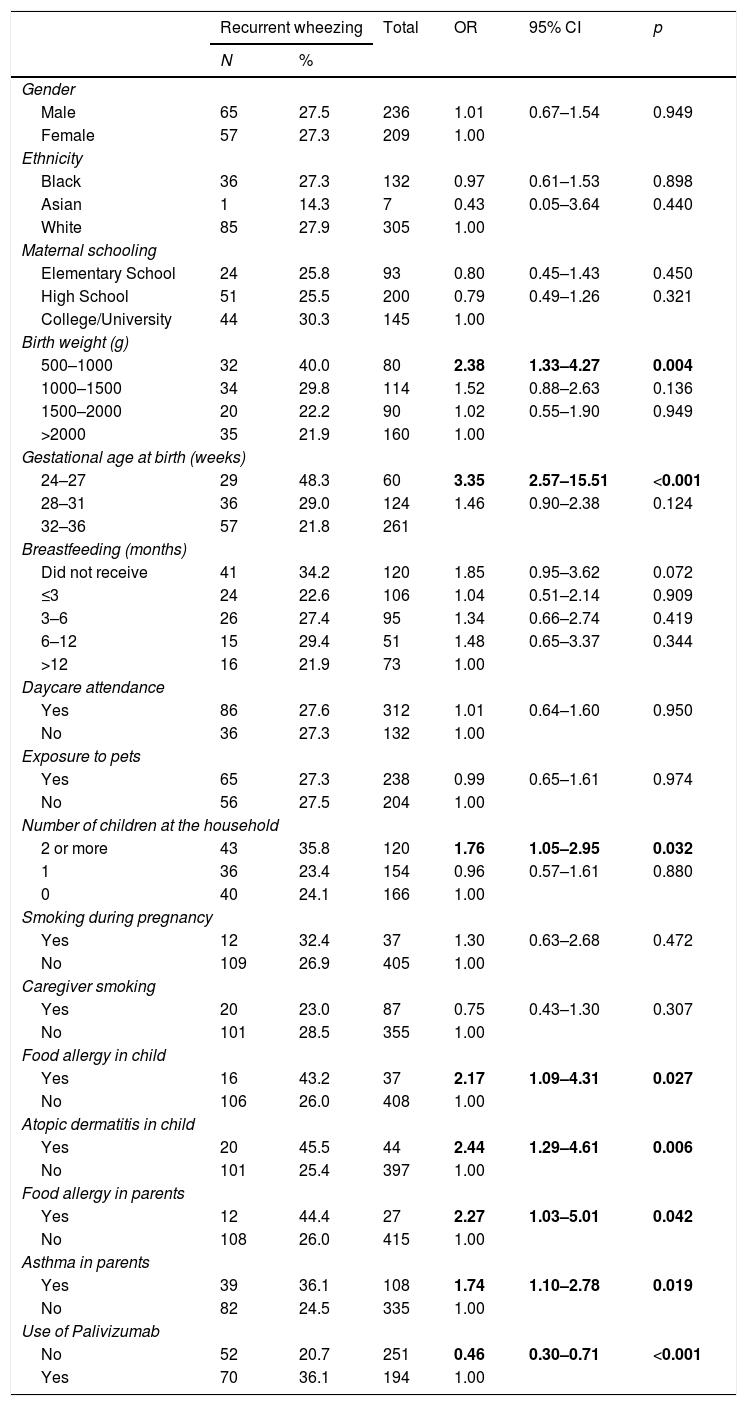
The analysis of risk factors for RW by univariate logistic regression did not find a statistically significant difference in relation to the following factors: gender, ethnicity, maternal schooling, birth weight>1000g, gestational age>28 weeks, breastfeeding, daycare attendance, exposure to pets, fewer than two children at home, maternal smoking, and caregiver smoking (Table 2).
Risk factors for recurrent wheezing.
| Recurrent wheezing | Total | OR | 95% CI | p | ||
|---|---|---|---|---|---|---|
| N | % | |||||
| Gender | ||||||
| Male | 65 | 27.5 | 236 | 1.01 | 0.67–1.54 | 0.949 |
| Female | 57 | 27.3 | 209 | 1.00 | ||
| Ethnicity | ||||||
| Black | 36 | 27.3 | 132 | 0.97 | 0.61–1.53 | 0.898 |
| Asian | 1 | 14.3 | 7 | 0.43 | 0.05–3.64 | 0.440 |
| White | 85 | 27.9 | 305 | 1.00 | ||
| Maternal schooling | ||||||
| Elementary School | 24 | 25.8 | 93 | 0.80 | 0.45–1.43 | 0.450 |
| High School | 51 | 25.5 | 200 | 0.79 | 0.49–1.26 | 0.321 |
| College/University | 44 | 30.3 | 145 | 1.00 | ||
| Birth weight (g) | ||||||
| 500–1000 | 32 | 40.0 | 80 | 2.38 | 1.33–4.27 | 0.004 |
| 1000–1500 | 34 | 29.8 | 114 | 1.52 | 0.88–2.63 | 0.136 |
| 1500–2000 | 20 | 22.2 | 90 | 1.02 | 0.55–1.90 | 0.949 |
| >2000 | 35 | 21.9 | 160 | 1.00 | ||
| Gestational age at birth (weeks) | ||||||
| 24–27 | 29 | 48.3 | 60 | 3.35 | 2.57–15.51 | <0.001 |
| 28–31 | 36 | 29.0 | 124 | 1.46 | 0.90–2.38 | 0.124 |
| 32–36 | 57 | 21.8 | 261 | |||
| Breastfeeding (months) | ||||||
| Did not receive | 41 | 34.2 | 120 | 1.85 | 0.95–3.62 | 0.072 |
| ≤3 | 24 | 22.6 | 106 | 1.04 | 0.51–2.14 | 0.909 |
| 3–6 | 26 | 27.4 | 95 | 1.34 | 0.66–2.74 | 0.419 |
| 6–12 | 15 | 29.4 | 51 | 1.48 | 0.65–3.37 | 0.344 |
| >12 | 16 | 21.9 | 73 | 1.00 | ||
| Daycare attendance | ||||||
| Yes | 86 | 27.6 | 312 | 1.01 | 0.64–1.60 | 0.950 |
| No | 36 | 27.3 | 132 | 1.00 | ||
| Exposure to pets | ||||||
| Yes | 65 | 27.3 | 238 | 0.99 | 0.65–1.61 | 0.974 |
| No | 56 | 27.5 | 204 | 1.00 | ||
| Number of children at the household | ||||||
| 2 or more | 43 | 35.8 | 120 | 1.76 | 1.05–2.95 | 0.032 |
| 1 | 36 | 23.4 | 154 | 0.96 | 0.57–1.61 | 0.880 |
| 0 | 40 | 24.1 | 166 | 1.00 | ||
| Smoking during pregnancy | ||||||
| Yes | 12 | 32.4 | 37 | 1.30 | 0.63–2.68 | 0.472 |
| No | 109 | 26.9 | 405 | 1.00 | ||
| Caregiver smoking | ||||||
| Yes | 20 | 23.0 | 87 | 0.75 | 0.43–1.30 | 0.307 |
| No | 101 | 28.5 | 355 | 1.00 | ||
| Food allergy in child | ||||||
| Yes | 16 | 43.2 | 37 | 2.17 | 1.09–4.31 | 0.027 |
| No | 106 | 26.0 | 408 | 1.00 | ||
| Atopic dermatitis in child | ||||||
| Yes | 20 | 45.5 | 44 | 2.44 | 1.29–4.61 | 0.006 |
| No | 101 | 25.4 | 397 | 1.00 | ||
| Food allergy in parents | ||||||
| Yes | 12 | 44.4 | 27 | 2.27 | 1.03–5.01 | 0.042 |
| No | 108 | 26.0 | 415 | 1.00 | ||
| Asthma in parents | ||||||
| Yes | 39 | 36.1 | 108 | 1.74 | 1.10–2.78 | 0.019 |
| No | 82 | 24.5 | 335 | 1.00 | ||
| Use of Palivizumab | ||||||
| No | 52 | 20.7 | 251 | 0.46 | 0.30–0.71 | <0.001 |
| Yes | 70 | 36.1 | 194 | 1.00 | ||
N, absolute frequency; %, relative frequency; p, probability of Wald test.
Regarding birth weight, the chance of the child having RW was 2.38 times greater if he/she was in the birth weight group between 500 and 1000g (OR: 2.38; 95% CI: 1.33–4.27; p=0.004) in relation to the group weighing more than 2000g. A gestational age of 24–27 weeks meant a 3.35-fold higher chance of belonging to the RW group (OR: 3.35; 95% CI: 1.86–6.01; p<0.001) compared to the group with gestational age at birth >37 weeks (Table 2).
The chance of RW was 76% higher when the child lived with two or more children in the same house (OR: 1.76; 95% CI: 1.05–2.95; p=0.032) (Table 2).
There was an association of RW and history of atopy in the children and family members. Food allergy in the child increased the chance of recurrent wheezing by 2.17 times (OR: 2.17; 95% CI: 1.09–4.31; p=0.027); atopic dermatitis in the child, by 2.44 times (OR: 2.44; 95% CI: 1.29–4.61; p=0.006); food allergy in the parents, by 2.27 times (OR 2.27; 95% CI 1.03–5.01; p=0.042); and asthma in the parents, by 74% (OR: 1.74; 95% CI: 1.10–2.78; p=0.019) (Table 2).
The group that did not receive passive immunization, with a higher gestational age, had a lower chance of recurrent wheezing (OR: 0.46; 95% CI: 0.30–0.71; p<0.001) (Table 2).
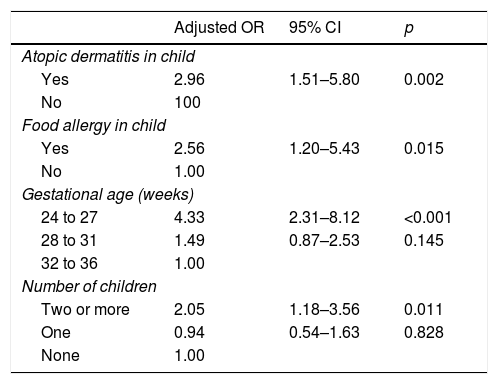
For the multivariate logistic regression analysis, the birth weight “risk factor” was not included due to the strong association with gestational age. After the final adjustments, the following variables remained in the model: gestational age between 24 and 27 weeks, in which the chance of having RW is 4.33-fold greater than in the group with 32–36 weeks of GA (adjusted OR=4.33; 95% CI: 2.31–8.12; p<0.001); living with two or more children, with a chance 2.05-fold greater than in families without other children (adjusted OR=2.05; 95% CI: 1.18–3.56; p=0.011); atopic dermatitis in the child, a group where the chance is 2.96-fold greater (adjusted OR=2.96; 95% CI: 1.51–5.8; p=0.002); and food allergy in the child, with a 2.56-fold greater chance of developing RW (adjusted OR=2.56; 95% CI: 1.20–5.43; p=0.015) (Table 3).
Multivariate analysis.
| Adjusted OR | 95% CI | p | |
|---|---|---|---|
| Atopic dermatitis in child | |||
| Yes | 2.96 | 1.51–5.80 | 0.002 |
| No | 100 | ||
| Food allergy in child | |||
| Yes | 2.56 | 1.20–5.43 | 0.015 |
| No | 1.00 | ||
| Gestational age (weeks) | |||
| 24 to 27 | 4.33 | 2.31–8.12 | <0.001 |
| 28 to 31 | 1.49 | 0.87–2.53 | 0.145 |
| 32 to 36 | 1.00 | ||
| Number of children | |||
| Two or more | 2.05 | 1.18–3.56 | 0.011 |
| One | 0.94 | 0.54–1.63 | 0.828 |
| None | 1.00 | ||
p, probability of Wald test; Adjusted OR, adjusted odds ratio; 95% CI, 95% confidence interval of the adjusted odds ratio.
The decision to evaluate RW in a group of preterm infants was motivated by the need to understand the risk factors associated with RW in this specific population.
The prevalence of RW found in this study was 27.4%, evaluated in a population of preterm infants heterogeneous in regard to the presence of comorbidities, with a high risk of severe RSV infection. In the study by Mallol et al.,1 published in 2016, there was a prevalence of 21.7% among children from the general population in the city of São Paulo, not considering prematurity and its comorbidities, but with demographic characteristics similar to the individuals in this study.1 A systematic review article on the risk of wheezing in preterm infants shows a prevalence rate of 31.6% of RW in preterm infants aged 4 years.18 Another systematic review and meta-analysis study showed a higher chance of RW in preterm patients, especially in children born with GA <32 weeks.19 We did not find any articles addressing the RW prevalence in individuals with characteristics similar to those evaluated in this study.
There was no association in this study group between RW and the risk factors of gender, ethnicity, maternal schooling, breastfeeding, daycare attendance, exposure to pets, maternal smoking, caregiver smoking, and Palivizumab immunization. However, an association was demonstrated between RW and birth weight <1000g, gestational age <28 weeks, living with two or more children in the same household, a personal history of atopy, and atopy in the parents. Hospital admission due to a wheezing crisis was more frequent in the group with recurrent wheezing, a finding that was similar to a study by the EISL group.1 This characteristic was not studied as a risk factor, and only its association was assessed.
A study performed with preterm infants shows that male gender is an independent risk factor associated with RW.20 Studies by the EISL group also demonstrate that male gender is associated with RW,21,22 but there are studies that found no difference between genders.23
EISL studies do not define ethnicity as a factor associated with RW,22,23 but one American study considered an African ancestry to be an independent factor associated with RW.24
Also, in some EISL studies, the level of maternal schooling was not a factor associated with a greater chance of RW.22,23 Other authors who analyzed this risk factor showed that a college- or university-degree level of schooling was a protective condition.21
The results of breastfeeding effects on RW are controversial in the literature, partly due to the fact that many studies are only observational.6 There is an association between RW and lack of breastfeeding in EISL studies.25 A publication evaluating this risk factor in preterm infants found no association between maternal breastfeeding and RW,20 as in this study.
Contrary to this study, several authors of studies using the EISL questionnaire showed an association between RW and daycare attendance.1,21–23,25
This study showed that the fact that two or more children live with the patient in the same household is significant, with a lower number of siblings not being important. Other EISL studies showed that there is no difference between the chance of RW and living with any number of siblings in the same household.22,23 A study carried out in Spain with preterm infants defines the presence of school-aged siblings as an independent risk factor associated with RW.26
Exposure to pets is not significant for RW according to the findings of EISL.22,23 However, the association between living with pets and RW has been previously published.25
Regarding the smoking risk factor, a systematic review that assessed the association between wheezing, maternal smoking in pregnancy, and postnatal exposure to cigarettes found an association between prenatal exposure to smoking and the occurrence of RW at 3–4 years of age.27 Another study showed that preterm birth associated with maternal smoking establishes a 3.8-fold higher probability of RW when compared to children born at term.28 A study carried out with preterm infants with GA <28 weeks did not find an association between smoking and RW, and its justification was the possibility that this factor was overcome by the inherent morbidity of extreme prematurity.29
Regarding the birth weight evaluation, this study showed that the lower the birth weight, the higher the chance of RW, which is similar to the findings of a French study carried out with preterm infants20 and a large EISL study.30 There is an association of low birth weight with preterm birth and intrauterine growth restriction, which are factors associated with pulmonary development impairment and reduction of pulmonary function.30 Systematic reviews have also associated preterm birth with RW.18,19 Simões et al.12 demonstrated an inversely proportional variation of RW with GA and birth weight.
Atopy is an important risk factor associated with RW in this study, as a personal history of food allergy and atopic dermatitis has been confirmed as providing a significant increase in the chance of RW in the study population. Studies have reported the association between food allergy and RW and asthma.15 Atopic dermatitis is a factor associated with RW in many EISL studies,21,23 as well as asthma in the parents.1,15,21–23,25
There is a complex association between severe RSV infection and RW development. This study evaluated several risk factors for RW in preterm infants, as previously discussed. The prevalence of RW was higher in the group of children who received passive immunization against RSV, which can be explained by their lower GA and lower weight.
In the literature, some articles associate the use of passive immunization against RSV and RW indices. Simões et al.12 report that the use of Palivizumab reduced the incidence of RW in a non-randomized cohort of preterm infants with GA <36 weeks without chronic lung disease. However, in another article15 by the same study group, when the presence of atopy in the family was evaluated the authors only found a reduction in RW indices in children with no family history of atopy. Yoshihara et al.14 also showed lower RW values in an observational study with preterm infants with GA of 33–35 weeks, whose outcome was the RW assessed in medical consultations. Blanken et al.13 carried out a randomized study that showed a reduction in days of wheezing in the first year of life of preterm infants who received passive immunization, without comorbidities, but Scheltema et al.16 reassessed this same cohort and showed that there was no change in the risk of asthma or pulmonary function at 6 years of age in relation to the use of Palivizumab. In its guidelines for Palivizumab use, the American Academy of Pediatrics (AAP) emphasizes its important role in reducing severe RSV infection; however, based on the articles described above, the AAP recommends that this prophylaxis should not be used either for the purpose of reducing subsequent episodes of RW or for asthma prevention.11
The multivariate analysis defined that atopic dermatitis, food allergy in the child, GA <28 weeks, and living with more than two children were risk factors for RW. This study confirmed that atopy is a risk factor associated with RW.
The smaller airway caliber related to GA is also one of the main risk factors associated with RW, as demonstrated by other authors.15,18,19 This is confirmed by the higher prevalence of RW in children with lower gestational age, even though they are protected against severe RSV infection by the passive immunization against RSV.
Despite the use of standardized tools in similar populations, the prevalence and risk factors associated with RW may vary, thus demonstrating that there is an interference of genetic, environmental, and cultural factors in each studied population.
As a study limitation, the association between the severity of wheezing crises and evolution with RW was not analyzed because there was no documentation regarding the presence of RSV as a causal agent in these events.
The high number of children whose parents or guardians were not interviewed was due to the great difficulty in locating them through the contact phones found in the CAISM and CRIE databases, as these registers were created 2–3 years before the interviews.
Conflicts of interestThe authors declare no conflicts of interest.
We would like to thank the staff at the Special Immunobiological Reference Center of Hospital das Clínicas of Unicamp for their support and collaboration in data collection.
Please cite this article as: Simões MC, Inoue Y, Matsunaga NY, Carvalho MR, Ribeiro GL, Morais EO, et al. Recurrent wheezing in preterm infants: Prevalence and risk factors. J Pediatr (Rio J). 2019;95:720–7.
Study conducted at Centro de Investigações em Pediatria (CIPED), Faculdade de Ciências Médicas (FCM), Universidade Estadual de Campinas (Unicamp), Campinas, SP, Brazil.