To evaluate the Western blotting method for the detection of IgG anti-Toxoplasma gondii (T. gondii) (IgG-WB) in the serum of children with suspected congenital toxoplasmosis.
MethodsWe accompanied 47 mothers with acquired toxoplasmosis in pregnancy and their children, between June of 2011 and June of 2014. The IgG-WB was done in house and the test was considered positive if the child had antibodies that recognized at least one band on IgG blots different from the mother's or with greater intensity than the corresponding maternal band, during the first three months of life.
Results15 children (15.1%) met the criteria for congenital toxoplasmosis and 32 (32.3%) had the diagnosis excluded. The symptoms were observed in 12 (80.0%) children and the most frequent were cerebral calcification in 9 (60.0%), chorioretinitis in 8 (53.3%), and hydrocephalus in 4 (26.6%). IgM antibodies anti-T. gondii detected by chemiluminescence (CL) were found in 6 (40.0%) children and the polymerase chain reaction (PCR) for detection of T. gondii DNA was positive in 5 of 7 performed (71.4%). The sensitivity of IgG-WB was of 60.0% [95% confidence interval (CI) 32.3–83.7%] and specificity 43.7% (95% CI 26.7–62.3%). The sensitivity of IgG-WB increased to 76.0 and 89.1% when associated to the research of IgM anti-T. gondii or PCR, respectively.
ConclusionsThe IgG-WB showed greater sensitivity than the detection of IgM anti-T. gondii; therefore, it can be used for the diagnosis of congenital toxoplasmosis in association with other congenital infection markers.
Avaliar o método Western Blotting para detecc¸ão de IgG anti-Toxoplasma gondii(T. gondii) (IgG-WB) no soro de crianc¸as com suspeita de toxoplasmose congênita
MétodosAcompanhamos 47 mães com toxoplasmose adquirida na gravidez e seus filhos, entrejunho de 2011 e junho de 2014. O IgG-WB foi feito internamente e o teste foi consideradopositivo quando a crianc¸a apresentava anticorpos que reconheciam pelo menos uma bandanas manchas de IgG diferente das bandas da mãe ou com maior intensidade do que a bandamaterna correspondente, durante os primeiros 3 meses de vida
ResultadosAtenderam aos critérios para diagnóstico de toxoplasmose congênita 15crianc¸as (15,1%) e 32 (32,3%) tiveram o diagnóstico excluído. Os sintomas foram observados em12 crianc¸as (80%) e os mais frequentes foram calcificac¸ão cerebral em nove (60%), coriorretiniteem oito (53,3%) e hidrocefalia em quatro (26,6%). Os anticorpos IgM anti-T. gondii detectadospor quimiluminescência (QL) foram encontrados em seis crianc¸as (40%) e a reac¸ão em cadeiada polimerase (RCP) para detecc¸ão do DNA de T. gondii foi positiva em cinco de sete reac¸ões(71,4%). A sensibilidade do IgG-WB foi de 60% [intervalo de confianc¸a (IC) de 95%, 32,3 a 83,7%]e a especificidade foi de 43,7% (IC de 95%, 26,7 a 62,3%). A sensibilidade do IgG-WB aumentoupara 76 e 89,1% quando relacionada à pesquisa de IgM anti-T. gondii ou à RCP, respectivamente
ConclusõesO IgG-WB mostrou maior sensibilidade do que a detecc¸ão de IgM anti-T. gondii;portanto, pode ser usado para o diagnóstico de toxoplasmose congênita em associac¸ão comoutros marcadores de infecc¸ão congênita.
Most children with congenital toxoplasmosis (CT) show no signs or symptoms at birth, yet there is a risk of developing late sequelae, particularly ocular and neurological impairment.1
All children are considered suspect whose mothers had acute toxoplasmosis in the course of pregnancy; therefore, these children must be subjected to serological investigation with antibody detection for anti-Toxoplasma gondii (T. gondii).1,2
However, a confirmed serological diagnosis of T. gondii infection through the detection of specific IgM and/or IgA antibodies against the parasite does not occur in all newborns.1,3–5 Therefore, IgG antibodies against T. gondii in serial serum samples must be analyzed and the child remains under outpatient follow-up, which can take months until the definitive diagnosis.1,6
In an infected fetus, IgG and IgM antibodies produced against the antigenic determinants of T. gondii may differ from those anti-T. gondii IgG and IgM antibodies detected in the maternal serum, suggesting a neosynthesis of specific antibodies. Thus, children with CT with non-reactivity in conventional tests for IgM detection have been diagnosed in the first months of life through the Western blot method (WB).7–9
There is a need for early and rapid diagnosis using a low complexity method that allows the reference laboratories for toxoplasmosis to differentiate the dubious results obtained using the routine conventional serological methods, such as indirect immunofluorescence (IIF), enzyme-linked immunoassay (ELISA), and immunoassay of microparticles using chemiluminescence (CL). The objective of this study was to evaluate the WB method for the detection of IgG antibodies against T. gondii (IgG-WB) in the serum of the child and his/her mother with acquired toxoplasmosis in pregnancy to assist in the early diagnosis of CT.
MethodsSubjectsThe population was comprised of women with suspected acquired toxoplasmosis in pregnancy and their children, assisted at the Pediatric Infectious Disease Outpatient, between June of 2011 and June of 2014. The study included children whose mothers demonstrated reactivity for anti-T. gondii IgM during the pre-natal care. The blood samples of child/mother pairs were collected simultaneously during the first three months of child's life and stored at −20°C for WB. The control group consisted of children whose mothers showed suspected acute toxoplasmosis during pregnancy, but did not meet the criteria for CT.2,10,11 In this period, 47 children and their mothers were monitored. Of these, 15 (15.1%) children were diagnosed with CT and in 32 (32.3%), this diagnosis was excluded. Pregnant women received spiramycin or sulfadiazine plus pyrimethamine and folinic acid until childbirth. The suspected infected children received sulfadiazine plus pyrimethamine and folinic acid until the definitive diagnosis.
Diagnostic criteria in childrenCT was considered when the child presented elevation of specific anti-T. gondii IgG titers in sequential samples in the first months of his life and/or the persistence of T. gondii IgG titers after 12 months of life and/or reactivity for anti-T. gondii IgM and/or chorioretinitis and/or lesion of central nervous system (CNS) with reactivity for IgG and/or positivity for the T. gondii DNA using polymerase chain reaction (PCR).2,10,11
Serological test for the detection of anti-T. gondii IgM and IgGThe serological tests performed during pre-natal care and on samples from children were performed by conventional routine laboratory methods. Anti-T. gondii IgG antibodies were determined by Indirect immunofluorescence (IIF)12 (Immunoblot, Biolab-Mérieux, Rio de Janeiro, RJ, Brazil), with T. gondii obtained from ascitic fluid of infected mice, and chemiluminescence (CL) (Architect, System Abbott–Wiesbaden, Germany) with p30 (SAG1) and p35 (GRA8) recombinant antigens of T. gondii. Anti-T. gondii IgM antibodies were also detected using CL (Architect, System Abbott–Wiesbaden, Germany) with p30 antigen and total lysate of T. gondii.
T. gondii antigensFive albino female mice, aged from 45 to 60 days and weighing between 25 and 40g were used for obtaining RH strain tachyzoites of T. gondii. The animals were inoculated by intraperitoneal route with a suspension of live tachyzoites (105/mL) in sterile saline solution. Forty-eight hours after the inoculation, the exudate was obtained by washing the peritoneal cavity with 3.0mL of sterile saline solution. The samples were passed through a 27G needle for rupture of the host cells. Later, they were centrifuged and the sediment was standardized at 109tachyzoites/mL by counting in a Neubauer chamber.13
The IgG-WB MethodThe proteins of T. gondii to be used in IgG-WB were quantified using the Lowry et al. method,14 and polyacrylamide gel 12% was used for electrophoretic separation of proteins with subsequent transfer of the proteins to a nitrocellulose membrane (iBlot® Gel Transfer System, California, USA). The nitrocellulose paper was stained with Ponceau and, after washing with distilled water, the nitrocellulose strips were cut and blocked with skim milk 5% plus tris buffered saline (TBS) with Tween®. Washes were subsequently performed with TBS and skim milk 5% and then, the patient's serum was added diluted in TBS with Tween (Sigma–Aldrich, MO, USA) and skim milk 5%, carried out washes, and added conjugated anti-human IgG (Invitrogen, Life Technologies – CA, USA) diluted in TBS with Tween (Sigma–Aldrich, MO, USA) and skim milk 5%. After further washes the authors added the chromogen substrate diaminobenzidine (DAB) 0.2% (Acrös Organics, Thermo Fisher Scientific – Geel, Belgium) and hydrogen peroxide (100μL). When the bands were visualized, the reaction was stopped with distilled water. Positive and negative control samples for anti-T. gondii were included in each test.
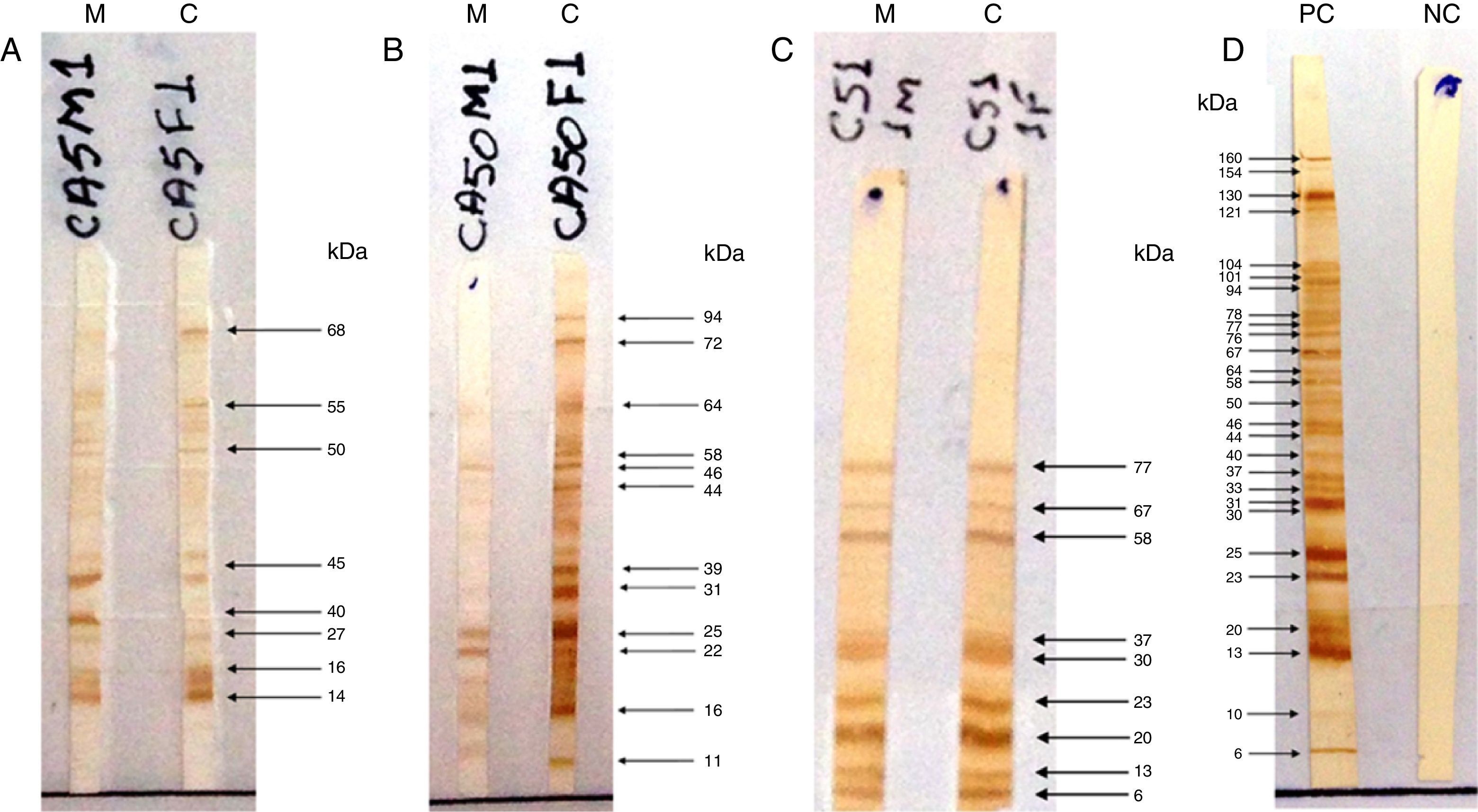
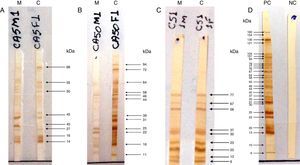
The test was considered positive if the child produced antibodies that recognized at least one band of protein different from the mother or with higher intensity than the corresponding maternal band (Fig. 1), characterizing the neosynthesis of anti-T. gondii IgG antibodies.15–18 To control the subjectivity of reading, two independent observers read the IgG-WB patterns blindly and without knowledge of the previous serological results of the conventional tests; there was concordance in 100.0% of the results.
Pattern recognition of RH strain proteins of Toxoplasma gondii by IgG antibodies using the Western blotting (IgG-WB) method performed with serum samples from mothers with acquired toxoplasmosis during pregnancy and their children with suspected congenital toxoplasmosis. (A) Positive IgG-Western blotting for congenital toxoplasmosis. Equal bands recognized by IgG antibodies, with stronger intensity in the child sample compared to maternal sample. (B) Positive IgG-Western blotting for congenital toxoplasmosis. Different bands recognized by IgG from the child sample as compared to the maternal sample. (C) Negative IgG-Western blotting for congenital toxoplasmosis. Equal bands recognized by IgG antibodies from child and mother samples. M, mother; C, child; PC, positive control; NC, negative control.
The specificity of the IgG-WB method was determined with 26 serum samples obtained from individuals seronegative for toxoplasmosis (anti-T. gondii IgG and IgM non-reactive by CL) and with seroreactivity to antibodies against other pathogens, such as Treponema pallidum (n=5), Trypanosoma cruzi (n=5), Leishmania spp. (n=2), Paracoccidioides brasilienses (n=5), or seroreactivity to serological markers of autoimmunity disorders, such as antinuclear antibodies (n=5) and anti-DNA double strand antibodies (n=4).
PCR for detection of T. gondii DNAThe DNA was extracted from peripheral blood cells of children, collected with EDTA as the anticoagulant up to three months after birth, using the kit EasyPrep DNA Mini I (EasyGen, Favorgen Biotech – Austria). The DNA amplification of T. gondii was performed using the method described by Homan et al.19 Primers Tox4 (CGCTGCAGGGAGGAAGACGAAAGTTG) and Tox5 (CGCTGCAGACACAGTGCATCTGGATT) were used for the amplification of a fragment of 529 base pairs (bp; GenBank No. AFI46527) of T. gondii DNA. PCR was performed in a final volume of 22.5μL, containing 2.5μL of DNA extracted from the sample; 1.0μL of 1.0mM for each primer, 100mM of dNTP (Invitrogen, Life Technologies – CA, United States), 60mM Tris–HCl (pH 9.0), 15mM (NH4)2SO4, 2mM MgCl2, and 0.25μL Taq DNA polymerase (Invitrogen, Life Technologies – CA, United States), also completed with 7.75μL of Milli Q water. The amplification was performed with 35 cycles in a thermal cycler (PTC-100, MJ Research – CA, United States), using the following cycling condition: 7min at 94°C for denaturation in the 1st cycle, followed by 33 cycles of 1min at 94C for denaturation, 1min at 55°C for annealing, and 1min at 72°C for extension; cycle 35 was followed by a final extension of 10min at 72°C. Aliquots of each PCR product were subjected to electrophoresis in 2% agarose gel. RH strain Tachyzoites (107/mL) had their DNA extracted to be used as positive control. The water was regarded as the negative control. Positive and negative control samples for T. gondii were included in each test.
Statistical analysisThe statistical analysis was done on GraphPad Prism 5 (GraphPad Software, Inc. – San Diego, United States). Categorical variables were expressed as absolute number (n) and percentage (%) and analyzed by the chi-squared test or Fisher's exact test. Parameters of sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated, with a confidence interval (CI) of 95.0%. Values of p<0.05 were considered statistically significant.
The study was approved by the Ethics Committee for Research Involving Human Beings. All mothers participated voluntarily and signed the informed consent.
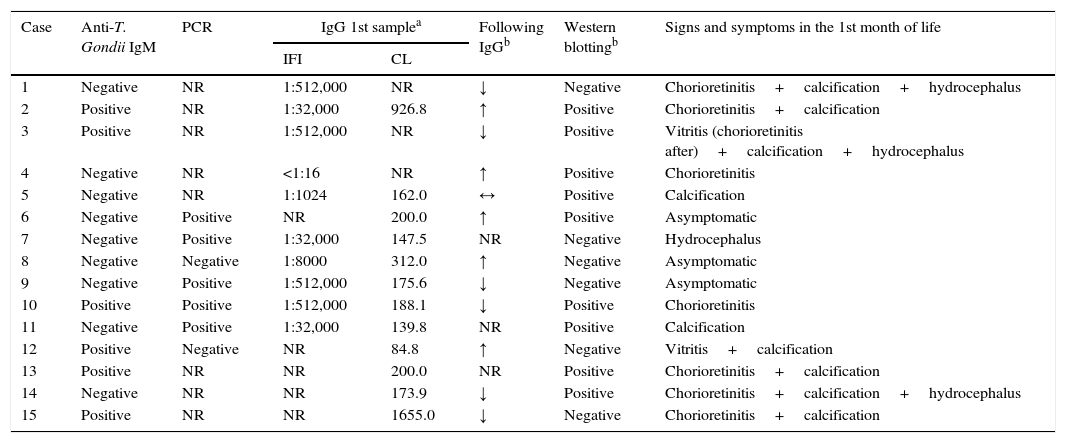
ResultsOf the 15 children with CT, 12 (80.0%) were symptomatic and met the clinical criteria for the definition of the disease (nine with cerebral calcification, eight with chorioretinitis, and four with hydrocephalus), six (40.0%) presented reactivity for anti-T. gondii IgM, five (33.3%) showed elevation of anti-T. gondii IgG in the first months of life, and one (6.7%) showed persistence of anti-T. gondii IgG titers in sequential samples. Five of the seven children (71.4%) who underwent PCR to detect T. gondii DNA showed positive results (Table 1).
Clinical and laboratory characteristics of 15 children with congenital toxoplasmosis, from June 2011 to June 2014.
| Case | Anti-T. Gondii IgM | PCR | IgG 1st samplea | Following IgGb | Western blottingb | Signs and symptoms in the 1st month of life | |
|---|---|---|---|---|---|---|---|
| IFI | CL | ||||||
| 1 | Negative | NR | 1:512,000 | NR | ↓ | Negative | Chorioretinitis+calcification+hydrocephalus |
| 2 | Positive | NR | 1:32,000 | 926.8 | ↑ | Positive | Chorioretinitis+calcification |
| 3 | Positive | NR | 1:512,000 | NR | ↓ | Positive | Vitritis (chorioretinitis after)+calcification+hydrocephalus |
| 4 | Negative | NR | <1:16 | NR | ↑ | Positive | Chorioretinitis |
| 5 | Negative | NR | 1:1024 | 162.0 | ↔ | Positive | Calcification |
| 6 | Negative | Positive | NR | 200.0 | ↑ | Positive | Asymptomatic |
| 7 | Negative | Positive | 1:32,000 | 147.5 | NR | Negative | Hydrocephalus |
| 8 | Negative | Negative | 1:8000 | 312.0 | ↑ | Negative | Asymptomatic |
| 9 | Negative | Positive | 1:512,000 | 175.6 | ↓ | Negative | Asymptomatic |
| 10 | Positive | Positive | 1:512,000 | 188.1 | ↓ | Positive | Chorioretinitis |
| 11 | Negative | Positive | 1:32,000 | 139.8 | NR | Positive | Calcification |
| 12 | Positive | Negative | NR | 84.8 | ↑ | Negative | Vitritis+calcification |
| 13 | Positive | NR | NR | 200.0 | NR | Positive | Chorioretinitis+calcification |
| 14 | Negative | NR | NR | 173.9 | ↓ | Positive | Chorioretinitis+calcification+hydrocephalus |
| 15 | Positive | NR | NR | 1655.0 | ↓ | Negative | Chorioretinitis+calcification |
PCR, polymerase chain reaction for DNA detection of Toxoplasma gondii; IFI, indirect immunofluorescence for detection of IgG anti-T. gondii; CL, microparticle chemiluminescence immunoassay for the detection of anti-T. gondi IgG, expressed in UI/mL; NR, unrealized; ↑, increased antibody levels of anti-T. gondii IgG in serial serum samples; ↔, persistence of levels of anti-T. gondii IgG in serum samples during follow-up; ↓, decline of antibodies.
Among the 15 children with CT, the IgG-WB test was positive in nine (60.0%), and among the 32 children without CT, the test was positive in 18 (56.3%), which resulted in a sensitivity of 60.0% (95% CI: 32.3–83.7%), specificity of 43.7% (95% CI: 26.4–62.3%), PPV of 33.3% (95% CI: 16.5–54.0%), and NPV of 70.0% (95% CI: 45.7–88.1%) (p=0.05875). Among the children with CT, two presented negative IgG-WB and were positive for anti-T. gondii IgM; five children presented positive IgG-WB and were negative for anti-T. gondii IgM, four presented both tests as positive, and four presented both tests as negative.
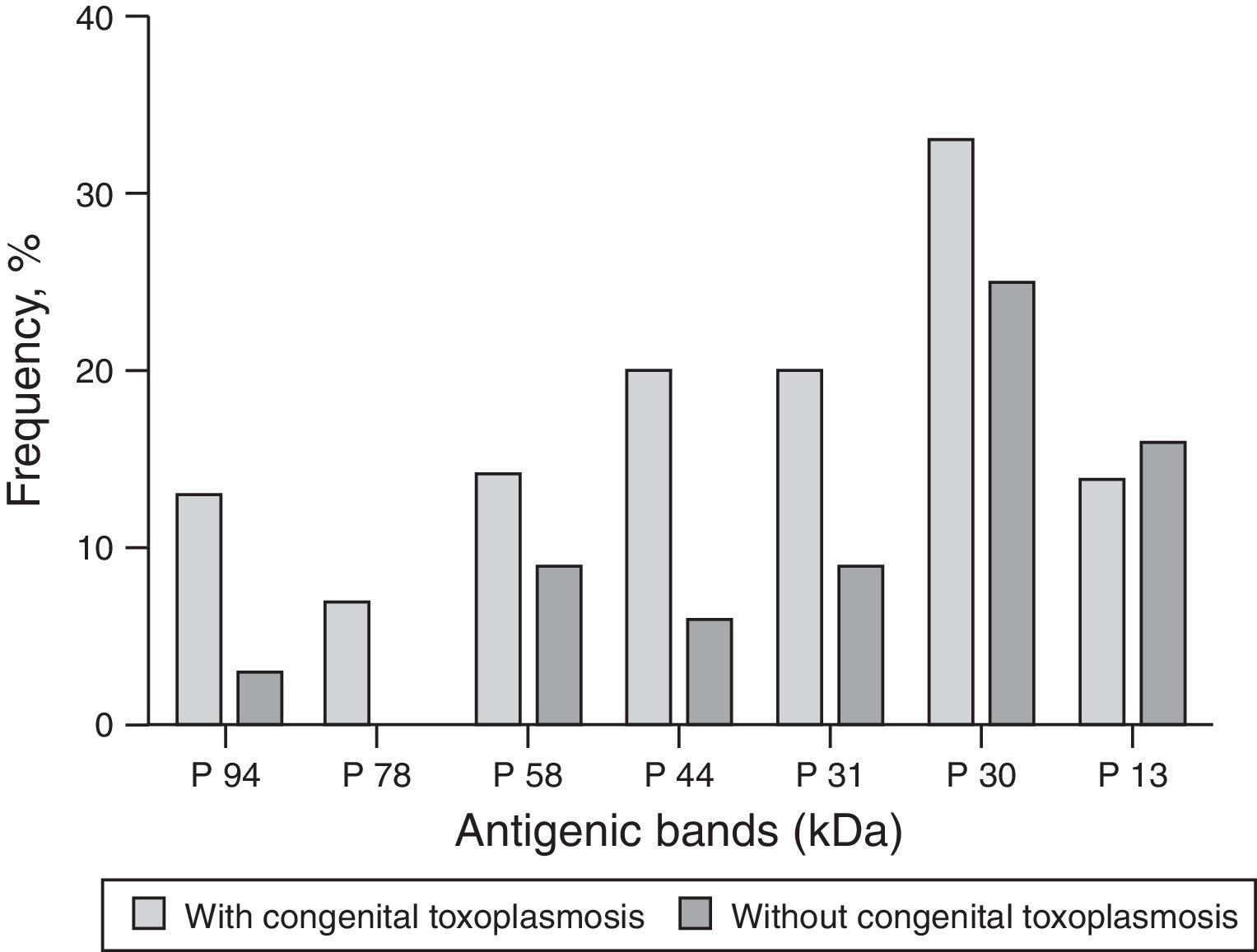
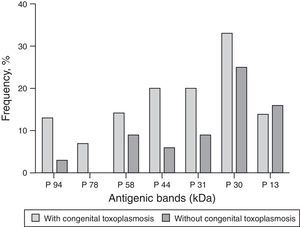
The molecular weight (MW) of the protein recognized by anti-T. gondii IgG in the serum of children with CT ranged from 2 to 94kDa, and regarding the proteins recognized by IgG antibodies in the serum of the non-infected, the MW ranged from 1 to 160kDa. Among the nine children diagnosed through IgG-WB, seven presented different bands from those observed in the maternal serum and two presented bands of greater intensity than those presented by their mother's sample. The dominant bands that were recognized by IgG antibodies from samples of children with CT and the non-infected are demonstrated in Fig. 2.
As for the 26 samples from patients with other diseases, two showed no reactivity and 24 showed reactivity for IgG antibodies that recognized proteins with MW ranging from 17 to 118kDa. Those most frequently recognized were p22 (11/40.7%), p34 (9/33.3%), p38 (8/29.6%), p94 (6/22.2%), p56 (5/18.5%), and p30 (5/18.5%).
The analysis of the results of anti-T. gondii IgM using CL showed sensitivity of 40.0% (95% CI: 16.3–67.6%), specificity of 100.0% (95% CI: 89.1–100.0%), PPV of 100.0% (95% CI: 54.1–100.0%), and NPV of 78.1% (95% CI: 62.4–89.4%; p=0.0005). For the detection of the parasite's DNA using PCR, the sensitivity was 71.4% (95% CI: 29.0–96.3%), specificity of 100.0% (95% CI: 69.2–100.0%), PPV of 100.0% (95% CI: 47.8–100.0%), and NPV of 83.3% (95% CI: 51.6–98.0%; p=0.0034).
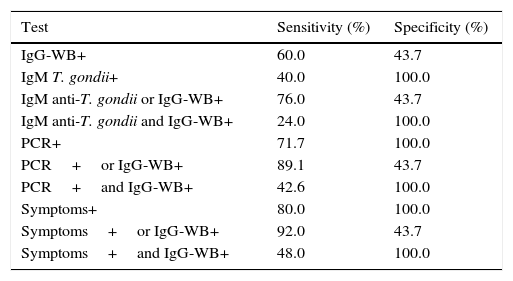
The sensitivity and specificity of IgG-WB when associated with other markers of CT – such as the presence of anti-T. gondii IgM, positive PCR, and the clinical symptoms – are shown in Table 2.
Sensitivity and specificity of the Western blotting method for the detection of anti-Toxoplasma gondii IgG (IgG-WB), anti-Toxoplasma gondii IgM test, the polymerase chain reaction (PCR) to Toxoplasma gondii, and the presence of clinical symptoms compatible with congenital toxoplasmosis, assessed in series and in parallel, according to the presence or absence of congenital toxoplasmosis.
| Test | Sensitivity (%) | Specificity (%) |
|---|---|---|
| IgG-WB+ | 60.0 | 43.7 |
| IgM T. gondii+ | 40.0 | 100.0 |
| IgM anti-T. gondii or IgG-WB+ | 76.0 | 43.7 |
| IgM anti-T. gondii and IgG-WB+ | 24.0 | 100.0 |
| PCR+ | 71.7 | 100.0 |
| PCR+or IgG-WB+ | 89.1 | 43.7 |
| PCR+and IgG-WB+ | 42.6 | 100.0 |
| Symptoms+ | 80.0 | 100.0 |
| Symptoms+or IgG-WB+ | 92.0 | 43.7 |
| Symptoms+and IgG-WB+ | 48.0 | 100.0 |
IgG-WB, Western blotting for diagnosis of congenital infection with detection of IgG antibodies; anti-Toxoplasma gondii IgM detected by chemiluminescence; Symptoms, presence of symptoms and/or clinical signs consistent with congenital toxoplasmosis; +, positive test.
IgG-WB was positive in six of nine patients (66.7%) with eye injuries and in three of six patients (50.0%) with CT without eye injuries (p=0.6224), with sensitivity of 66.7% (95% CI: 29.9–92.5%), specificity of 50.0% (95% CI: 11.8–88.2%), PPV of 66.7% (95% CI: 29.9–92.5%), and NPV of 50% (95% CI: 11.8–88.2%).
DiscussionThe diagnosis of CT is a challenge in clinical practice, because the sensitivity of anti-T. gondii IgM using conventional serological methods varies widely, from 48.3% to 75.0%.3–5 The absence of anti-T. gondii IgM can be justified by the fact that fetal infection occurred at the beginning of pregnancy or as a result of maternal treatment for toxoplasmosis carried out during the first two trimesters of pregnancy, which would cause blockage or delay of the immune response.20 Moreover, the delay or the absence of detectable immune response by standard methods (IgG and IgM dosage or WB) could be related to differences in the individual immune response. Another disadvantage of this marker is the delay in the sample collection, with decreased positivity of IgM after the first 30 days of life.21 Therefore, it is often necessary to monitor the serological results with the identification of stable or increasing titers of anti-T. gondii IgG, which delays the diagnosis and causes uncertainty to the family.16 In this context, the IgG-WB can be used to compare the patterns of antibodies against T. gondii in serum samples from the mothers and their children, and determine if the antibodies are transmitted passively or synthesized by the fetus or infant in cases of CT.15
Other authors have observed a higher contribution of IgG-WB to the diagnosis of CT in the first months of life.22,23 In addition, the association of IgG-WB with other serological methods can increase the probability of diagnosis. In a cohort of children, the sensitivity of IgG-WB was 82.4% and increased to 85.7% when IgG-WB was associated with the presence of IgM and/or IgA anti-T. gondii using ELISA.16 Another study showed that the combination of IgA and IgM immunocapture tests, the analysis of IgG an IgM WB patterns, and the combination of both techniques allowed the detection of 94.0%, 94.0%, and 100.0% of cases, respectively.24
The IgG-WB results obtained in the present study are close to those found by other authors, who reported sensitivity of 73.5%.18 These same authors reported that the combination of IgG-WB and IgM-WB increased the sensitivity to 86.5%.18
The lower sensitivity obtained in this study can be explained, in part, because all the patients with suspected CT were treated with sulfadiazine, pyrimethamine, and folinic acid during the investigation, which could inhibit the neosynthesis of antibodies.20
Another strategy to improve the sensitivity of this proposed method is the repetition of IgG-WB throughout the first three months of life.8 In a study with samples collected at birth, the sensitivity of anti-T. gondii IgM by conventional methods (ISAGA and ELISA) was 52.0% and for WB (with IgG and IgM) was 67.0%. By combining the two methods, the sensitivity increased to 78.0% at birth and to 85.0% at three months of life, with the detection of 94.0% of the cases of CT.17
The present study analyzed some sequential samples of IgG-WB. Among infected children, with false-negative result in the first sample by IgG-WB, two of six (33.3%) children have different recognized proteins in relation to the maternal sample in the second collection, characterizing neosynthesis of anti-T. gondii IgG, which justifies the need for repetition of IgG-WB in serial samples.
In a previous analysis, the time of maternal sample collection influenced the outcome of the IgG-WB test, because mothers who received treatment for toxoplasmosis during pregnancy may no longer react to the antigens of T. gondii in the late after birth period, with false-negative result.16 In the present study, this phenomenon was observed in one mother of an infected child. However, the majority of mothers showed an increase in the number of bands recognized in the samples collected immediately after the interruption of treatment at delivery.
In a previous study, the MW of antigenic bands that were recognized by IgG of neonates with CT varied from 21 to 116kDa.18 In the present study, similar to that found by other authors,15,16 the antibodies found with high frequency in infected children were those against the proteins with MW higher than 30kDa. The differences in the MW of recognized proteins reported by previous studies can be explained by different methodological requirements, such as the preparation of the T. gondii antigen, conditions of electrophoresis on acrylamide gel, and the serum dilutions.20 Another explanation is the genetic diversity among strains of T. gondii, which can lead to the recognition of different proteins, besides the fact that some individuals present IgG that recognizes multiple proteins, while others, a single protein.25
In the present study, IgG-WB was also useful in demonstrating the active synthesis of anti-T. gondii IgG in patients with IgG non-reactive by conventional methods. In Patient 4, with anti-T. gondii IgG titer<1:16 by IIF in the first sample and a slight increase in sequential samples (maximum of 1:64), disappearance of IgG antibodies was observed when assayed by CL and IIF methods after treatment. However, by the IgG-WB method, it was possible to demonstrate anti-T. gondii IgG, which recognized the proteins p22 and p46, which were absent in the maternal serum. After 12 months of life, this child, who showed chorioretinitis scarring, remained non-reactive to anti-T. gondii IgG antibodies evaluated by CL and IIF; however, positivity was shown for IgG-WB.
CT with non-reactivity for anti-T. gondii IgG and IgM may result from toxoplasmosis treatment on the mother and neonate.20 The treatment of the child for a year after birth may also cause antibody non-reactivity. However, in most of these cases there is detection of anti-T. gondii IgG soon after treatment interruption, called the rebound effect.1,7,20
In this present study, Patient 1 showed non-reactivity, but presented detectable anti-T. gondii antibodies after interruption of the treatment. Similarly to Patient 4, Patients 6, 8, and 9 also showed a drop in antibodies up to the point of non-reactivity and remained thus after interruption of treatment. It is possible that these patients no longer recognize the proteins p30 and p35 present in the CL test, which justifies the non-reactivity in this test with the evolution of the infection.
Therefore, instead of evaluating only the persistence of anti-T. gondii IgG by CL, the authors suggest the use of IgG-WB in serological monitoring, since the infected child can produce antibodies against other proteins of the parasite and thus remains positive after 12 months age. In this case, the absence of anti-T. gondii IgG in the CL may not exclude CT, which would also justify the lower specificity of IgG-WB found during the present study compared to previous studies.16–18,22,23 However, more studies should be done before using IgG-WB in serological monitoring.
According to a study carried out in a Brazilian population, patients with IgM reactive by WB method (IgM-WB) showed greater risk for active macular injury than patients with negative IgM-WB.18 However, no significant difference was found between positivity of IgG-WB and the presence of macular injury, as in the present study. It is not clear whether there are specific proteins of T. gondii strains associated with eye injuries and if they could explain the greater frequency of eye injury on Brazilian children with CT than in other countries.26 The main limitation to this present study was the absence of evaluation of the samples with IgM-WB, which could improve the diagnostic sensitivity of the test.
The technical procedure for IgG-WB is of low complexity and with available cost, which makes it viable in the laboratory routine. The drawback of the in-house method is its slowness, which could be reduced with standardization of a set of reagents to be produced in Brazil. The semi-automation of the method with the use of a program for the reading of band intensity viewed though WB would facilitate the reading and the reproducibility of results.20
The results reinforce the usefulness of the IgG-WB method for serological evaluation of patients with CT, with greater sensitivity than the detection of anti-T. gondii IgM by conventional methods. Therefore, IgG-WB can be used for the early diagnosis of CT in combination with other markers of the congenital T. gondii infection.
FundingProgram of Support to the University Extension of the Ministry of Education of Brazil (PROEXT – MEC/SESu) and the National Council of Scientific and Technological Development (CNPq).
Conflicts of interestThe authors declare no conflicts of interest.
To all the patients and family members who contributed to the fulfillment of this work, to the Postgraduate Program in Health Sciences of the Universidade Estadual de Londrina (UEL), and to the Postgraduate Program in Animal Sciences of the UEL.
Please cite this article as: Capobiango JD, Monica TC, Ferreira FP, Mitsuka-Breganó R, Navarro IT, Garcia JL, et al. Evaluation of the Western blotting method for the diagnosis of congenital toxoplasmosis. J Pediatr (Rio J). 2016;92:616–23.
Study conducted at Universidade Estadual de Londrina, Londrina, PR, Brazil.