This narrative review aimed to provide practitioners a synthesis of the current knowledge on the role of a low Fermentable Oligosaccharides Disaccharides Monosaccharides and Polyols diet in reducing symptoms associated with functional abdominal pain disorders in children. This review is focused on the pathophysiology, efficacy and criticism of low Fermentable Oligosaccharides Disaccharides Monosaccharides and Polyols diet in children.
SourcesCochrane Database, Pubmed and Embase were searched using specific terms for Fermentable Oligosaccharides Disaccharides Monosaccharides and Polyols diet interventions and functional abdominal pain disorders.
Summary of the findingsIn children, only one Randomized Control Trial and one open-label study reported positive results of low Fermentable Oligosaccharides Disaccharides Monosaccharides and Polyols diet; one Randomized Control Trial showed exacerbation of symptoms with fructans in children with Irritable Bowel Syndrome; no effect was found for the lactose-free diet whilst fructose-restricted diets were effective in 5/6 studies.
ConclusionsIn children there are few trials evaluating low Fermentable Oligosaccharides Disaccharides Monosaccharides and Polyols in functional abdominal pain disorders, with encouraging data on the therapeutic efficacy particularly of fructose-restricted diet. Additional efforts are still needed to fill this research gap and clarify the most efficient way for tailoring dietary restrictions based on the patient's tolerance and/or identification of potential biomarkers of low Fermentable Oligosaccharides Disaccharides Monosaccharides and Polyols efficacy, to maintain nutritional adequacy and to simplify the adherence to diet by labeling Fermentable Oligosaccharides Disaccharides Monosaccharides and Polyols content in commercial products.
Nos últimos anos, foram feitos esforços consideráveis para esclarecer o papel da dieta com baixo teor de oligossacarídeos fermentáveis, dissacarídeos, monossacarídeos e polióis (FODMAPs) para o tratamento de distúrbios gastrintestinais funcionais (DGIFs). Esta revisão narrativa teve como objetivo fornecer aos profissionais uma síntese do conhecimento atual sobre o papel de uma dieta com baixo teor de FODMAPs (BFM) na redução dos sintomas associados a distúrbios funcionais de dor abdominal (DFDA) em crianças. Esta revisão está focada na fisiopatologia, eficácia e crítica da dieta BFM em crianças.
FontesO banco de dados Cochrane, Pubmed e Embase foram pesquisados com o uso dos termos específicos para intervenções na dieta FODMAP e DFDA.
Resumo dos achadosEm crianças, apenas um estudo controlado randomizado e um estudo aberto relataram resultados positivos da dieta BFM; um estudo controlado randomizado mostrou exacerbação dos sintomas com frutanos em crianças com síndrome do intestino irritável nenhum efeito foi encontrado para a dieta livre de lactose, enquanto dietas com restrição de frutose foram eficazes em 5/6 estudos.
ConclusõesExistem poucos estudos que avaliam BFM em DFDA em crianças, com dados encorajadores sobre a eficácia terapêutica, particularmente de dietas com restrição de frutose. Esforços adicionais ainda são necessários para preencher essa lacuna de pesquisa e esclarecer a maneira mais eficiente de adaptar as restrições dietéticas com base na tolerância do paciente e/ou identificação de biomarcadores potenciais de eficácia da BFM, para manter a adequação nutricional e simplificar a adesão à dieta, ao incluir informações sobre conteúdo de FODMAPs em rótulos de produtos comerciais.
The role of diet is increasingly emerging in different gastrointestinal disorders such as functional gastrointestinal (GI) disorders (FGIDs), irritable bowel syndrome (IBS), constipation, diverticular disease, and inflammatory bowel disease.1 FGIDs are common disorders characterized by chronic or recurrent GI symptoms not explained by biochemical or structural abnormalities.2 The pathogenesis of FGIDs remains poorly understood, although a complex altered interaction between gut and brain has been recently advocated.3 In the absence of specific biomarkers, FGIDs are clinically classified according to the Rome IV diagnostic criteria, mainly based on a thorough patient history.4,5 In children, FGIDs may be classified in three main categories: disorders of nausea and vomiting, disorders of defecation or pain-based (such as functional abdominal pain disorders [FAPDs]). FAPDs comprise four distinct disorders: IBS, functional dyspepsia, abdominal migraine, and FAP-not otherwise specified (FAP-NOS), meaning that the FAP does not fit the specific diagnostic pattern of one of the first three disorders.6 Functional dyspepsia and IBS frequently occur both in children and adults. The prevalence of IBS ranges from 10 to 25% of adults7 and 0 to 45% of children, respectively.8,9 IBS strongly impairs quality of life, social functioning, school attendance, and work productivity; it also substantially increases health care costs. In the pathogenesis of FGIDs different mechanisms have been proposed: increased pain sensitivity or visceral hypersensitivity,10,11 abnormal gut motility,12 small intestinal bacterial overgrowth,13 low-grade intestinal inflammation,14 infections,15–17 psychosocial factors,18 early-life events,19,20 cow's milk protein allergy,21,22 and dysregulated gut–brain axis.23,24 Familiar aggregation of FGIDs is commonly found and may be secondary to social learning and genetic factors.25 Gene variants coding for disaccharidases with defective or reduced enzymatic activity has been recently found to predispose to IBS in a subset of adult patients.26 The gut microbiome is also recognized as a key player in FGID and intestinal dysbiosis has been reported in patients with IBS compared to healthy subjects.27 This microbial diversity could lead to enhanced intestinal permeability, mucosal immune activation, altered gut motility, and visceral hypersensitivity.27 The concept of a strict relation between microbiota and immunity, motility, nervous system, stress, and behavior has resulted in the joined term of microbiome–gut–brain axis. This axis has emerged as responsible for the control and regulation of different GI and neurological functions.28 In this context, the importance of early adverse events and diet has long been recognized. The perinatal period with its pivotal influence in the maturation of both gut and brain is a critical period in which different determinants may predispose to the development of FGIDs.27
Most patients with FGIDs report that their GI symptoms are generically or specifically related to food, although evidence of this relation is often difficult to prove, particularly in children.29–31 Multiple interacting and confounding mechanisms may be involved in the patient's perceived “food intolerance.” In IBS patients, postprandial symptoms can be triggered by overfeeding, hyperactive gastrocolonic response, visceral sensitivity,32,33 dysbiosis, disordered gut–brain signaling,34 anticipation, allergies, and malabsorption. In the absence of readily available, easy to administer, reliable office-based tests, empirical dietary restrictions are often indicated in the absence of proven intolerance, malabsorption, or a confirmed diagnosis of food allergies.35 This is relevant, as changes in diet can interfere with the individual metabolism, intestinal motility, secretions, sensitivity, barrier function, gut microbiota,36 and adequate nutrition. As expected, given the heterogeneity of FGIDs, individual patients, and underlying factors, no single treatment has shown to be effective in all patients. Unnecessary diet restrictions are of particular concern in growing children. Therefore, it is of great importance to have a deep understanding of the evidence behind each dietary recommendation given to children, in order to design personalized treatment plans.
In the last years, great interest has been focused on dietary fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) for the treatment of FGIDs in adults and children. The low FODMAPs (LFM) diet restricts the intake of several fermentable carbohydrates, including foods containing fructans (wheat and onion), galacto-oligosaccharides (legumes and cabbage), lactose (dairy products), fructose in excess of glucose (pears and apples), and sugar polyols, such as sorbitol and mannitol (stone fruits and artificial sweeteners).18 Promising effect of the low FODMAP diet in reducing functional GI symptoms in adult patients has been shown,37 but there is limited evidence in children.38
This narrative review explores the available literature on LFM diets and aims to provide practitioners with a critical synthesis of the pathophysiology, mechanism of action, efficacy, and limits to help in identifying a diet-tailored intervention for FAPDs in children.
MethodsA literature search of MEDLINE (via PubMed), Embase, and the Cochrane Library databases was conducted, from inception to August 2018, focusing on LFM diet in relation with FAPDs. The following terms were queried: “FODMAP,” “FODMAPs,” “fermentable oligosaccharides, disaccharides, monosaccharides, and polyols,” “fermentable, poorly absorbed, short chain carbohydrates,” “lactose-free diet,” “fructose,” “fructans,” “sorbitol” AND “functional gastrointestinal disorders,” OR “functional abdominal pain,” “recurrent abdominal pain,” “irritable bowel syndrome,” “IBS.” The authors restricted the search to English language and children (aged 0–18 years). Because the purpose of this review was not to duplicate a recent systematic review on FODMAPs but rather to critically review the available literature and provide the clinicians a practical summary of LFD in children, the search was expanded to include prospective and retrospective studies, randomized controlled trials (RCTs), reviews, and editorials.
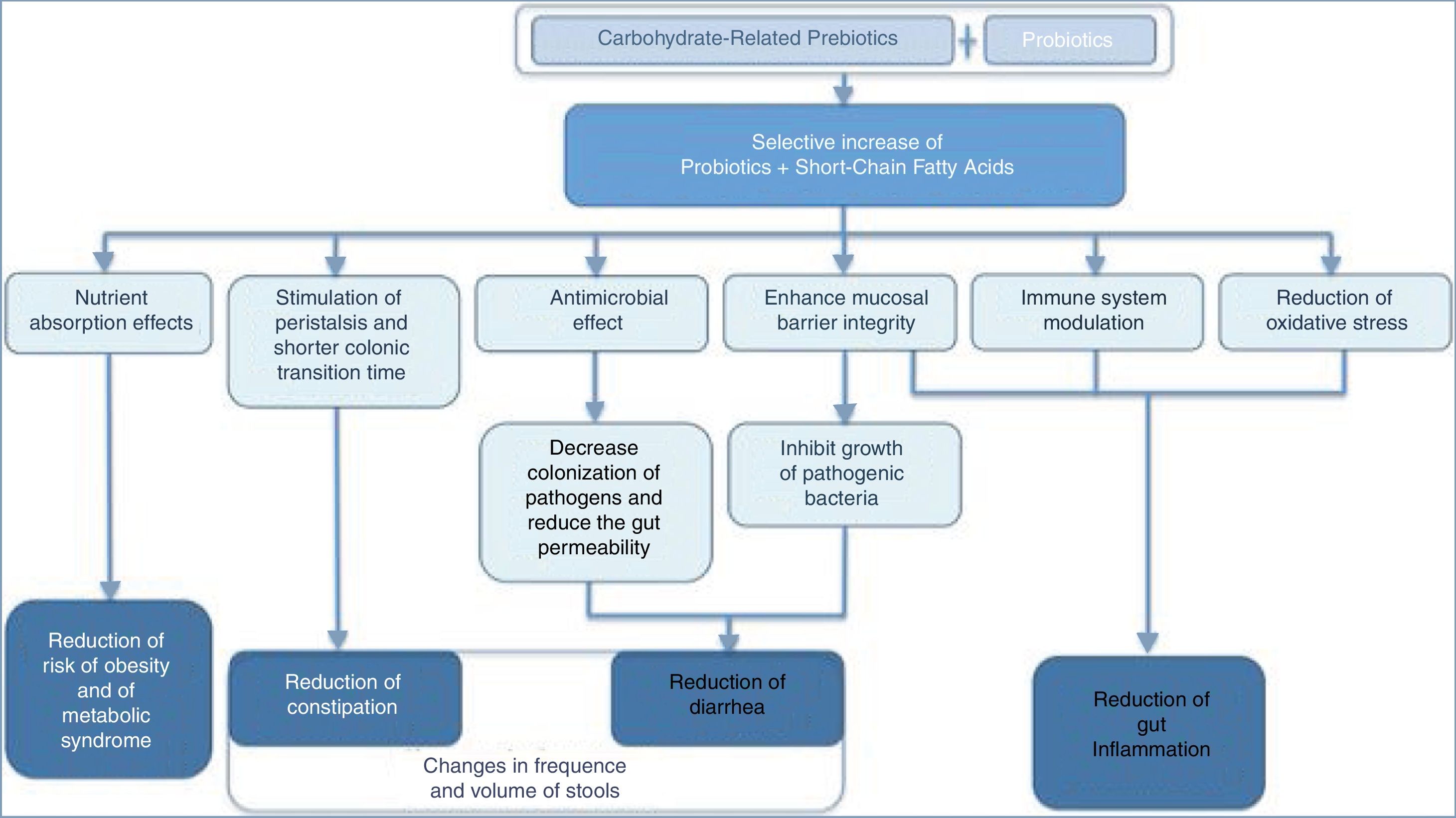
FODMAPsThroughout the 1980s and 1990s, clinical studies focused primarily on the role of lactose, fructose, and sorbitol intake as triggers of GI symptoms including abdominal pain, discomfort, bloating, distension, and diarrhea. In recent years, the focus has shifted to fructo-oligosaccharides (FOS) (fructans) and galacto-oligosaccharides (GOS) ingestion as possible culprits of IBS.39 Grouping all these fermentable short-chain carbohydrates and sugar alcohols together led to the acronym FODMAPs. Poor absorption, osmotic activity, and rapid fermentation by the luminal microbiome40 may result in excessive gas production,41 luminal distension, loose stools, and visceral hypersensitivity, features that are consistent with IBS. Moreover, small intestinal bacterial overgrowth that may occur in some patients with IBS can increase the metabolism of these carbohydrates and consequent gas production.39 Symptoms related to FODMAPs may also derive from changes in the gut microbiota and metabolism, endocrine cells, immune function, and intestinal barrier.42–45 Intriguingly, a non-restrictive FODMAP diet may be both associated with loose stools and with delayed gut transit time and constipation, as occurs in individuals with methane-producing microbiota.46,47 Furthermore, dietary intake of carbohydrate-related prebiotics (fibers) interacting with intestinal probiotics may have beneficial effects on human health48,49 (Fig. 1) .
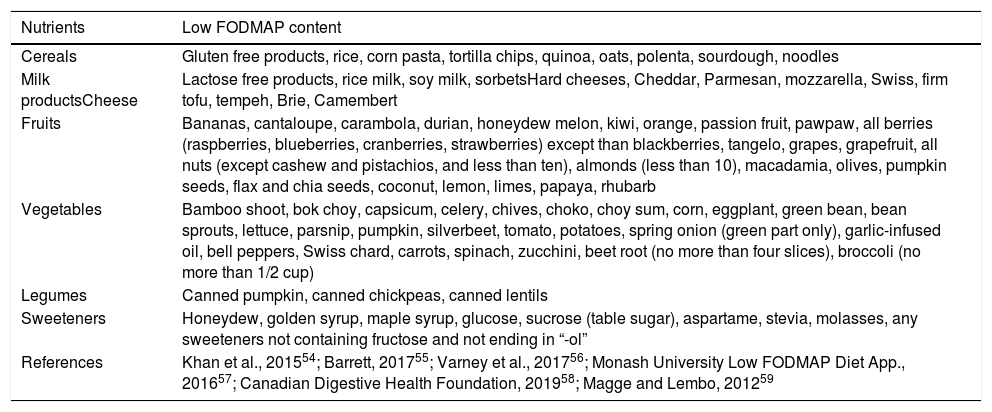
The LFM diet was first developed in 1999 by Dr. Gibson and Dr. Shepard at Monash University in Melbourne. In 2005, the same authors proposed that a diet high in FODMAPs might perpetuate (functional) gastrointestinal symptoms in patients with Crohn's disease.50 This concept was soon extrapolated to IBS patients. Early studies showed the positive effects of fructose and short-chain fructans restriction in adults with fructose malabsorption. Moreover, the co-ingestion of fructose and glucose counteracts the detrimental effect of the excess of free fructose in the small intestine of IBS patients with fructose malabsorption due to facilitated combined transport.51 Since then, multiple trials have been conducted and LFM is currently considered an effective dietary approach in adults with IBS,39 particularly when symptoms persist despite lifestyle changes and other dietary advice.52 FODMAPs are present in foods containing wheat, rye, barley, legumes, lentils, chickpeas, Brussels sprouts, asparagus, artichokes, beets, broccoli, cabbage, fennel, onions, garlic, leeks, okra, peas, shallots, apples, peaches, persimmon, watermelon, and pistachios.53 A list of food with low FODMAP content is shown in Table 1.54–59
List of foods with low fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAP) content.
| Nutrients | Low FODMAP content |
|---|---|
| Cereals | Gluten free products, rice, corn pasta, tortilla chips, quinoa, oats, polenta, sourdough, noodles |
| Milk productsCheese | Lactose free products, rice milk, soy milk, sorbetsHard cheeses, Cheddar, Parmesan, mozzarella, Swiss, firm tofu, tempeh, Brie, Camembert |
| Fruits | Bananas, cantaloupe, carambola, durian, honeydew melon, kiwi, orange, passion fruit, pawpaw, all berries (raspberries, blueberries, cranberries, strawberries) except than blackberries, tangelo, grapes, grapefruit, all nuts (except cashew and pistachios, and less than ten), almonds (less than 10), macadamia, olives, pumpkin seeds, flax and chia seeds, coconut, lemon, limes, papaya, rhubarb |
| Vegetables | Bamboo shoot, bok choy, capsicum, celery, chives, choko, choy sum, corn, eggplant, green bean, bean sprouts, lettuce, parsnip, pumpkin, silverbeet, tomato, potatoes, spring onion (green part only), garlic-infused oil, bell peppers, Swiss chard, carrots, spinach, zucchini, beet root (no more than four slices), broccoli (no more than 1/2 cup) |
| Legumes | Canned pumpkin, canned chickpeas, canned lentils |
| Sweeteners | Honeydew, golden syrup, maple syrup, glucose, sucrose (table sugar), aspartame, stevia, molasses, any sweeteners not containing fructose and not ending in “-ol” |
| References | Khan et al., 201554; Barrett, 201755; Varney et al., 201756; Monash University Low FODMAP Diet App., 201657; Canadian Digestive Health Foundation, 201958; Magge and Lembo, 201259 |
Note: FODMAP composition is affected by food processing techniques. Processes that involve heating and water can reduce the content of water soluble FODMAPs (such as fructans and GOS) leaching into the surrounding liquid (canned legumes are lower in FODMAP, GOS, than boiled legumes). Servings should also be considered because an excessive intake may increase the content of FODMAP diet (i.e., almonds and other nuts, if less than ten pieces, are low in FODMAP; large bananas have increased FODMAP).a Gluten free products are low-FODMAP except if they contain honey or fructose, or polyol sweeteners.
A study in ileostomates clarified that the fermentable load and volume of liquid delivered to the large intestine are increased by FODMAPs.40 The gut microbiota rapidly ferment carbohydrates, resulting in luminal distension and abdominal pain in adults with visceral hypersensitivity. A scintigraphic study demonstrated that fructose–sorbitol ingestion reduced the oro-cecal transit time by approximately 3h in healthy individuals.60 Other studies showed that FODMAPs can change the microbiota composition, decrease urinary histamine,61 and increase pro-inflammatory cytokines62 and visceral nociception.63 A diet high in FODMAPs (HFM) increased rat fecal Gram-negative bacteria, elevated lipopolysaccharides, and induced intestinal barrier dysfunction and visceral hypersensitivity.63 A four-week LFM diet was able to reverse these manifestations, improving IBS symptoms and reducing fecal lipopolysaccharides levels. Intracolonic administration of fecal supernatant from IBS patients to rats caused visceral hypersensitivity in the animals, which was not transferred if the patients were on an LFM diet.63
The LFM diet: practical stepsA LFM diet can be implemented following two different approaches: bottom-up and top-down.64 The bottom-up approach is a progressive gradual elimination of single products (or groups of products) from the diet until the symptoms are alleviated and allows specifying the patient's limit of tolerance for FODMAPs. This method is preferred in patients: (1) who have not yet been diagnosed with IBS but experience suggestive symptoms affecting their quality of life; (2) who are already on other elimination diet; or (3) who struggle to follow a full LFM.65 The second approach, top-down, more commonly used in experimental studies, require transient reduction or elimination of all foods high in FODMAPs from the diet, being therefore far more restrictive. Next, products containing FODMAPs are gradually reintroduced into the diet.65,66
In an LFM diet, there are three distinct phases: elimination, determination of sensitivities, and personalization.36,67,68 The majority of the studies conducted on the LFM diet have focused on the elimination phase, usually prescribed for two to six weeks, followed by assessment of symptom improvement. After completing the first phase of FODMAP restriction, if a therapeutic response is achieved, patients should undergo a structured reintroduction phase (ideally with the help of a dietitian) to determine the type and amount of FODMAPs that can be tolerated before experiencing symptoms, thus creating a personalized LFM diet.67 With this approach, based on tailored dietary restrictions demonstrated by the patient's tolerance, nutritional adequacy is more likely to be maintained and alterations of the luminal microbiota may be partially offset.69
FODMAP content guidance is available via scientific and lay publications for many types of foods70; the majority of information and analysis originated in Australia.71 The discordance of FODMAP content in other countries food lists could be partly related to the lack of clear FODMAP content guidance in different geographical areas.72 Chumpitazi et al.70 found an excessive FODMAP content in several processed foods, previously considered as LFM foods, such as gluten-free baked products and manufactured beverages. Also, in Australian manufactured gluten-free bread, the fructose content is sometimes higher than the nongluten-free counterparts.73 Appropriate assessment of FODMAPs and fructose and/or fructans may be helpful for patients with different tolerance to different carbohydrate.74 The development of technologies enabling the reduction of FODMAPs in processed food is also recommended.64
Criticisms of the low FODMAP dietDespite the demonstrated effectiveness of the LFM diet in a subset of patients with IBS, multiple concerns have been raised.64,75 Those include: (1) the lack of high-quality, randomized, placebo-controlled trials in children76–78; (2) the complexity and difficulty of teaching the diet; (3) the lack of specific cut-off levels for FODMAP content; (4) the paucity of data on safety and long-term efficacy; and (5) its effect on the gut microbiota.79
In addition to the impact on nutrient intake, the LFM diet may have psychosocial impacts. Patients have reported finding the diet ‘too demanding to follow’,80 and a questionnaire study reported difficulty in eating out and traveling for those following a long-term FODMAP diet.81
However, the beneficial effects of the LFM diet on quality of life have been demonstrated.82,83 Despite the perceived complexity of following such a restrictive diet,83 57% of patients reported adequate relief of symptoms. Of 90 patients enrolled in a prospective observational study, 60% stated that the LFM diet was easy to follow.84
In terms of safety, major concerns regard possible nutrient deficiencies, particularly during the initial elimination phase of high FODMAP containing foods.79 Reduced intake of grains, fruits, vegetables, and dairy products can restrict dietary choices, and may result, especially in growing children, in weight loss, failure to thrive, risk of fiber and micronutrient deficiencies (mainly iron, calcium, retinol, thiamin, and riboflavin),83,85–87 and eating disorders such as poor eating habits and food aversions. In the largest RCT on LFM diets79 the energy intake was not different to those following normal diet, and the change in body weight was minimal (mean<0.5kg) and not different between both groups.83,85 Conversely, two other 4-week RCTs reported reduction in energy intake in the LFM group.44,86,87 Moreover, some FODMAP-rich vegetables (e.g., cauliflower, onion, garlic) contain natural antioxidants, such as flavonoids, carotenoids, and vitamin C; some fruits and blackberries contain phenolic acid and anthocyanins, and wheat is a major source of phenolic acids.88,89 However, a recent follow-up study demonstrated no nutritional inadequacies following the reintroduction period in IBS patients on an ‘adapted FODMAP’ diet.69 Additionally, the only two long-term studies in patients with IBS following a personalized FODMAP diet with FODMAP reintroduction according to patients’ tolerance, found that calcium,81,82 iron, and other micronutrients81 were not compromised at 6–18 months.
Two RCTs in adults with IBS29,86 and a small, uncontrolled trial in patients with radiation-induced gastrointestinal symptoms80 reported reductions in fiber intake during the LFM diet compared with baseline. Inadequate substitution of high FODMAP grains and fruit and vegetables with suitable LFM/high-fiber replacements could explain these findings. Moreover another large RCT found no difference in fiber or macronutrient intake after a four-week LFM diet in IBS.85 The nutritional impact may vary mostly due to the availability of alternative food choices, and the completeness of dietary advice given.
About the influence of diet on the gut microbiome, species considered beneficial for host health, such as bifidobacteria and Faecalibacterium prausnitzii, were reduced in IBS patients receiving a LFM diet, most likely as a consequence of reducing prebiotic intake.34 The abundance of several bacteria (F. prausnitzii, Actinobacteria, and Bifidobacterium) rebounded after ten days of FOS supplementation62 and the strain diversity did not decrease with FODMAP restriction in four studies.61,87,90,91 Moreover, Staudacher et al.83 found that the reduction in Bifidobacterium induced by a LFM diet was counteracted through a specific probiotic preparation containing bifidobacteria.
Evidence for using LFM diets to manage gastrointestinal symptoms in adultsA recent systematic review38 has found that in 12 out of 13 trials in adults, a FODMAPs-restricted diet was an effective dietary intervention for reducing IBS symptoms. However, a recent RCT has shown that although the LFM diet reduced symptoms of IBS, it was no better than traditional dietary advice.44 Another trial found that the proportion of subjects reporting adequate relief of IBS-D symptoms by ≥50% (primary end point) during weeks three and four did not significantly differ from those not receiving an LFM diet.87
In athletes with a self-reported history of persistent exercise associated GI distress, a short-term LFM resulted in lower daily GI symptoms compared with a high FODMAP diet.92
Evidence for using LFM diets to manage FAPDs in childrenThe pediatric literature search retrieved 156 records and, according to the selected inclusion criteria, 24 records were included in this review (Fig. S1).
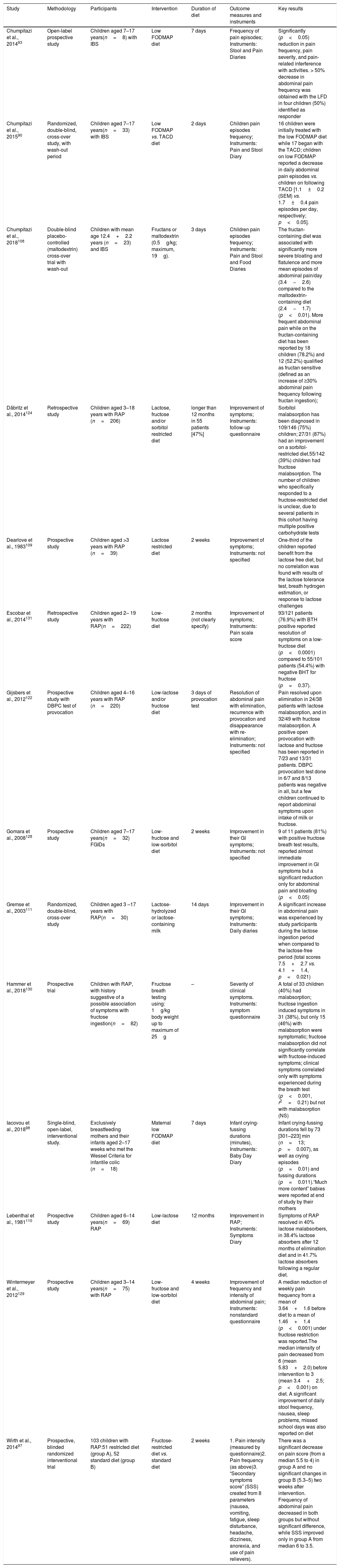
Evidence for comprehensive LFM diet in children with FAPDsThe summary of the studies in children is shown in Table 2.
Characteristics of the fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAP) pediatric studies included in the review.
| Study | Methodology | Participants | Intervention | Duration of diet | Outcome measures and instruments | Key results |
|---|---|---|---|---|---|---|
| Chumpitazi et al., 201493 | Open-label prospective study | Children aged 7–17 years(n=8) with IBS | Low FODMAP diet | 7 days | Frequency of pain episodes; Instruments: Stool and Pain Diaries | Significantly (p<0.05) reduction in pain frequency, pain severity, and pain-related interference with activities. > 50% decrease in abdominal pain frequency was obtained with the LFD in four children (50%) identified as responder |
| Chumpitazi et al., 201590 | Randomized, double-blind, cross-over study, with wash-out period | Children aged 7–17 years(n=33) with IBS | Low FODMAP vs. TACD diet | 2 days | Children pain episodes frequency; Instruments: Pain and Stool Diary | 16 children were initially treated with the low FODMAP diet while 17 began with the TACD; children on low FODMAP reported a decrease in daily abdominal pain episodes vs. children on following TACD [1.1±0.2 (SEM) vs. 1.7±0.4 pain episodes per day, respectively; p<0.05]. |
| Chumpitazi et al., 2018108 | Double-blind placebo-controlled (maltodextrin) cross-over trial with wash-out | Children with mean age 12.4+2.2 years (n=23) and IBS | Fructans or maltodextrin (0.5g/kg; maximum, 19g). | 3 days | Children pain episodes frequency; Instruments: Pain and Stool and Food Diaries | The fructan-containing diet was associated with significantly more severe bloating and flatulence and more mean episodes of abdominal pain/day (3.4−2.6) compared to the maltodextrin-containing diet (2.4−1.7) (p<0.01). More frequent abdominal pain while on the fructan-containing diet has been reported by 18 children (78.2%) and 12 (52.2%) qualified as fructan sensitive (defined as an increase of ≥30% abdominal pain frequency following fructan ingestion); |
| Däbritz et al., 2014124 | Retrospective study | Children aged 3–18 years with RAP (n=206) | Lactose, fructose and/or sorbitol restricted diet | longer than 12 months in 55 patients [47%] | Improvement of symptoms; Instruments: follow-up questionnaire | Sorbitol malabsorption has been diagnosed in 109/146 (75%) children; 27/31 (87%) had an improvement on a sorbitol-restricted diet.55/142 (39%) children had fructose malabsorption. The number of children who specifically responded to a fructose-restricted diet is unclear, due to several patients in this cohort having multiple positive carbohydrate tests |
| Dearlove et al., 1983109 | Prospective study | Children aged >3 years with RAP (n=39) | Lactose restricted diet | 2 weeks | Improvement of symptoms; Instruments: not specified | One-third of the children reported benefit from the lactose free diet, but no correlation was found with results of the lactose tolerance test, breath hydrogen estimation, or response to lactose challenges |
| Escobar et al., 2014131 | Retrospective study | Children aged 2– 19 years with RAP(n=222) | Low-fructose diet | 2 months (not clearly specify) | Improvement of symptoms; Instruments: Pain scale score | 93/121 patients (76.9%) with BTH positive reported resolution of symptoms on a low-fructose diet (p<0.0001) compared to 55/101 patients (54.4%) with negative BHT for fructose (p=0.37). |
| Gijsbers et al., 2012122 | Prospective study with DBPC test of provocation | Children aged 4–16 years with RAP (n=220) | Low-lactose and/or fructose diet | 3 days of provocation test | Resolution of abdominal pain with elimination, recurrence with provocation and disappearance with re- elimination; Instruments: not specified | Pain resolved upon elimination in 24/38 patients with lactose malabsorption, and in 32/49 with fructose malabsorption. A positive open provocation with lactose and fructose has been reported in 7/23 and 13/31 patients. DBPC provocation test done in 6/7 and 8/13 patients was negative in all, but a few children continued to report abdominal symptoms upon intake of milk or fructose. |
| Gomara et al., 2008128 | Prospective study | Children aged 7–17 years(n=32) FGIDs | Low-fructose and low-sorbitol diet | 2 weeks | Improvement in their GI symptoms; Instruments: not specified | 9 of 11 patients (81%) with positive fructose breath test results, reported almost immediate improvement in GI symptoms but a significant reduction only for abdominal pain and bloating (p<0.05) |
| Gremse et al., 2003111 | Randomized, double-blind, cross-over study | Children aged 3 –17 years with RAP(n=30) | Lactose-hydrolyzed or lactose-containing milk | 14 days | Improvement in their GI symptoms; Instruments: Daily diaries | A significant increase in abdominal pain was experienced by study participants during the lactose ingestion period when compared to the lactose-free period (total scores 7.5+2.7 vs. 4.1+1.4, p=0.021) |
| Hammer et al., 2018130 | Prospective trial | Children with RAP, with history suggestive of a possible association of symptoms with fructose ingestion(n=82) | Fructose breath testing using: 1g/kg body weight up to maximum of 25g | – | Severity of clinical symptoms. Instruments: symptom questionnaire | A total of 33 children (40%) had malabsorption; fructose ingestion induced symptoms in 31 (38%), but only 15 (46%) with malabsorption were symptomatic; fructose malabsorption did not significantly correlate with fructose-induced symptoms; clinical symptoms correlated only with symptoms experienced during the breath test (p<0.001, r2=0.21) but not with malabsorption (NS) |
| Iacovou et al., 201898 | Single-blind, open-label, interventional study. | Exclusively breastfeeding mothers and their infants aged 2–17 weeks who met the Wessel Criteria for infantile colic (n=18) | Maternal low FODMAP diet | 7 days | Infant crying-fussing durations (minutes), Instruments: Baby Day Diary | Infant crying-fussing durations fell by 73 [301–223] min (n=13; p=0.007), as well as crying episodes (p=0.01) and fussing durations (p=0.011).“Much more content” babies were reported at end of study by their mothers |
| Lebenthal et al., 1981110 | Prospective study | Children aged 6–14 years(n=69) RAP | Low-lactose diet | 12 months | Improvement in RAP; Instruments: Symptoms Diary | Symptoms of RAP resolved in 40% lactose malabsorbers, in 38.4% lactose absorbers after 12 months of elimination diet and in 41.7% lactose absorbers following a regular diet. |
| Wintermeyer et al., 2012129 | Prospective study | Children aged 3–14 years(n=75) with RAP | Low-fructose and low-sorbitol diet | 4 weeks | Improvement of frequency and intensity of abdominal pain; Instruments: nonstandard questionnaire | A median reduction of weekly pain frequency from a mean of 3.64+1.6 before diet to a mean of 1.46+1.4 (p<0.001) under fructose restriction was reported.The median intensity of pain decreased from 6 (mean 5.83+2.0) before intervention to 3 (mean 3.4+2.5; p<0.001) on diet. A significant improvement of daily stool frequency, nausea, sleep problems, missed school days was also reported on diet |
| Wirth et al., 201497 | Prospective, blinded randomized interventional trial | 103 children with RAP:51 restricted diet (group A), 52 standard diet (group B) | Fructose-restricted diet vs. standard diet | 2 weeks | 1. Pain intensity (measured by questionnaire)2. Pain frequency (as above)3. “Secondary symptoms score” (SSS) created from 8 parameters (nausea, vomiting, fatigue, sleep disturbance, headache, dizziness, anorexia, and use of pain relievers). | There was a significant decrease on pain score (from a median 5.5 to 4) in group A and no significant changes in group B (5.3–5) two weeks after intervention. Frequency of abdominal pain decreased in both groups but without significant difference, while SSS improved only in group A from median 6 to 3.5. |
IBS, irritable bowel syndrome; RAP, recurrent abdominal pain; TACD, typical American childhood diet; LFD, low FODMAP diet; BHT, breath hydrogen test; DBPC, double blind placebo controlled.
In a open-label study,93 conducted in a small sample of eight children with IBS, abdominal pain severity, intensity, and interference with daily activities were significantly reduced after one week of LFM diet. Four out of eight children had ≥50% decrease in abdominal pain frequency as compared to the baseline.93 In a randomized double-blind crossover trial,90 33 children with IBS were randomized to receive a LFM diet (0.15g/kg/day, maximum 9g/day of FODMAPs) or a typical American childhood diet (TACD) containing 0.7g/kg/day (maximum 50g/day) of FODMAPs for 48h. After a five-day washout period, the children were “crossed over” to the other diet for another 48h. Children on LFM diet reported significantly lower number of daily episodes of abdominal pain compared to children following TACD. Children who had significant improvement on the LFM diet had a distinct microbiota profile showing enriched taxa with a major saccharolytic metabolic function (e.g. Bacteroides, Ruminococcaceae, F. prausnitzii). No difference in α-diversity (number of operational taxonomic units, i.e., number of species) has been found after a one-week LFM diet. In a subsequent editorial,94 the authors hypothesize that a larger effect size could have been found with a longer trial comparing the LFM and the TACD.
A recent study in adults with IBS confirmed that pre-treatment levels of selected gut microbial DNA markers (higher levels of Bacteroides fragilis, Acinetobacter, Ruminiclostridium, Streptococcus, and Eubacterium) were associated with higher probability to respond to FODMAP restriction.95
Two recent reviews38,96 highlighted the limited data available in children,90,97 and the need of larger, high quality studies to test the effectiveness of the LFM diet.
A recent pediatric single-blind, open-label, interventional proof-of-concept study98 assessed the efficacy of an innovative, alternative approach. In this study, 18 exclusively breastfeeding healthy full-term infants aged 2–17 weeks who met criteria for infantile colic and their mothers were recruited for a dietary intervention trial. The infant's mothers were delivered a seven-day LFM diet and completed the Baby Day Diary on days five through seven to assess clinical study outcomes, and used a stopwatch to measure duration of sleep, feeding, crying, fussiness, and awake and content times. FODMAP content of breast milk and infant fecal samples for pH were analyzed at baseline and at the end of the dietary intervention. Crying duration decreased by 52min compared to baseline [142min] by the end of the dietary trial. Significant reductions in duration of fussiness and in number of episodes of crying were also reported. The analysis of breast milk lactose content was found to be stable throughout the intervention. Stool pH of infants was unchanged from baseline. Despite the lack of evidence, many breastfeeding mothers tend to practice a dietary change as a common strategy to relieve their infant's colic symptoms,99–102 in some cases avoiding gas-producing foods (e.g., onions, garlic, cabbage, or legumes/pulses).101,102
Restricted diets, such as the LFM diet, need to be managed and supervised appropriately in both breastfeeding mothers and growing children, and in consultation with a specialized dietitian, as they can lead to a compromised nutritional adequacy and also to the development of poor eating behaviors and food fears.
Fructans-related studies in children with FAPDsFructans are FODMAPs commonly present in the usual diet of adults and children.103 Fructans are rich in fructosyl–fructose linkages that reach intact the colon intact, as they cannot be hydrolyzed by human enzymes.103–105 These oligosaccharides undergo colonic fermentation, increasing gas production and subsequent luminal distension, which can exacerbate symptoms in individuals with IBS.42,106 However, limiting fructans may reduce healthy foods (i.e., fibers) and Bifidobacteria.104,107
In a recent double-blind, randomized, placebo-controlled crossover trial,108 23 children with IBS completed a one-week baseline abdominal pain and stool diaries and three-day food diaries. Depression, anxiety, and somatization scores were measured through validated questionnaires. Children were randomly assigned to receive meals containing either fructans or maltodextrin (0.5g/kg; maximum, 19g) for 72h followed by a washout period of at least ten days.
Breath hydrogen and methane production were tested at baseline and during each study period. Fructan-sensitive and fructan-insensitive subjects were similar in baseline symptoms and diet, psychosocial evaluation, IBS subtype, and gas production. There were a significantly higher number of daily abdominal pain episodes, more severe bloating, and flatulence during the fructan-containing diet compared to the maltodextrin-containing diet. Hydrogen (but not methane) production was significantly higher during the fructan period).
Lactose-related studies in children with FAPDsThree RCT on a restricted lactose diet have been completed109–111 in children with FAPDs112 as well as a larger number of observational or uncontrolled trials.113–124 Most of the older studies were conducted using old terminology of recurrent abdominal pain (RAP). Two out of the three RCT trials on lactose-free diet that were conducted in children with RAP109,110 were evaluated by a Cochrane Review,125 and included in a systematic review by Rutten et al.126
Lebenthal et al.110 studied the effect of lactose intolerance in RAP through an uncontrolled treatment and a randomized controlled challenge. Thirty-eight of 69 children with lactose intolerance received six weeks of either lactose-containing or lactose-free infant formula. Lactose intake increased symptoms in 48% of the lactose malabsorbers and in 24% of the lactose absorbers. Forty of the 69 children continued with lactose free diet for 12-months. After 12 months, improvement of abdominal pain was similar in both groups regardless if they were lactose absorbers or malabsorbers (40% vs. 38%).
In another double blind, single crossover design trial conducted in 39 children with RAP,109 children were instructed to continue with their usual diet for the first two weeks, while during the third and fourth weeks they received a lactose-free diet, and during the fifth and sixth weeks the children randomly received either lactose (2g/kg) or a similarly flavored placebo. One-third of the children were reported to have benefitted from the lactose-free diet, but there was no correlation between the improvement and the results of the lactose tolerance blood test, breath hydrogen test, or clinical response to lactose challenge.
In 2003, Gremse et al.111 conducted a randomized, double-blinded, cross-over study. Thirty children, aged 3–17 years, affected by RAP and lactose maldigestion defined by >10-ppm increase in lactose breath hydrogen test received either lactose-hydrolyzed or lactose-containing milk for two weeks. Abdominal pain, bloating, flatulence, and diarrhea scores were similar in subjects who had >10-ppm or >20-ppm increase in breath hydrogen testing after ingesting lactose. Due to the conflicting results of the above studies, further prospective RCTs are necessary to clarify the efficacy of a lactose-restricted diet in children with AP-FGIDs who have lactose malabsorption,93 whereas, according to a recent systematic review38 the current evidence in the literature does not encourage the use of a lactose-restricted diet in all children with IBS. Data in adult studies are also inconclusive, and it is still unclear if the lactose malabsorption is part of the IBS symptoms, or if the two conditions may simply coexist in some patients.38
Fructose-related studies in children with FAPDsFructose is a monosaccharide, of which American children consume a mean of 54.7g/day (accounting for approximately 10% of their daily caloric intake).127 Fructose is dependent on the glucose transporter 5 (GLUT5) and glucose transporter 2 (GLUT2) for passive absorption.112 One prospective study on the effect of low lactose and/or fructose diet,116 four prospective studies on the effect of a low fructose diet97,128,129 or fructose ingestion,130 and two retrospective studies124,131 on the effect of a low-fructose diet have been conducted in children (Table 2).
In a prospective controlled trial,97 103 children with AP-FGIDs were randomized to either a fructose-restricted diet (n=51) or a no dietary intervention group (n=52) for two weeks. Lower abdominal pain intensity, but not frequency, was reported by those children on the fructose-restricted diet (irrespective of their fructose hydrogen breath test result). In a prospective observational trial,129 75 children with AP-FGIDs and positive fructose breath test on a restricted fructose diet showed an overall reduction on the abdominal pain frequency and pain severity.129 Gomara et al.128 performed fructose hydrogen breath testing using various doses of fructose, including 1g, 15g, and 45g in 32 children with an AP-FGID. They found that 11 (34%) of the 32 children studied had fructose malabsorption either with the 15g or 45g doses. Following a two-week dietitian-recommended fructose-restricted diet, nine out of these 11 (82%) had a significant improvement.128
Recently, 82 children with functional abdominal pain disorders whose history was suggestive for a possible correlation with ingestion of fructose revealed that only 40% of them had malabsorption defined by increased breath hydrogen; just half of them with malabsorption were symptomatic. The authors suggested that visceral hypersensitivity, rather than malabsorption per se, may correlate with symptoms in some patients.130 Escobar et al.131 completed fructose breath testing using 1g/kg (up to 25g) in 222 children with AP-FGIDs and found that 121 out of 222 children (55%) had fructose malabsorption, and 93 (77%) of them improved on a dietitian-recommended low-fructose diet.
In a retrospective study,124 55/142 (39%) children had fructose malabsorption identified by fructose hydrogen breath testing, but several also showed multiple positive carbohydrate tests, making the response to diet unclear. In another study122 involving 220 children with RAP, some children still complained of abdominal symptoms when using milk or fructose-containing food despite a negative double-blind placebo-controlled provocation test with lactose or fructose (25% with milk and 48% with fructose, respectively).
Similarly to lactose,38,132 an improvement in abdominal symptoms has been reported on fructose-restricted diet regardless of the presence or absence of fructose malabsorption.91,133,134
Sorbitol-related studies in children with FAPDsNo prospective studies with only sorbitol restriction have been completed in children with AP-FGIDs,110 while two prospective studies128,129 evaluated a combination of low–fructose and low-sorbitol diet (Table 2).
A case report135 described a 15-year-old girl with chronic abdominal pain, most likely due to sorbitol ingestion from sugar-free gum, which improved with elimination of the sorbitol source. In a retrospective study, Däbritz et al.124 found that 109/146 (75%) children with RAP who underwent hydrogen breath testing had sorbitol malabsorption, and 27/31 (87%) who started a sorbitol-restricted diet reported symptom improvement.
DiscussionA significant beneficial effect of an LFM diet on clinical symptoms has been reported by several studies in adults with IBS,37,97 while in children there is currently very limited data, and only one small randomized double-blind study. The evidence to support a change in maternal diet for the treatment of infantile colic is also weak. Thus more research is needed before recommendations in children can be made.
Due to the complexity of designing LFM diets and the potential nutritional imbalances, guidance by professionals with expertise in dietary management is important, particularly in children to ensure nutritional adequacy and growth potential. It is currently difficult to predict which patients would benefit from LFM diets because of the lack of a specific biomarker and of a relation between breath tests and improvement of symptoms. When clinicians consider LFM diets, they should be aware of short-term and long-term limitations, including the impact on quality of life determined by multiple restrictions, the possible changes in gut microbiota, and the lack of knowledge of the relative efficacy in children.
Gradual reintroduction of FODMAPs into the diet after the elimination phase is currently recommended.82 This approach allows a personalized diet based on individual tolerance and avoids over-restriction with potential nutritional imbalances. Supplementation with specific probiotics could also restore the gut microbiota altered by an LFM diet.83
Increasing the knowledge regarding FODMAP content, improving the foods labeling, and analysis are important70 for clinicians and dieticians to design a tailored LFM diet and for the patients to ease the dietary compliance.
Further research is still needed to identify the best way to reintroduce foods containing FODMAPs and to determine which food is responsible of symptoms for each patient.34 Moreover, additional data on long-term adherence, effectiveness, and safety are also needed.
Current evidence does not support the use of a lactose-restricted diet in all children with IBS. Further work is needed to elucidate the role of exclusively restricting fructans and fructose for the treatment of pediatric FAPDs.
In conclusion, a low FODMAP diet is a promising dietary therapeutic intervention in adults with IBS, but the effectiveness of this approach in children with IBS and FAPDs remains unclear. Additional efforts are still needed to clarify which patients and which kind of FODMAP restriction would benefit to ensure nutritional adequacy, to facilitate recognition of FODMAP content, and to simplify the adherence to diet.
Availability of data and materialData sharing not applicable to this article, as no datasets were generated or analyzed during the current study.
Conflicts of interestThere are no conflicts of interest related to this study. S. Salvatore has participated as consultant and/or speaker for Deca, IMS-Health, Danone, Nestlé, and Menarini; Nikhil Thapar has participated as an advisory board member and/or speaker for Nutricia and Danone. Yvan Vandenplas has participated as a clinical investigator, and/or advisory board member, and/or consultant, and/or speaker for Abbott Nutrition, Aspen, Biocodex, Danone, Nestle Health Science, Nestle Nutrition Institute, Nutricia, Mead Johnson Nutrition, Merck, Phacobel, Rontis, United Pharmaceuticals, Wyeth, and Yakult, Annamaria Staiano has participated as a clinical investigator, and/or advisory board member, and/or consultant, and/or speaker for D.M.G., Valeas, Angelini, Miltè, Danone, Nestlé, Sucampo, and Menarini. Miguel Saps has served as a Scientific Consultant for Forest, Quintiles, Ardelyx, IM HealthScience, QOL Medical, and Sucampo. All the above manufacturers and companies have had no input or involvement in any aspect of this study. The other authors have no conflicts of interest to disclose.
Please cite this article as: Pensabene L, Salvatore S, Turco R, Tarsitano F, Concolino D, Baldassarre ME, et al. Low FODMAPs diet for functional abdominal pain disorders in children: critical review of current knowledge. J Pediatr (Rio J). 2019;95:642–56.
Study conducted at University Magna Graecia of Catanzaro, Department of Medical and Surgical Sciences, Catanzaro, Italy.