Community-acquired pneumonia is an important cause of morbidity in childhood, but the detection of its causative agent remains a diagnostic challenge. The authors aimed to evaluate the role of the chest radiograph to identify cases of community-aquired pneumonia caused by typical bacteria.
MethodsThe frequency of infection by Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis was compared in non-hospitalized children with clinical diagnosis of community acquired pneumonia aged 2–59 months with or without radiological confirmation (n=249 and 366, respectively). Infection by S. pneumoniae was diagnosed by the detection of a serological response against at least one of eight pneumococcal proteins (defined as an increase ≥2-fold in the IgG levels against Ply, CbpA, PspA1 and PspA2, PhtD, StkP-C, and PcsB-N, or an increase ≥1.5-fold against PcpA). Infection by H. influenzae and M. catarrhalis was defined as an increase ≥2-fold on the levels of microbe-specific IgG.
ResultsChildren with radiologically confirmed pneumonia had higher rates of infection by S. pneumoniae. The presence of pneumococcal infection increased the odds of having radiologically confirmed pneumonia by 2.8 times (95% CI: 1.8–4.3). The negative predictive value of the normal chest radiograph for infection by S. pneumoniae was 86.3% (95% CI: 82.4–89.7%). There was no difference on the rates of infection by H. influenzae and M. catarrhalis between children with community-acquired pneumonia with and without radiological confirmation.
ConclusionsAmong children with clinical diagnosis of community-acquired pneumonia submitted to chest radiograph, those with radiologically confirmed pneumonia present a higher rate of infection by S. pneumoniae when compared with those with a normal chest radiograph.
O objetivo deste estudo foi avaliar o papel do raio-X de tórax na identificação de casos de pneumonia adquirida na comunidade (PAC) causada por agentes bacterianos.
MétodosA frequência de infecção por Streptococcus pneumoniae, Haemophilus influenzae e Moraxella catarrhalis em crianças com PAC não hospitalizadas foi comparada com a presença de confirmação radiológica da pneumonia (n=249 crianças com pneumonia radiologicamente confirmada e 366 crianças com raio X de tórax normal). Infecção por S. pneumoniae foi diagnosticada com base na resposta sorológica a pelo menos uma dentre oito proteínas pneumocócicas investigadas (aumento ≥2 vezes nos níveis de IgG em relação a Ply, CbpA, PspA1 e 2, PhtD, StkP-C e PcsB-N ou aumento≥1,5 vezes em relação aPcpA). Infecção por H. influenzae e M. catarrhalis foi definida por aumento≥2 vezes nos níveis de IgG específica a antígenos de cada agente.
ResultadosCrianças com pneumonia radiologicamente confirmada apresentaram maior taxa de infecção pelo pneumococo. Além disso, a presença de infecção pneumocócica foi um fator preditor de pneumonia radiologicamente confirmada, aumentando sua chance de detecção em 2,8 vezes (IC 95%: 1,8-4,3). O valor preditivo negative do raio X normal para a infecção por S. pneumoniae foi 86,3% (IC95%: 82,4%-89,7%). Não houve diferença nas frequências de infecção por H. influenzae e M. catarrhalis entre crianças com PAC com ou sem confirmação radiológica.
ConclusõesCrianças com diagnóstico clínico de PAC submetidas a um raio X de tórax que apresentam confirmação radiológica tem maior taxa de infecção por S. pneumoniae, comparado às crianças com raio X normal.
Community acquired-pneumonia (CAP) is an important cause of morbidity and mortality in childhood.1 However, the etiologic diagnosis of CAP is challenging. Chest radiographs have been used as a diagnostic tool by the identification of radiologic patterns suggestive of an inflammatory process, such as pulmonary infiltrates. Nevertheless, the role of chest radiograph in pediatric CAP remains controversial, due to problems observed in the routine use of this exam, such as poor inter-observer concordance2 and the inability to distinguish between distinct etiologic agents.3,4 In turn, a significant proportion of children with a clinical diagnosis of CAP present normal chest radiograph upon admission,5 and important differences in admission and evolution have been reported among children with CAP with or without radiological confirmation.6–9 Altogether, these data suggest that the disease in children with or without radiologically confirmed pneumonia might be caused by distinct mechanisms and/or different etiologic agents.
In Brazil, Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis have been reported as important bacterial agents of pediatric pneumonia in hospitalized children.10 Herein, the presence of infection by S. pneumoniae, H. influenzae, and M. catarrhalis was investigated in non-hospitalized Brazilian children aged 2–59 months with clinical diagnosis of pneumonia with or without radiological confirmation. In doing so, the authors aimed to evaluate the role of the chest radiograph to identify probable cases of CAP caused by typical bacteria.
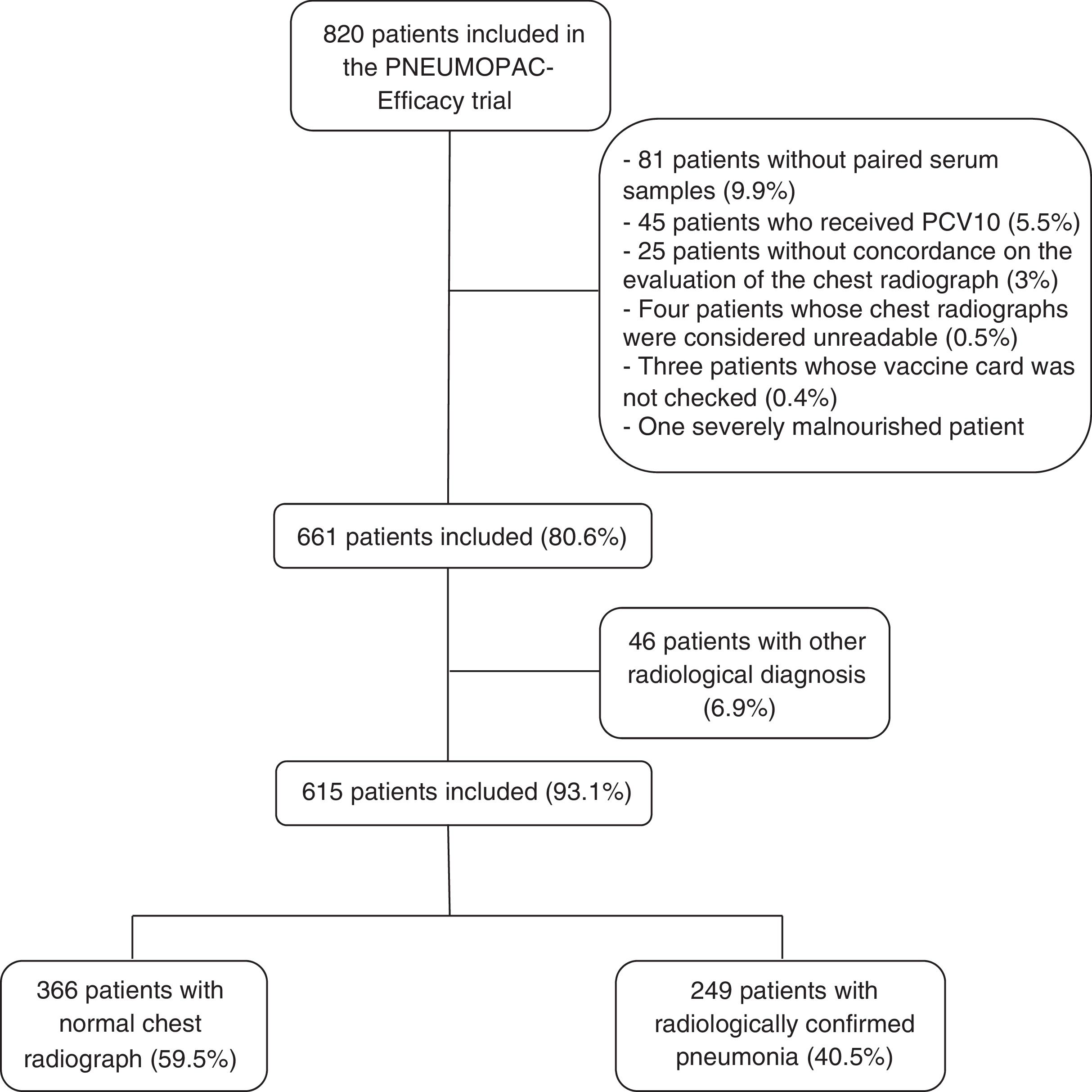
MethodsStudy design and participantsThis prospective cohort study was part of a clinical trial that evaluated the use of oral amoxicillin given thrice or twice daily to 2–59 months-old children diagnosed with CAP (PNEUMOPAC-Efficacy trial, ClinicalTrials.gov NCT01200706).11 In that trial, 820 children were enrolled in the Emergency Department of the Universidade Federal da Bahia, in Salvador, Northeast Brazil, from November 2006 to May 2011. All children had a chest radiograph (frontal and lateral views) taken on admission, and blood samples were collected both at admission and at the follow-up visit, two to four weeks later. Inclusion criteria comprised the report of respiratory complaints and detection of lower respiratory findings, along with the presence of pulmonary infiltrate/consolidation on the chest radiograph according to the interpretation of the pediatrician on duty. Legal guardians of the included patients signed an informed consent upon enrollment.
All chest radiographs were independently read by two pediatric radiologists (CAA-N and SCA), who were blinded to the clinical data. An overall agreement of 78.7% by these two pediatric radiologists was previously demonstrated.5 If there was no concordance on the final diagnosis of any exam, this chest radiograph was then evaluated by a third radiologist (RVB). The radiologic findings were registered according to the standardized interpretation for epidemiological studies previously published by the World Health Organization.12 Radiologically confirmed pneumonia was defined as the presence of pulmonary infiltrate or consolidation in two independent assessments.
The use of pneumococcal conjugate vaccine-10 (PCV10) was universally implemented in Salvador, Brazil, in July 2010, for children aged <2 years.13 Every child included in the PNEUMOPAC-efficacy trial who could have received PCV10 had the vaccine card checked personally by one of the researchers (ICB) after the trial was completed. Patients who received any dose of PCV10 and those whose vaccine status could not be identified were excluded from this analysis. Patients with severe malnutrition, defined as Z-score for weight-for-age under −3.00,14 were also excluded. Nutritional evaluation was performed using the Anthro software. Children with lower-chest in-drawing or danger signs (inability to drink, convulsions, central cyanosis, grunting in a calm child) were excluded from the PNEUMOPAC-efficacy trial, as well as those with underlying chronic diseases.
This study was approved by the Ethics Committee of the Universidade Federal da Bahia and was conducted in accordance with the principles of the Declaration of Helsinki.
Laboratory proceduresFluorescent multiplexed bead-based immunoassay was used to quantify the levels of antibodies against protein antigens from S. pneumoniae, H. influenzae and M. catarrhalis using Luminex xMAP® technology (Luminex Corporation, TX, USA).15 This assay included eight recombinant proteins from S. pneumoniae (pneumolysin [Ply], choline binding protein A [CbpA], pneumococcal surface protein A families 1 and 2 [PspA1 and PspA2], pneumococcal choline binding protein A [PcpA], pneumococcal histidine triad protein D [PhtD], serine/threonine protein kinase [StkP-C, SP1732-3], and protein required for cell wall separation of group B streptococcus [PcsB-N, SP2216-1]), three recombinant proteins from H. influenzae (NTHi Protein D, NTHi0371-1, and NTHi0830), and five recombinant proteins from M. catarrhalis (MC Omp CD, MC_RH4_2506, MC_RH4_1701, MC_RH4_3729-1, and MC_RH4_4730). Nine bead sets were created using the aforementioned proteins in the following combination: Ply, CbpA, PcpA, PhtD, StkP-C, and PcsB-N were conjugated in one bead region each; PspA1 and PspA2 were conjugated in the same bead region; and all H. influenzae and all M. catarrhalis proteins were conjugated in one bead region per bacterium.
This assay provided the mean fluorescence intensity (MFI) values for each antigen and serum evaluated. The MFI value represents an indirect measure of the IgG concentration against the studied antigens. True duplicates were used throughout the procedure and their fluorescence readings were averaged. To ensure the repeatability of the assays, high and low controls were analyzed on each plate. Furthermore, acute and convalescent samples were always analyzed on the same plate. All samples were tested using 1:400 and 1:1600 dilutions and, if necessary, further dilutions were performed. The occurrence of a serological response against S. pneumoniae was defined as an increase in the antibody levels ≥2-fold for IgG against Ply, CbpA, PspA1 and PspA2, PhtD, StkP-C, and PcsB-N, or an increase ≥1.5-fold for IgG against PcpA, based on the validation of a sensitive and specific serological test for the diagnosis of invasive pneumococcal disease.16 The diagnosis of infection by S. pneumoniae was established by the detection of serological response against any of the evaluated antigens, based on the specificity of the assay and good correlation with ELISA.15 The sensitivity and specificity for a serological response against each antigen were previously published.16 The occurrence of infection by H. Influenzae or M. catarrhalis was defined as an increase in antibody levels ≥2-fold between acute and convalescent samples.15,17 All serological tests were performed by DCA and ICB at the National Institute for Health and Welfare, in Helsinki, Finland. The frequency of these infections analyzed by age distribution, interval of sample collection, and duration of disease has been published elsewhere.17
Statistical analysisCategorical variables were compared using the chi-squared or Fisher's exact tests as appropriate, and continuous variables were evaluated using Mann–Whitney's U test, as they presented non-parametric distribution. The negative predictive value of the normal chest radiograph for the diagnosis of infection by S. pneumoniae was calculated. Multivariate logistic regression was performed using the presence of radiologically confirmed pneumonia as the dependent variable and infection by S. pneumoniae as the independent variable. This model was adjusted by age and infection by H. influenzae or M. catarrhalis. All statistical tests were two-tailed, with a significance level of 0.05. The software Stata/SE 12.0 (StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX, USA) was used to calculate the negative predictive value of the normal chest radiograph, and the software SPSS (SPSS Inc., version 9.0. Chicago, USA) was used for the remaining analyzes.
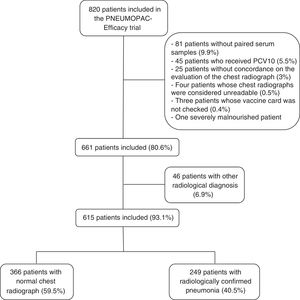
ResultsOut of 820 patients included in the PNEUMOPAC-efficacy trial, 615 were included in this study, of whom 249 (40.5%) had radiologically confirmed pneumonia and 366 (59.5%) had normal chest radiograph. Fig. 1 shows the flowchart of the included and excluded cases in this investigation. Overall, 311 (50.6%) were males and the median age was 27.2 months (25th–75th percentile: 14.9–41.4 months). Consolidation was detected by radiologists 1, 2, and 3 in 84.6%, 79.8%, and 67.3% of the cases with concordant radiologically confirmed pneumonia, respectively. The remaining cases of radiologically confirmed pneumonia were diagnosed based on the detection of pulmonary infiltrates.
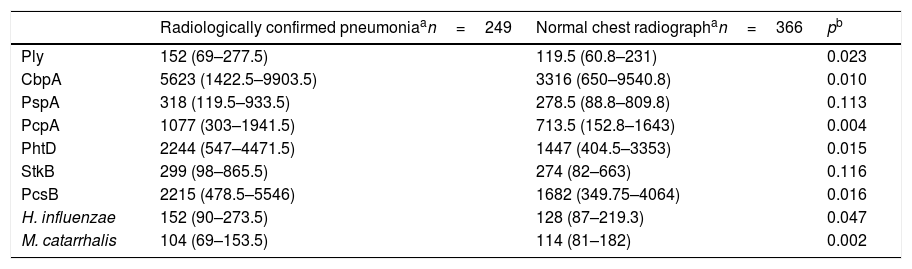
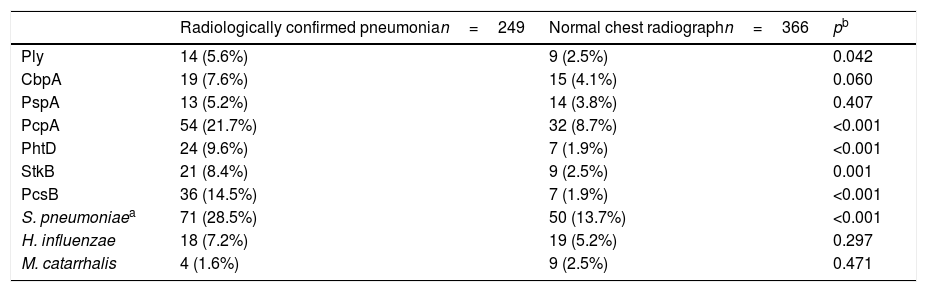
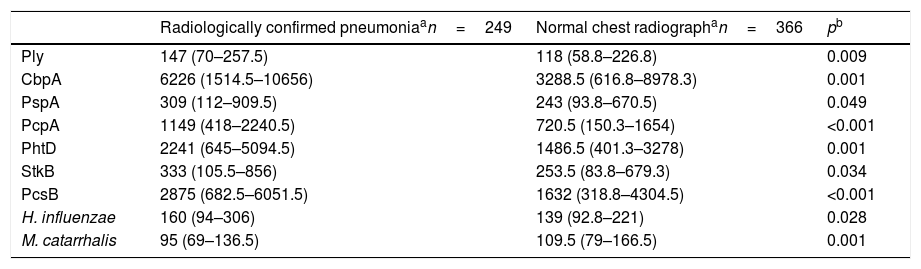
The comparison of the levels of antibodies on admission (first serum sample) against the studied antigens using a 1:1600 dilution factor is shown in Table 1. Children with radiologically confirmed pneumonia had significantly higher levels of antibodies against several protein antigens from S. pneumoniae and H. influenzae, and lower levels of antibodies against M. catarrhalis proteins. Similar results were obtained when using a 1:400 dilution factor (data not shown). Children with radiologically confirmed pneumonia also presented a higher frequency of infection by S. pneumoniae. Antibody responses against S. pneumoniae proteins were detected in 28.5% of the children with radiologically confirmed pneumonia and in 13.7% of children with a normal chest radiograph (p<0.001). Antibody responses against PcpA, PhtD, and PcsB were most frequently detected in children with radiologically confirmed pneumonia. These results are shown in Table 2. When the levels of antibodies against the studied antigens on the second serum sample were compared using a 1:1600 dilution factor, higher levels of IgG against all proteins from S. pneumoniae and H. influenzae were observed, as well as lower levels of antibodies against M. catarrhalis, as shown in Table 3. Similar results were obtained when using a 1:400 dilution factor (data not shown).
Comparison of the median fluorescence intensity (MFI) values from the first serum sample from children with radiologically confirmed pneumonia or those with a normal chest radiograph, using a 1:1600 dilution factor.
| Radiologically confirmed pneumoniaan=249 | Normal chest radiographan=366 | pb | |
|---|---|---|---|
| Ply | 152 (69–277.5) | 119.5 (60.8–231) | 0.023 |
| CbpA | 5623 (1422.5–9903.5) | 3316 (650–9540.8) | 0.010 |
| PspA | 318 (119.5–933.5) | 278.5 (88.8–809.8) | 0.113 |
| PcpA | 1077 (303–1941.5) | 713.5 (152.8–1643) | 0.004 |
| PhtD | 2244 (547–4471.5) | 1447 (404.5–3353) | 0.015 |
| StkB | 299 (98–865.5) | 274 (82–663) | 0.116 |
| PcsB | 2215 (478.5–5546) | 1682 (349.75–4064) | 0.016 |
| H. influenzae | 152 (90–273.5) | 128 (87–219.3) | 0.047 |
| M. catarrhalis | 104 (69–153.5) | 114 (81–182) | 0.002 |
Comparison of the frequencies of antibody response against protein antigens for children with a clinical diagnosis of CAP and either radiologically confirmed pneumonia or a normal chest radiograph.
| Radiologically confirmed pneumonian=249 | Normal chest radiographn=366 | pb | |
|---|---|---|---|
| Ply | 14 (5.6%) | 9 (2.5%) | 0.042 |
| CbpA | 19 (7.6%) | 15 (4.1%) | 0.060 |
| PspA | 13 (5.2%) | 14 (3.8%) | 0.407 |
| PcpA | 54 (21.7%) | 32 (8.7%) | <0.001 |
| PhtD | 24 (9.6%) | 7 (1.9%) | <0.001 |
| StkB | 21 (8.4%) | 9 (2.5%) | 0.001 |
| PcsB | 36 (14.5%) | 7 (1.9%) | <0.001 |
| S. pneumoniaea | 71 (28.5%) | 50 (13.7%) | <0.001 |
| H. influenzae | 18 (7.2%) | 19 (5.2%) | 0.297 |
| M. catarrhalis | 4 (1.6%) | 9 (2.5%) | 0.471 |
Comparison of the median fluorescence intensity (MFI) values from the second serum sample from children with radiologically confirmed pneumonia or those with a normal chest radiograph, using a 1:1600 dilution factor.
| Radiologically confirmed pneumoniaan=249 | Normal chest radiographan=366 | pb | |
|---|---|---|---|
| Ply | 147 (70–257.5) | 118 (58.8–226.8) | 0.009 |
| CbpA | 6226 (1514.5–10656) | 3288.5 (616.8–8978.3) | 0.001 |
| PspA | 309 (112–909.5) | 243 (93.8–670.5) | 0.049 |
| PcpA | 1149 (418–2240.5) | 720.5 (150.3–1654) | <0.001 |
| PhtD | 2241 (645–5094.5) | 1486.5 (401.3–3278) | 0.001 |
| StkB | 333 (105.5–856) | 253.5 (83.8–679.3) | 0.034 |
| PcsB | 2875 (682.5–6051.5) | 1632 (318.8–4304.5) | <0.001 |
| H. influenzae | 160 (94–306) | 139 (92.8–221) | 0.028 |
| M. catarrhalis | 95 (69–136.5) | 109.5 (79–166.5) | 0.001 |
A multivariate logistic regression was performed to assess the effect of infection by S. pneumoniae on the presence of radiologically confirmed pneumonia, adjusting this model by infection by H. influenzae or M. catarrhalis and age. The presence of infection by S. pneumoniae increased the odds of radiologically confirmed pneumonia by 2.8 (95% CI: 1.8–4.3). The presence of infection by either H. influenzae or M. catarrhalis or the age of the child did not affect the odds for detection of radiologically confirmed pneumonia (odds radio [95% CI]: 1.42 [0.7–2.9]; 0.4 [0.1–1.6]; and 0.9 [0.9–1], respectively). Furthermore, the negative predictive value of the normal chest radiograph for the diagnosis of infection by the pneumococcus was 86.3% (95% CI: 82.4–89.7%).
DiscussionThis study demonstrated that children with radiologically confirmed pneumonia have a higher frequency of infection by S. pneumoniae than those with a normal chest radiograph. The presence of infection by pneumococcus was independently associated to radiologically confirmed pneumonia among non-hospitalized children with clinical CAP. Furthermore, the presence of a normal chest radiograph had a high negative predictive value for the detection of antibody responses against S. pneumoniae.
A higher frequency of antibody response against several antigens from S. pneumoniae was observed in the group of children with radiologically confirmed pneumonia when compared with those with a normal chest radiograph. This finding corroborates the results from previous studies, which demonstrated that the presence of alveolar infiltrates on chest radiographs was associated with bacterial pneumonia.18 For instance, Nascimento-Carvalho et al. also reported that infection by S. pneumoniae was more frequently detected among hospitalized children with CAP who presented radiographic pneumonia rather than those with a normal chest radiograph.19 In turn, children with a normal chest radiograph had a higher incidence of viral infection.19 This is the first report of the association between pneumococcal infection and radiologically confirmed pneumoniae among non-hospitalized children with clinical CAP.
Accordingly, the negative predictive value of the normal chest radiograph for the detection of pneumococcal infection was high (86.3% [95% CI: 82.4–89.7%]). Although an association between bacterial infection and alveolar infiltrates/consolidation has been previously described,18 these findings cannot reliably establish the etiologic diagnosis of CAP.4,5 Therefore, the present finding that the normal chest radiograph has a high negative predictive value for pneumococcal infection may aid in the interpretation of this exam. The high negative predictive value observed for the normal chest radiograph in a population with high prevalence of pneumococcal infection is noteworthy,10 thereby reinforcing the present results. Altogether, the present data indicate that children with non-severe CAP with radiologically confirmed pneumonia have a higher chance of infection by S. pneumoniae, whereas children with a normal chest radiograph are not likely to present infection by this agent and might not benefit from empiric antibiotic therapy.
Data from vaccine trials reinforce the relationship between pneumococcal infection and radiologically confirmed pneumonia, as a differential effect of pneumococcal vaccination was found on the rates of pediatric CAP depending on the applied diagnostic criteria. For instance, the efficacy of the PCV10 was significantly higher for children with consolidation on the chest radiograph than for children either with alveolar infiltrates or solely with a clinical diagnosis of CAP.20 Therefore, the greater impact of pneumococcal vaccination on children with consolidation on chest radiographs suggests that patients with this radiological diagnosis present a higher incidence of pneumococcal infection. These findings are consistent with those reported by Lucero et al., who demonstrated a good vaccine efficacy of PCV11 on children with radiographic pneumonia defined as consolidation and a practicably negligible vaccine efficacy for children with a clinical diagnosis of pneumonia.21 These vaccine trials provide indirect evidence regarding the etiology of pneumonia in children with distinct radiological patterns, indicating that children with radiologically confirmed pneumonia indeed present a higher frequency of infection by S. pneumoniae.
The role of the chest radiograph in the management of children with CAP, however, has been largely debated. Importantly, Bradley et al. recommend that the chest radiograph should only be used in children who are hospitalized or with hypoxemia, significant respiratory distress, suspected complications, or therapy failure.22 This position is corroborated by Harris et al., who stated that children with signs and symptoms suggesting pneumonia who are not admitted to hospital should not routinely have a chest radiograph.23 These recommendations are partly due to previous studies that have shown that bacterial pneumonia cannot be differentiated from non-bacterial pneumonia based solely on the findings of an abnormal chest radiograph.3,4,24 Furthermore, the current evidence suggests that the use of a chest radiograph does not improve the outcome of pediatric patients with CAP.25 Nonetheless, it is important to emphasize that when the impact of the chest radiograph on the management of children with CAP was evaluated, the patients received antibiotics at the discretion of the attending physician, regardless of the radiologic findings, thereby limiting the potential benefit of a radiological study in these patients as a diagnostic tool with therapeutic implications.25 Accordingly, Harris et al. recommend the use of antibiotics for all children with a clear diagnosis of CAP.23 Both guidelines agree, however, that young children do not require routine use of antibiotics, as most present viral acute lower respiratory infection.22,23 In this scenario, although the chest radiograph does not unequivocally distinguish etiologic agents of CAP, it may help differentiating distinct patterns of lower respiratory infections. Recent evidence has demonstrated important differences between children with or without radiologically confirmed pneumonia in the clinical presentation and evolution. Children with radiologically confirmed pneumonia have a higher frequency and longer persistence of fever,6–8 and also evolve more severely, with longer hospitalization, higher need of respiratory support, and higher rates of treatment failure.9 These differences indicate that children with and without radiologically confirmed pneumonia may have different patterns of lower respiratory tract infection, and the chest radiograph, when performed, may aid the management of doubtful cases of non-severe CAP.
It was also observed that children with radiologically confirmed pneumonia had higher levels of antibodies against several pneumococcal proteins both at admission and in convalescence. Lower levels of anti-pneumococcal antibodies on admission have been associated with a higher frequency of antibody responses against S. pneumoniae due to particularities of the serological methods.17 Therefore, the level of antibodies at admission probably was not responsible for the higher rate of antibody responses against the pneumococcus in children with radiological pneumonia. The higher level of antibodies at admission in this group of children, in turn, might have been caused by previous colonization by S. pneumoniae. Nasopharyngeal colonization has been recognized as part of the natural history of pneumococcal disease, which ensues if immunological barriers are crossed by the colonizing bacteria.26 Also, children with clinical and radiological pneumonia are also more frequently colonized with S. pneumoniae when compared with healthy controls.27 Therefore, it is possible that a higher rate of carriage of S. pneumoniae in children with radiologically confirmed pneumonia elicited the higher levels of anti-pneumococcal antibodies found in this subgroup.
No difference was observed on the rates of antibody response against H. influenzae and M. catarrhalis in this study, possibly due to the low numbers of responders within the study group. However, discretely higher levels of antibodies against H. influenzae were found in children with radiologically confirmed pneumonia, as well as lower levels of antibodies against M. catarrhalis. It is known that several bacterial agents compete to colonize the nasopharyngeal tract of pediatric patients, creating a dynamic process of turnover of colonizing agents.27 Increased rates of colonization by S. pneumoniae might also have contributed to lower the levels of antibodies against M. catarrhalis on the samples collected from children with radiologically confirmed pneumonia at admission. In turn, a positive correlation between colonization by S. pneumoniae and H. influenzae has already been described, which may have contributed to the high levels of antibodies at admission found against H. influenzae.28
The limitations of the present study must be emphasized. Firstly, data on the colonization status of the evaluated children were not available, and the putative effect of pneumococcal carriage on the antibody levels at admission was not evaluated. Secondly, the study was composed of unvaccinated children, which does not represent the reality in most countries in the post-PCV era. Nevertheless, recent evidence suggests that the use of PCV does not interfere with the result of protein-based serological assays in children with CAP,29 which favors the generalization of the present results. Also, data on the use of other vaccines that could have influenced the results presented herein, such as the H. influenzae type b vaccine, was not available. However, the coverage of the H. influenzae type b vaccine among the pediatric population in Brazil is high (>80%), so differential rates of vaccination probably did not affect the present results.30 Finally, as all antigens from H. influenzae and M. catarrhalis were conjugated in one bead region per bacterium, individual fluorescence readings were not obtained for these antigens.
In conclusion, this study demonstrated that, among non-hospitalized children with clinical CAP who were submitted to a chest radiograph, those with radiologically confirmed pneumonia had a higher frequency of infection by S. pneumoniae compared to children with a normal chest radiograph. Furthermore, the presence of pneumococcal infection was independently associated with radiologically confirmed pneumonia; normal chest radiograph has a high negative predictive value for pneumococcal infection.
FundingSanofi Pasteur (Lyon, France) supplied PcpA and PhtD; Prof. Elaine Tuomanen at St. Judes Children's Research Hospital (Memphis, United States) supplied Ply, CbpA, PspA1; Profs. Susan Hollingshead, David Briles, and Pat Coan at University of Alabama (Birmingham, United States) supplied PspA2; and Valneva Austria GmbH (Vienna, Austria) supplied StkP-C, PcsB-N, NTHi Protein D, NTHi0371-1, NTHi0830, MC Omp CD, MC_RH4_2506, MC_RH4_1701, MC_RH4_3729-1, and MC_RH4_4730.
This work was supported by the Bahia State Agency for Research Funding (Fundação de Amparo à Pesquisa do Estado da Bahia [FAPESB]) and the Brazilian Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico [CNPq]).
Conflicts of interestAndreas Meinke is an employee of Valneva Austria GmbH. The others authors declare no conflicts of interest.
The authors would like thank Sanofi Pasteur (Lyon, France) for supplying PcpA and PhtD; Prof. Elaine Tuomanen at St. Judes Children's Research Hospital (Memphis, United States) for supplying Ply, CbpA, PspA1; Profs. Susan Hollingshead, David Briles, and Pat Coan at University of Alabama (Birmingham, United States), for supplying PspA2; and Valneva Austria GmbH (Vienna, Austria) for supplying SP1732-3, SP2216-1, NTHi Protein D, NTHi0371-1, NTHi0830, MC Omp CD, MC_RH4_2506, MC_RH4_1701, MC_RH4_3729-1, and MC_RH4_4730.
Please cite this article as: Andrade DC, Borges IC, Vilas-Boas AL, Fontoura MS, Araújo-Neto CA, Andrade SC, et al. Infection by Streptococcus pneumoniae in children with or without radiologically confirmed pneumonia. J Pediatr (Rio J). 2018;94:23–30.