Volumetric capnography provides the standard CO2 elimination by the volume expired per respiratory cycle and is a measure to assess pulmonary involvement. Thus, the objective of this study was to evaluate the respiratory dynamics of healthy control subjects and those with cystic fibrosis in a submaximal exercise protocol for six minutes on the treadmill, using volumetric capnography parameters (slope 3 [Slp3], Slp3/tidal volume [Slp3/TV], and slope 2 [Slp2]).
MethodsThis was a cross-sectional study with 128 subjects (cystic fibrosis, 64 subjects; controls, 64 subjects]. Participants underwent volumetric capnography before, during, and after six minutes on the treadmill. Statistical analysis was performed using the Friedman, Mann–Whitney, and Kruskal–Wallis tests, considering age and sex. An alpha=0.05 was considered.
ResultsSix minutes on the treadmill evaluation: in cystic fibrosis, volumetric capnography parameters were different before, during, and after six minutes on the treadmill; the same was observed for the controls, except for Slp2. Regarding age, an Slp3 difference was observed in cystic fibrosis patients regardless of age, at all moments, and in controls for age≥12 years; a difference in Slp3/TV was observed in cystic fibrosis and controls, regardless of age; and an Slp2 difference in the cystic fibrosis, regardless of age. Regarding sex, Slp3 and Slp3/TV differences were observed in cystic fibrosis regardless of sex, and in controls in male participants; an Slp2 difference was observed in the cystic fibrosis and female participants. The analysis between groups (cystic fibrosis and controls) indicated that Slp3 and Slp3/TV has identified the CF, regardless of age and sex, while the Slp2 showed the CF considering age.
ConclusionsCystic fibrosis showed greater values of the parameters before, during, and after exercise, even when stratified by age and sex, which may indicate ventilation inhomogeneity in the peripheral pathways in the cystic fibrosis.
A capnografia volumétrica fornece o padrão de eliminação do CO2, pelo volume expirado por ciclo respiratório, e avalia o comprometimento pulmonar. O objetivo do estudo foi avaliar a dinâmica respiratória de indivíduos controles saudáveis e em indivíduos com fibrose cística, em um protocolo de exercício submáximo por seis minutos em esteira, por parâmetros da capnografia volumétrica [slope 3(Slp3), Slp3/volume corrente (Slp3/TV) e slope 2(Slp2)].
MétodosEstudo de corte transversal com 128 indivíduos [(fibrose cística) 64 (controles) 64]. Os participantes realizaram capnografia volumétrica antes, durante e após seis minutos em esteira. Análise estatística foi realizada pelos testes de Friedman, Mann-Whitney e Kruskal-Wallis, considerado a idade e o sexo. Alpha=0,05.
ResultadosAvaliação de seis minutos em esteira: na fibrose cística, os parâmetros da capnografia volumétrica foram diferentes antes, durante e após seis minutos em esteira, o mesmo ocorreu nos controles, exceto para o Slp2. Considerando a idade: (Slp3) diferença na FC, independentemente da idade, em todos os momentos e nos controles apenas para ≥12 anos; (Slp3/TV) diferença para fibrose cística e controles independentemente da idade; (Slp2) diferença apenas para o grupo fibrose cística, independentemente da idade. Considerando o sexo: (Slp3 e Slp3/TV) diferença para fibrose cística, independentemente do sexo, e controles apenas no sexo masculino; (Slp2) diferença para fibrose cística e sexo feminino. Análise entre grupos (fibrose cística versus controles): Slp3 e Slp3/TV identificou a fibrose cística, independentemente da idade e sexo, enquanto o Slp2 evidenciou a fibrose cística considerando a idade.
ConclusãoA fibrose cística apresentou maiores valores dos parâmetros antes, durante e após exercício, inclusive quando se considerou idade e sexo, podendo indicar não homogeneidade da distribuição da ventilação nas vias periféricas.
In cystic fibrosis (CF; OMIM: #219700) there is a progressive decline of lung function that is determinant of patients’ mortality. However, recently, favorable CF prognosis have been demonstrated in subjects with better aerobic conditioning and nutritional status.1,2 For this reason, markers that show limitations to exercise and that evaluate progressive pulmonary deterioration have been studied.2,3
CF shows progressive involvement of peripheral airways (pulmonary silent zone), and conventional pulmonary function tests are ineffective to correctly assess the region.4,5 Thus, volumetric capnography (VCap) arises as an alternative to assess the partial pressure of carbon dioxide (VCO2) in the gas that comes in and out of the lungs over time and to detect homogenization of the pulmonary periphery at the beginning of the gas washout. VCap provides gas transport indexes in the alveolar airways of the lung periphery, slope 3. In summary, VCap is a low cost procedure, easy to administer, that is well tolerated and does not require a maneuver that is forced or hard to understand. In addition, it can detect early pulmonary alterations.5,6
Therefore, the objective of this study was to evaluate the respiratory dynamics of healthy control children and adolescents and those with CF in a submaximal exercise protocol on treadmill for six minutes (SMWT) considering VCap parameters (slope 3, slope 3/exhaled tidal volume [slope 3/TV], and slope 2).
MethodsThis was a cross-sectional study with 128 subjects, 64 with CF and 64 healthy control volunteers. The study included subjects with CF who agreed to participate in the study and who met the inclusion criteria. A signed informed consent was obtained from all participants included in the study. The study followed the ethical criteria of the Helsinki Convention and was approved by the Ethics Committee of the institution (No. 1182/2009).
CF groupInclusion criteria were: age between 6 and 25 years; CF diagnosis by two sweat tests with chloride values above 60 mEq/L and/or presence of two mutations in the cystic fibrosis transmembrane regulator (CFTR) gene3,7,8; and clinical stability determined by two clinical scores: Cystic Fibrosis Foundation Clinical Score and Cystic Fibrosis Clinical Score.9,10 Disease severity was classified in accordance with the Shwachman-Kulczycki score.11
Control groupHealthy subjects aged between 6 and 25 years were randomly selected among students of public and private schools located in the same neighborhood as this university. Participants answered a questionnaire about health conditions and did not present acute respiratory disease or modification in the spirometry variables.
Volumetric capnographyThe subjects underwent the VCap on a treadmill. In VCap, the CO2smo Plus Dx-8100 monitor (Novametrix, Medical Systems, USA) was used to evaluate the participants four minutes before SMWT, six minutes during it, and four minutes after the test. The system is not invasive and is composed of a monitor containing a capnograph, pulse oximetry, and pneumotachograph. VCap and pneumotachograph measurements were obtained in real time by the expired gas analysis. The CO2smo Plus Dx-8100 monitor was linked to a computer equipped with the software to record flow, volume, pressure, pressure-volume, volume flow, and VCap and curves measurements.
The sensor of the monitor was connected to a nozzle, and a nasal clip was used to avoid air escape through the nose. At the end of collection, an offline sequence of respiratory cycles was selected to admit a coefficient of variation for the TV lower than 25% of the mean TV, and a coefficient of variation lower than 10% in millimeters of mercury (mmHg) was admitted for EtCO2. Respiratory cycles with zero value for the slope 3 were eliminated. The result was obtained from the mean of variables during the minutes of monitorization.3,5,9
In this study, the following measures were assessed: (i) TV: volume of air exhaled in one breath, measured in milliliters (mL); (ii) Slope of phase II of the VCap curve (slope 2), measured in mmHg/L; (iii) Slope of phase III of VCap curve (slope 3): measured in mmHg/L.
Submaximal exercise of six minutes on the treadmill (SMWT)Before starting the SMWT on the treadmill, the anthropometric assessment was conducted, which included weight (kg), height (cm), and calculation of body mass index (BMI). Participants walked on a treadmill (Caloi Electronics® Pro CL 5004, SP, Brazil) for six minutes to analyze the behavior of the VCap variables. SMWT started with the treadmill paused. The speed was progressively increased every minute according to the individual's tolerance to walking, without running, and respecting the submaximal effort of each individual.3,10
Immediately before the SMWT, the participant remained at rest for four minutes to assess the VCap. Then, the SMWT was started, and the VCap data were measured during the SMWT.
The exercise was accompanied by verbal stimuli, which was provided on the first minute and at the end of each minute.
The exercise protocol was based on the SMWT and on protocols developed on treadmill.3,10–15
Statistical analysisFor each moment of evaluation during the exercise, the VCap data are presented by the mean, standard deviation, median, minimum, maximum, and the confidence interval for the mean. The moments evaluated were: (1) baseline; (2) 1st and 2nd minutes of SMWT; (3) 3rd and 4th minutes of SMWT; (4) 5th and 6th minutes of SMWT; and (5) after SMWT. Statistical analysis was performed in the software SPSS, version 24 (IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24 – Armonk, NY: IBM Corp.) using Friedman, Mann–Whitney, and Kruskal–Wallis tests. The analyses considered the age and sex of subjects. An alpha=0.05 was considered.
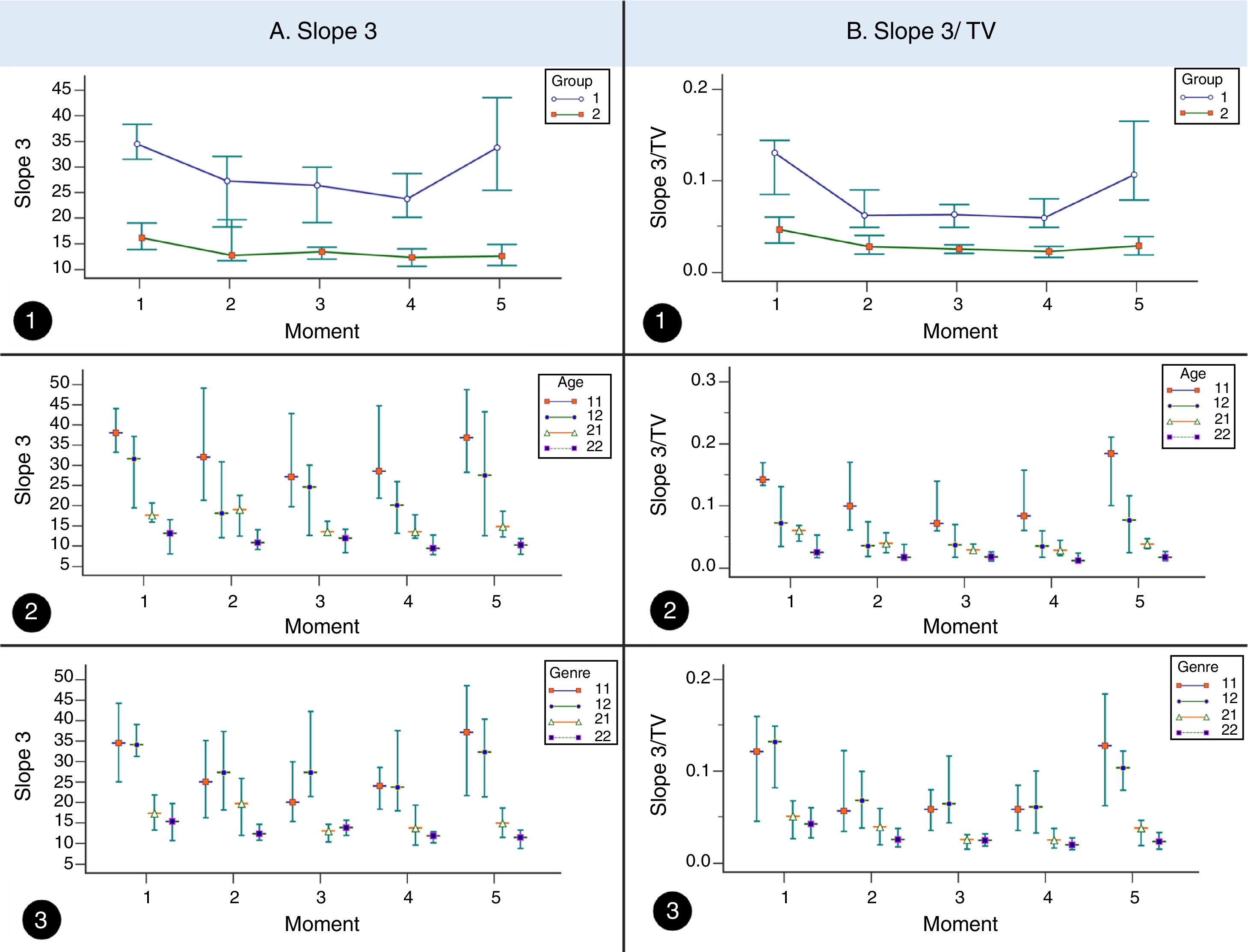
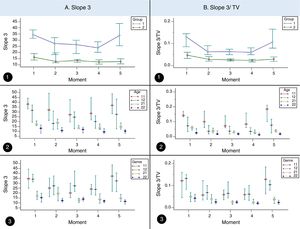
ResultsIn the CF and control groups, slope 3, slope 3/TV, and slope 2 presented differences among the moments analyzed (p<0.05). Slope 3 showed higher values in the CF than control groups at moments 1, 3, 4, and 5 (p<0.05), while slope 3/TV showed higher values in the CF than control groups for all the moments (p<0.05; Table 1 and Fig. 1).
Association of slope 3, slope 3/TV, and slope 2 among subjects with cystic fibrosis (n=64) and healthy controls (n=64) in different moments of the volumetric capnography.
| Markers | Group | Moment | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||||
| Slope 3 | Cystic fibrosis | Mean±SD | 37.61±3.01 | 35.8±4.52 | 31.2±2.75 | 30.98±2.8 | 38.05±3.75 | <0.001 (1≠2, 3 and 4; 4≠1 and 5; 5≠3 and 4) |
| Median Minimum Maximum 95% CI | 34.5 5 140.1 31.58–43.64 | 27.29 6.05 246.48 26.76–44.84 | 26.41 6.24 99.47 25.71–36.7 | 23.74 6.51 99.34 25.36–36.59 | 33.77 6.85 170.58 30.55–45.55 | |||
| Controls | Mean±SD | 20.95±2.25 | 20.44±2.65 | 18.49±2.6 | 17.55±2.01 | 18.24±2.77 | 0.019 (1≠3, 4 and 5) | |
| Median Minimum Maximum 95% CI | 16.15 1.5 91 16.45–25.45 | 12.75 4.63 147.71 15.13–25.76 | 13.4 4.58 97.61 13.28–23.7 | 12.31 3.5 78.08 13.52–21.58 | 12.57 1 163.67 12.71–23.78 | |||
| p-Value | <0.001 | 0.052 | <0.001 | <0.001 | <0.001 | |||
| Slope 3/tidal volume | Cystic fibrosis | Mean±SD | 0.16±0.02 | 0.15±0.04 | 0.1±0.01 | 0.1±0.01 | 0.17±0.03 | <0.001 (1≠2, 3 and 4; 4≠1 and 5; 5≠3 and 4) |
| Median Minimum Maximum 95% CI | 0.13 0.01 1.4 0.11–0.21 | 0.06 0.01 3.04 0.05–0.25 | 0.06 0 0.54 0.07–0.14 | 0.05 0 0.52 0.07–0.13 | 0.1 0.01 2.05 0.1–0.25 | |||
| Controls | Mean±SD | 0.07±0.01 | 0.07±0.02 | 0.05±0.01 | 0.05±0.01 | 0.07±0.02 | <0.001 (1≠3, 4 and 5) | |
| Median Minimum Maximum 95% CI | 0.04 0 0.67 0.04–0.09 | 0.02 0 1.74 0.01–0.12 | 0.02 0 0.76 0.02–0.09 | 0.02 0 0.85 0.02–0.08 | 0.02 0 1.41 0.02–0.11 | |||
| p-Value | <0.001 | 0.003 | <0.001 | <0.001 | <0.001 | |||
| Slope 2 | Cystic fibrosis | Mean±SD | 487.55±21.57 | 449.93±20.28 | 450.8±21.44 | 443.29±24.02 | 483.63±24.91 | <0.001 (1≠2, 3 and 4; 4≠1 and 5; 5≠3 and 4) |
| Median Minimum Maximum 95% CI | 480.63 140 940.17 444.43–530.68 | 439.04 147.49 1003.17 409.39–490.48 | 402.71 167.4 1070.96 407.95–493.65 | 385.18 161.66 1173.23 395.28–491.3 | 462.7 139 1366.24 433.84–533.42 | |||
| Controls | Mean±SD | 455.95±21.17 | 415.73±15.35 | 408.27±16.88 | 409.1±16.18 | 428.99±19.65 | 0.101 | |
| Median Minimum Maximum 95% CI | 440.06 225.88 1215.58 413.64–498.25 | 401.39 228.65 791.22 385.04–446.41 | 380.12 207.82 816.03 374.53–442.02 | 388.58 207.76 779.63 376.75–441.44 | 400.36 240.28 1184.37 389.72–468.26 | |||
| p-Value | 0.596 | 0.596 | 0.216 | 0.86 | 0.052 | |||
SD, standard deviation; CI, confidence interval; 1, baseline; 2, 1st and 2nd minutes of the SMWT; 3, 3rd and 4th minutes of the SMWT; 4, 5th and 6th minutes of the SMWT; 5, after the SMWT; SMWT, six-minute walk test on the treadmill. Alpha=0.05.
The Mann-Whitney and Friedman tests were used. Data with positive p-values are presented in bold.
Volumetric capnography association among subjects with cystic fibrosis. A. Slope 3 association among subjects with cystic fibrosis (CF, 1) (n=64) and healthy controls (2) (n=64) considering age and sex in different moments of volumetric capnography. 1. Association of slope 3 with CF and controls. (moment 1) 1=34.5, 2=16.15, (moment 2) 1=27.29, 2=12.75. (moment 3) 1=26.41, 2=13.4. (moment 4) 1=23.74, 2=12.31. (moment 5) 1=33.77, 2=12.57. 2. Association of slope 3 with CF and controls considering age. (moment 1) 11 (CF+<12 years)=38.04, 12 (CF+≥12 years)=31.6, 21 (controls+<12 years)=17.63, 22 (controls+≥12 years)=13.05. (moment 2) 11=32.01, 12=18.13, 21=19.04, 22=10.84. (moment 3) 11=27.09, 12=24.56, 21=13.5, 22=11.9. (moment 4) 11=28.51, 12=20.06, 21=13.54, 22=9.37. (moment 5) 11=36.84, 12=27.52, 21=14.84, 22=10.21. 3. Association of slope 3 with CF and controls considering sex. (moment 1) 11 (CF+masculine)=34.5, 12 (CF+feminine)=34.1, 21 (controls+masculine)=17.25, 22 (controls+feminine)=15.29. (moment 2) 11=24.96, 12=27.29, 21=19.67, 22=12.32. (moment 3) 11=27.97, 12=27.32, 21=12.96, 22=13.84. (moment 4) 11=24.02, 12=23.74, 21=13.72, 22=11.76. (moment 5) 11=37.12, 12=32.31, 21=14.87, 22=11.37. B. Slope 3/tidal volume association among subjects with CF (1) (n=64) and healthy controls (controls, 2) (n=64) considering the age and sex in different moments of volumetric capnography. 1. Association of slope 3 with CF and controls. (moment 1) 1=0.13, 2=0.04, (moment 2) 1=0.06, 2=0.02. (moment 3) 1=0.06, 2=0.02. (moment 4) 1=0.05, 2=0.02. (moment 5) 1=0.1, 2=0.02. 2. Association of slope 3 with CF and controls considering age. (moment 1) 11 (CF+<12 years)=0.14, 12 (CF+≥12 years)=0.07, 21 (controls+<12 years)=0.05, 22 (controls+≥12 years)=0.02. (moment 2) 11=0.09, 12=0.03, 21=0.03, 22=0.01. (moment 3) 11=0.07, 12=0.03, 21=0.02, 22=0.01. (moment 4) 11=0.08, 12=0.03, 21=0.02, 22=0.01. (moment 5) 11=0.18, 12=0.07, 21=0.03, 22=0.01. 3. Association of slope 3/tidal volume with CF and controls considering sex. (moment 1) 11 (CF+masculine)=0.14, 12 (CF+feminine)=0.07, 21 (controls+masculine)=0.05, 22 (controls+feminine)=0.02. (moment 2) 11=0.09, 12=0.03, 21=0.03, 22=0.01. (moment 3) 11=0.07, 12=0.03, 21=0.02, 22=0.01. (moment 4) 11=0.08, 12=0.03, 21=0.02, 22=0.01. (moment 5) 11=0.18, 12=0.07, 21=0.02, 22=0.01. (moments) 1, baseline time in the SMWT; 2, 1st and 2nd minutes of the SMWT; 3, 3rd and 4th minutes of the SMWT; 4, 5th and 6th minutes of the SMWT; 5, after the SMWT; SMWT, six-minute walk test on the treadmill. TV, tidal volume. We used the Mann–Whitney, Kruskal–Wallis and Friedman tests. Alpha=0.05. The medians and the 95% confidence interval are represented in the graphs.
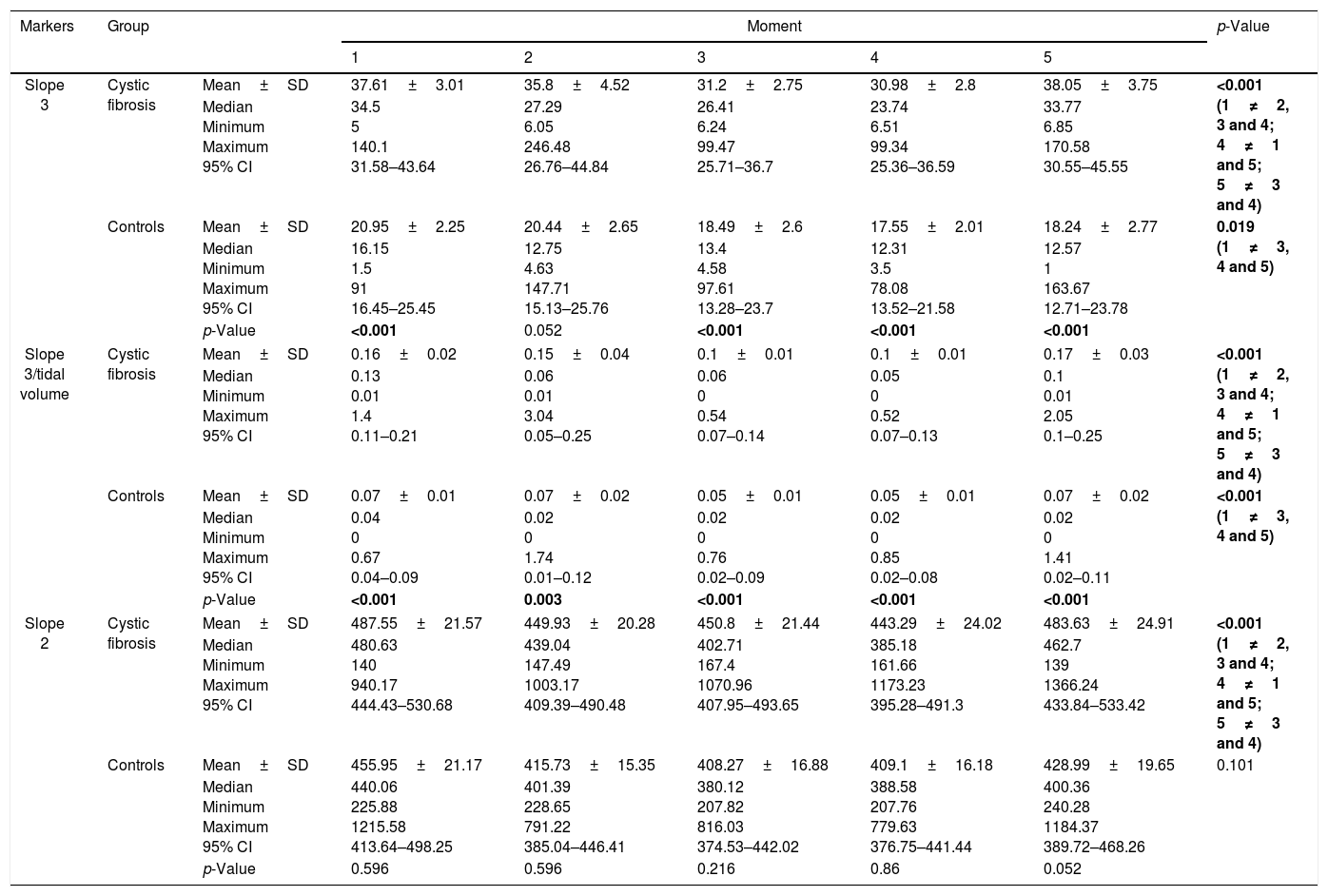
Considering the subjects by age group, the slope 3 showed difference in CF between the analyzed moments regardless of age, whereas the controls only showed difference in subjects aged≥12 years (p<0.05; Table 2 and Fig. 1). The slope 3/TV showed difference in the CF and control groups regardless of age, between all the moments analyzed (Table 2 and Fig. 1). However, for the slope 2, only the CF was different between the moments for the age groups (p<0.05) (Table 2). When stratified by age group, slope 3, slope 3/TV, and slope 2 were able to differentiate the groups (CF and controls), at all moments and in all the analyses (Table 2).
Association of slope 3, slope 3/TV, and slope 2 among subjects with cystic fibrosis (n=64) and healthy controls (n=64) considering the age in different moments of volumetric capnography.
| Markers | Group | Moment | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||||
| Slope 3 | Cystic fibrosis <12 years old (A) | Mean±SD | 44.69±4.91 | 45.79±8.84 | 36.29±4.54 | 37.76±4.47 | 44.38±5.1 | 0.034 (3≠1, 5) |
| Median Minimum Maximum 95% CI | 38.04 16.38 140.1 34.59–54.8 | 32.01 15.4 246.48 27.6–63.98 | 27.09 13.25 99.47 26.95–45.64 | 28.51 12.15 99.34 28.55–46.96 | 36.84 11.71 135.6 33.9–54.87 | |||
| Controls <12 years old (B) | Mean±SD | 24.57±3.51 | 25.32±4.3 | 23.35±4.3 | 21.63±3.16 | 22.85±4.56 | 0.445 | |
| Median Minimum Maximum 95% CI | 17.63 1.5 91 17.43–31.71 | 19.04 7.17 147.71 16.58–34.05 | 13.5 7.52 97.61 14.62–32.07 | 13.54 6.7 78.08 15.2–28.06 | 14.84 1 163.67 13.6–32.1 | |||
| Cystic fibrosis ≥12 years old (C) | Mean±SD | 32.44±3.61 | 28.51±4.15 | 27.49±3.33 | 26.03±3.41 | 33.43±5.24 | 0.006 (4≠1, 2, 3, 5) | |
| Median Minimum Maximum 95% CI | 31.6 5 102.12 25.11–39.78 | 18.13 6.05 123.5 20.10–36.93 | 24.56 6.24 84.87 20.73–34.25 | 20.06 6.51 85.1 19.09–32.96 | 27.52 6.85 170.58 22.80–44.07 | |||
| Controls ≥12 years old (D) | Mean±SD | 15.99±1.98 | 13.77±1.55 | 11.89±1 | 11.96±1.48 | 11.93±1.38 | 0.028 (1≠3, 4, 5) | |
| Median Minimum Maximum 95% CI | 13.05 4.79 39.04 11.91–20.07 | 10.84 4.63 36.72 10.57–16.96 | 11.9 4.58 23.6 9.77–13.9 | 9.37 3.5 36.25 8.92–15 | 10.21 1.21 32.95 9.08–14.77 | |||
| p-Value | <0.001 (A≠B, C, D; C≠A, B, D; B≠A, C; D≠A, C) | 0.004 (A≠B, C, D; C≠A, D; B≠A, D; D≠A, B, C) | <0.001 (A≠B, C, D; C≠A, D; B≠A, D; D≠A, B, C) | <0.001 (A≠B, C, D; C≠A, D; B≠A, D; D≠A, B, C) | <0.001 (A≠B, C, D; C≠A, B, D; B≠A, C, D; D≠A, B, C) | |||
| Slope 3/tidal volume | Cystic fibrosis <12 years old (A) | Mean±SD | 0.21±0.04 | 0.25±0.11 | 0.15±0.02 | 0.14±0.02 | 0.2±0.03 | <0.001 (1 and 5≠2, 3, 4; 2≠1, 3, 5; 3≠1, 2, 5; 4≠1, 5) |
| Median Minimum Maximum 95% CI | 0.14 0.04 1.4 0.11–0.32 | 0.09 0.02 3.04 0.02–0.48 | 0.07 0.02 0.54 0.09–0.2 | 0.08 0.02 0.52 0.09–0.2 | 0.18 0.02 0.98 0.12–0.27 | |||
| Controls <12 years old (B) | Mean±SD | 0.09±0.02 | 0.1±0.04 | 0.09±0.02 | 0.07±0.02 | 0.1±0.04 | 0.005 (1 and 5≠3, 4; 2≠4; 3≠1, 5; 4≠1, 2, 5) | |
| Median Minimum Maximum 95% CI | 0.05 0 0.67 0.05–0.13 | 0.03 0.01 1.74 0.01–0.2 | 0.02 0.01 0.76 0.03–0.14 | 0.02 0.01 0.85 0.02–0.12 | 0.03 0 1.41 0.02–0.18 | |||
| Cystic fibrosis ≥12 years old (C) | Mean±SD | 0.12±0.02 | 0.08±0.02 | 0.07±0.01 | 0.07±0.01 | 0.16±0.05 | <0.001 (1 and 5≠2, 3, 4; 2 and 3≠1, 4, 5; 4≠1, 2, 3, 5) | |
| Median Minimum Maximum 95% CI | 0.07 0.01 0.64 0.07–0.16 | 0.03 0.01 0.57 0.04–0.12 | 0.03 0 0.51 0.04–0.11 | 0.03 0 0.49 0.03–0.1 | 0.07 0.01 2.05 0.04–0.27 | |||
| Controls ≥12 years old (D) | Mean±SD | 0.04±0 | 0.02±0 | 0.01±0 | 0.01±0 | 0.02±0 | 0.001 (1≠2, 3, 4, 5; 2 and 5≠1, 4; 3≠1; 4≠1, 2, 5) | |
| Median Minimum Maximum 95% CI | 0.02 0.01 0.15 0.02–0.05 | 0.01 0 0.07 0.01–0.03 | 0.01 0 0.04 0.01–0.02 | 0.01 0 0.08 0.01–0.02 | 0.01 0 0.09 0.01–0.03 | |||
| p-Value | <0.001 (A≠B, C, D; C≠A, D; B≠A, D; D≠A, B, C) | <0.001 (A≠B, C, D; C≠A, D; B≠A, D; D≠A, B, C) | <0.001 (A≠B, C, D; C≠A, D; B≠A, D; D≠A, B, C) | <0.001 (A≠B, C, D; C≠A, D; B≠A, D; D≠A, B, C) | <0.001 (A≠B, C, D; C≠A, D; B≠A, D; D≠A, B, C) | |||
| Slope 2 | Cystic fibrosis <12 years old (A) | Mean±SD | 562.34±28.52 | 537.44±29.71 | 534±32.3 | 529.34±33.79 | 551.43±28.59 | 0.017 (1 and 5≠3, 4) |
| Median Minimum Maximum 95% CI | 548.63 332.84 940.17 503.7–620.97 | 554.58 340.24 1003.17 476.35–598.52 | 549.36 279.94 1070.96 467.59–600.41 | 566.16 277.46 919.83 459.87–598.8 | 553.21 304.51 871.09 492.66–610.2 | |||
| Controls <12 years old (B) | Mean±SD | 517.42±30.66 | 470.71±20.77 | 460.03±24.62 | 462.76±22.44 | 490.97±28.59 | 0.497 | |
| Median Minimum Maximum 95% CI | 476.5 273 1215.58 455.22–579.62 | 450.06 272.59 791.22 428.58–512.84 | 421.89 287.67 816.03 410.09–509.97 | 427 293.25 779.63 417.23–508.29 | 462.52 277.14 1184.37 432.98–548.96 | |||
| Cystic fibrosis ≥12 years old (C) | Mean±SD | 432.98±28.01 | 386.08±22.62 | 390.08±24.47 | 380.49±29.75 | 434.15±35.87 | 0.003 (4≠1, 3, 5) | |
| Median Minimum Maximum 95% CI | 400.21 140 833.67 376.17–489.79 | 381.25 147.49 708.09 340.18–431.97 | 347.12 167.4 924 340.45–439.72 | 366.42 161.66 1173.23 320.14–440.84 | 430.06 139 1366.24 361.39–506.92 | |||
| Controls ≥12 years old (D) | Mean±SD | 371.7±17.82 | 340.37±12.55 | 337.35±12.33 | 335.55±13.72 | 344.05±13.67 | 0.244 | |
| Median Minimum Maximum 95% CI | 351 225.88 602.15 335.07–408.33 | 334.85 228.65 464.55 314.56–366.18 | 338.1 207.82 471.01 311.99–362.71 | 340.32 207.76 519.02 307.34–363.76 | 341.68 240.28 501.23 315.94–372.17 | |||
| p-Value | <0.001 (A≠C, D; C≠A, B; B≠C, D; D≠A, B) | <0.001 (A≠C, D; C≠A, B; B≠C, D; D≠A, B) | <0.001 (A≠C, D; C≠A, B; B≠C, D; D≠A, B) | <0.001 (A≠C, D; C≠A, B; B≠C, D; D≠A, B) | <0.001 (A≠C, D; C≠A, B, D; B≠C, D; D≠A, B, C) | |||
SD, standard deviation; CI, confidence interval; 1, baseline; 2, 1st and 2nd minutes of the SMWT; 3, 3rd and 4th minutes of the SMWT; 4, 5th and 6th minutes of the SMWT; 5, after the SMWT; SMWT, six-minute walk test on the treadmill. Alpha=0.05. The Kruskal–Wallis and Friedman tests were used. Data with positive p-values are presented in bold.
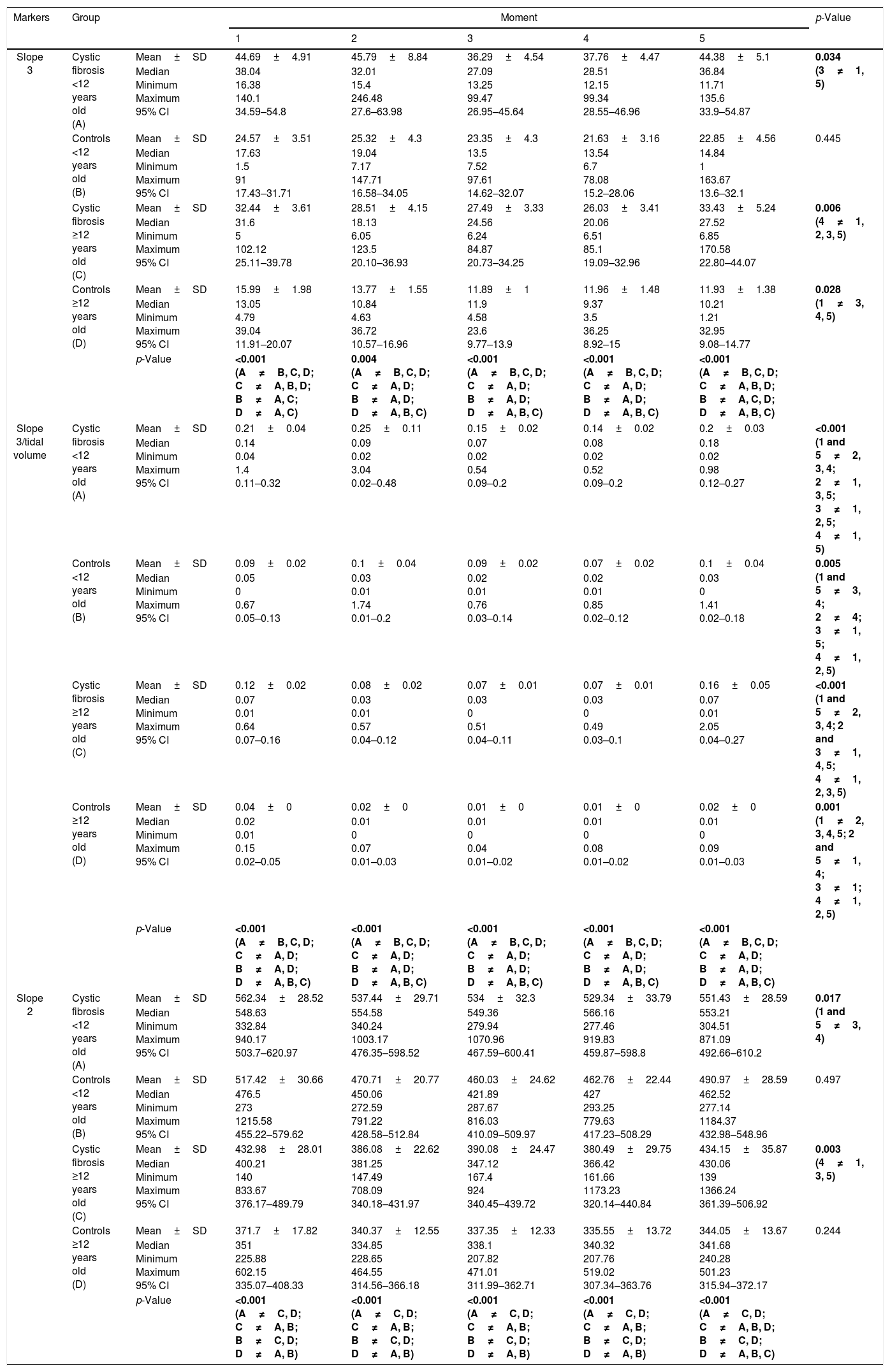
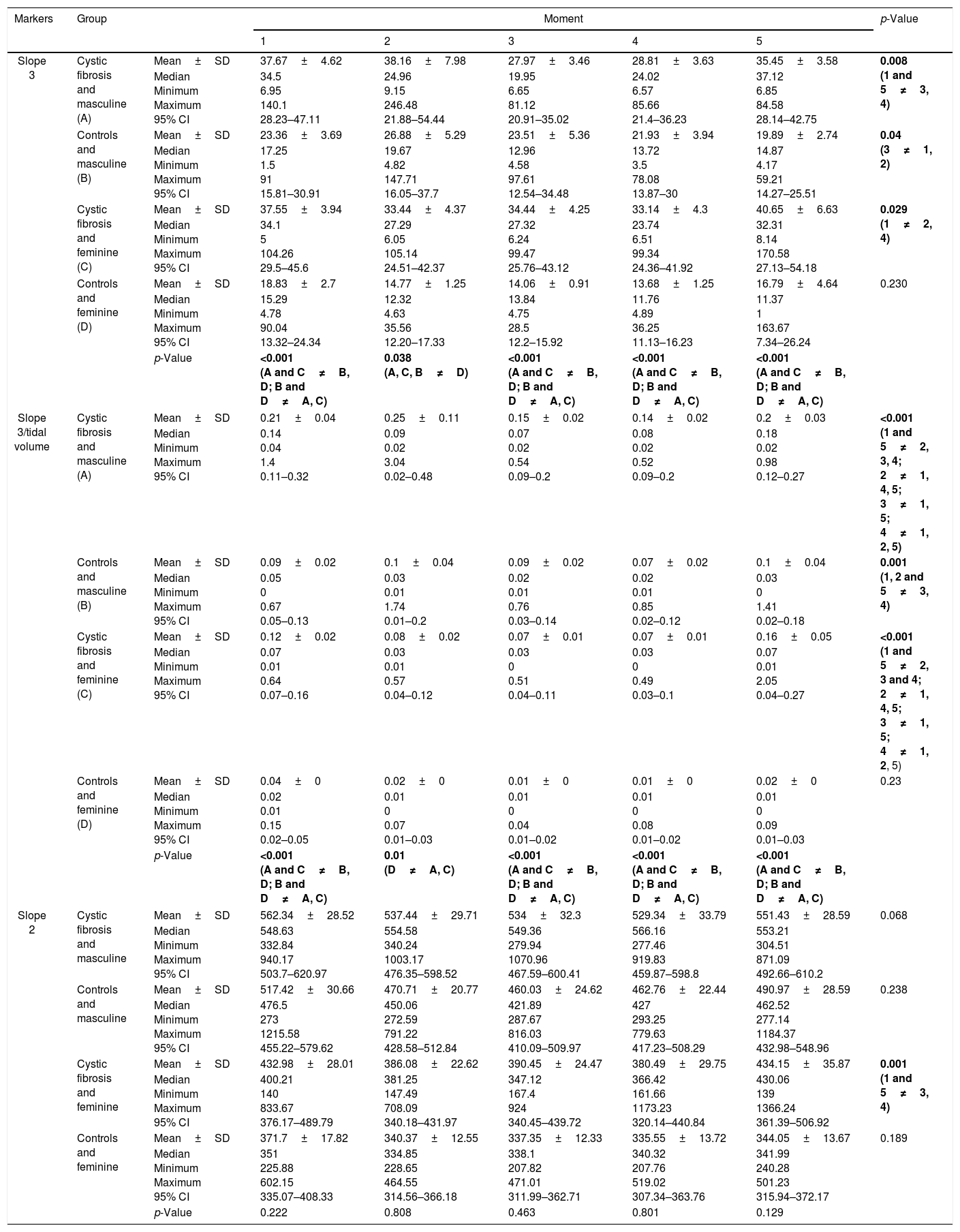
Regarding sex, no differences were observed between the moments in the parameters slope 3 and slope 3/TV for the controls of the female sex (p>0.05) (Table 3; Fig. 1). In turn, slope 2 only showed differences between the moments for the females from the CF group (p<0.05; Table 3). The slope 3 and slope 3/TV were able to differentiate the groups (CF and controls), at all moment and in all the analyses, when sex was considered (Table 3).
Association of slope 3, slope 3/TV, and slope 2 among subjects with cystic fibrosis (n=64), and healthy controls (n=64) considering the sex in different moments of volumetric capnography.
| Markers | Group | Moment | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||||
| Slope 3 | Cystic fibrosis and masculine (A) | Mean±SD | 37.67±4.62 | 38.16±7.98 | 27.97±3.46 | 28.81±3.63 | 35.45±3.58 | 0.008 (1 and 5≠3, 4) |
| Median Minimum Maximum 95% CI | 34.5 6.95 140.1 28.23–47.11 | 24.96 9.15 246.48 21.88–54.44 | 19.95 6.65 81.12 20.91–35.02 | 24.02 6.57 85.66 21.4–36.23 | 37.12 6.85 84.58 28.14–42.75 | |||
| Controls and masculine (B) | Mean±SD | 23.36±3.69 | 26.88±5.29 | 23.51±5.36 | 21.93±3.94 | 19.89±2.74 | 0.04 (3≠1, 2) | |
| Median Minimum Maximum 95% CI | 17.25 1.5 91 15.81–30.91 | 19.67 4.82 147.71 16.05–37.7 | 12.96 4.58 97.61 12.54–34.48 | 13.72 3.5 78.08 13.87–30 | 14.87 4.17 59.21 14.27–25.51 | |||
| Cystic fibrosis and feminine (C) | Mean±SD | 37.55±3.94 | 33.44±4.37 | 34.44±4.25 | 33.14±4.3 | 40.65±6.63 | 0.029 (1≠2, 4) | |
| Median Minimum Maximum 95% CI | 34.1 5 104.26 29.5–45.6 | 27.29 6.05 105.14 24.51–42.37 | 27.32 6.24 99.47 25.76–43.12 | 23.74 6.51 99.34 24.36–41.92 | 32.31 8.14 170.58 27.13–54.18 | |||
| Controls and feminine (D) | Mean±SD | 18.83±2.7 | 14.77±1.25 | 14.06±0.91 | 13.68±1.25 | 16.79±4.64 | 0.230 | |
| Median Minimum Maximum 95% CI | 15.29 4.78 90.04 13.32–24.34 | 12.32 4.63 35.56 12.20–17.33 | 13.84 4.75 28.5 12.2–15.92 | 11.76 4.89 36.25 11.13–16.23 | 11.37 1 163.67 7.34–26.24 | |||
| p-Value | <0.001 (A and C≠B, D; B and D≠A, C) | 0.038 (A, C, B≠D) | <0.001 (A and C≠B, D; B and D≠A, C) | <0.001 (A and C≠B, D; B and D≠A, C) | <0.001 (A and C≠B, D; B and D≠A, C) | |||
| Slope 3/tidal volume | Cystic fibrosis and masculine (A) | Mean±SD | 0.21±0.04 | 0.25±0.11 | 0.15±0.02 | 0.14±0.02 | 0.2±0.03 | <0.001 (1 and 5≠2, 3, 4; 2≠1, 4, 5; 3≠1, 5; 4≠1, 2, 5) |
| Median Minimum Maximum 95% CI | 0.14 0.04 1.4 0.11–0.32 | 0.09 0.02 3.04 0.02–0.48 | 0.07 0.02 0.54 0.09–0.2 | 0.08 0.02 0.52 0.09–0.2 | 0.18 0.02 0.98 0.12–0.27 | |||
| Controls and masculine (B) | Mean±SD | 0.09±0.02 | 0.1±0.04 | 0.09±0.02 | 0.07±0.02 | 0.1±0.04 | 0.001 (1, 2 and 5≠3, 4) | |
| Median Minimum Maximum 95% CI | 0.05 0 0.67 0.05–0.13 | 0.03 0.01 1.74 0.01–0.2 | 0.02 0.01 0.76 0.03–0.14 | 0.02 0.01 0.85 0.02–0.12 | 0.03 0 1.41 0.02–0.18 | |||
| Cystic fibrosis and feminine (C) | Mean±SD | 0.12±0.02 | 0.08±0.02 | 0.07±0.01 | 0.07±0.01 | 0.16±0.05 | <0.001 (1 and 5≠2, 3 and 4; 2≠1, 4, 5; 3≠1, 5; 4≠1, 2, 5) | |
| Median Minimum Maximum 95% CI | 0.07 0.01 0.64 0.07–0.16 | 0.03 0.01 0.57 0.04–0.12 | 0.03 0 0.51 0.04–0.11 | 0.03 0 0.49 0.03–0.1 | 0.07 0.01 2.05 0.04–0.27 | |||
| Controls and feminine (D) | Mean±SD | 0.04±0 | 0.02±0 | 0.01±0 | 0.01±0 | 0.02±0 | 0.23 | |
| Median Minimum Maximum 95% CI | 0.02 0.01 0.15 0.02–0.05 | 0.01 0 0.07 0.01–0.03 | 0.01 0 0.04 0.01–0.02 | 0.01 0 0.08 0.01–0.02 | 0.01 0 0.09 0.01–0.03 | |||
| p-Value | <0.001 (A and C≠B, D; B and D≠A, C) | 0.01 (D≠A, C) | <0.001 (A and C≠B, D; B and D≠A, C) | <0.001 (A and C≠B, D; B and D≠A, C) | <0.001 (A and C≠B, D; B and D≠A, C) | |||
| Slope 2 | Cystic fibrosis and masculine | Mean±SD | 562.34±28.52 | 537.44±29.71 | 534±32.3 | 529.34±33.79 | 551.43±28.59 | 0.068 |
| Median Minimum Maximum 95% CI | 548.63 332.84 940.17 503.7–620.97 | 554.58 340.24 1003.17 476.35–598.52 | 549.36 279.94 1070.96 467.59–600.41 | 566.16 277.46 919.83 459.87–598.8 | 553.21 304.51 871.09 492.66–610.2 | |||
| Controls and masculine | Mean±SD | 517.42±30.66 | 470.71±20.77 | 460.03±24.62 | 462.76±22.44 | 490.97±28.59 | 0.238 | |
| Median Minimum Maximum 95% CI | 476.5 273 1215.58 455.22–579.62 | 450.06 272.59 791.22 428.58–512.84 | 421.89 287.67 816.03 410.09–509.97 | 427 293.25 779.63 417.23–508.29 | 462.52 277.14 1184.37 432.98–548.96 | |||
| Cystic fibrosis and feminine | Mean±SD | 432.98±28.01 | 386.08±22.62 | 390.45±24.47 | 380.49±29.75 | 434.15±35.87 | 0.001 (1 and 5≠3, 4) | |
| Median Minimum Maximum 95% CI | 400.21 140 833.67 376.17–489.79 | 381.25 147.49 708.09 340.18–431.97 | 347.12 167.4 924 340.45–439.72 | 366.42 161.66 1173.23 320.14–440.84 | 430.06 139 1366.24 361.39–506.92 | |||
| Controls and feminine | Mean±SD | 371.7±17.82 | 340.37±12.55 | 337.35±12.33 | 335.55±13.72 | 344.05±13.67 | 0.189 | |
| Median Minimum Maximum 95% CI | 351 225.88 602.15 335.07–408.33 | 334.85 228.65 464.55 314.56–366.18 | 338.1 207.82 471.01 311.99–362.71 | 340.32 207.76 519.02 307.34–363.76 | 341.99 240.28 501.23 315.94–372.17 | |||
| p-Value | 0.222 | 0.808 | 0.463 | 0.801 | 0.129 | |||
SD, standard deviation; CI, confidence interval; 1, baseline; 2, 1st and 2nd minutes of the SMWT; 3, 3rd and 4th minutes of the SMWT; 4, 5th and 6th minutes of the SMWT; 5, after the SMWT; SMWT, six-minute walk test on the treadmill. Alpha=0.05. The Kruskal–Wallis and Friedman tests were used. Data with positive p-values are presented in bold.
The authors believe that this is the first time the VCap parameters during exercise are compared in healthy subjects and CF subjects. The study demonstrated the possibility of obtaining markers in a non-invasive way and through a submaximal effort protocol that is tolerable by participants.
Specifically, VCap presented numerous indexes, such as the slope 3, which provides data about the dynamics of gas transport in the alveolar airways of the lung periphery. The inclination of slope 3 is determined by the emptying nature of the alveolar units: synchronous or asynchronous.16,17
Emptying is synchronous when the gas simultaneously expires from the alveoli, resulting in phase III horizontal or with minimal tilt. In turn, sequential/late emptying occurs when the alveolar units are emptied in an asynchronous manner, i.e., with longer time constants, making the PCO2 higher, resulting in a growing inclination of slope 3. Slope 3 depends on the numerous alveoli emptying patterns with different ventilation/perfusion ratios (V/Q), as the continuous elimination of CO2 in the alveoli, making the VCap a useful tool to detect anomalies in the maladjustment between V/Q.9,18,19
Slope 3 presented higher values in the CF, indicating a ventilation inhomogeneity in the distal airspaces in comparison with controls.5,17 Veronez et al. described that the increase in slope 3 in subjects with bronchiectasis, related or not to the CF, indicated diffuse disease of small airways.6
The behavior of slope 3 during physical exercise observed in this research was similar in the CF and control groups. A decrease in the values of the index was noted during the physical exercise, followed by a quick recovery. However, higher values of slope 3 inclination probably occurred in the CF due to greater retention of exhaled carbon dioxide (VCO2), but it is necessary to consider other hypothesis, such as earlier emptying of alveolar dead space regions (V/Q relationships) and variations in cardiac output. Therefore, the CF group presented increased values of slope 3 during the evaluation, indicating initial ventilation inhomogeneity during the rest. This fact is justified in the CF by the largest CO2 production and retention, increase in airway resistance, decreased functional residual capacity, and changes in cardiac debit during physical activity – these factors can affect the V/Q ratio and influence the height/slope 3.20–23
Still considering the slope 3, some authors suggest that it should be standardized by TV.5,6,9 Almeida et al. noted increased slope3/TV in subjects with asthma, in comparison with controls, suggesting ventilation inhomogeneity in the distal airspaces and indicating chronic airway structural disorders and acute reversible changes observed in the bronchial challenge test.9 Moreover, Ribeiro et al., when using spirometry and the VCap to assess lung function in CF, identified an increase in slope 3/TV, even in subjects with normal spirometry.5 The present study corroborates those findings and identified an increased slope 3/TV during physical exercise in the CF when compared with controls.
The slope 3 and/or slope 3/TV show association even in the CF with age younger than 12 years. Thus, since a young age, the CF group present alterations in the respiratory dynamics, but does not compromise the physical activity performance.
Numerous features on the physiology of physical effort considering the age group and sex were observed. In this line, the higher values of slope 3 were observed in male participants from the CF group (moments 1, 4 and 5). Also, when normalizing by TV (slope 3/TV), the higher values were observed in male participants from the CF group. This observation is due to the increase of maximal oxygen consumption (VO2max) in absolute terms throughout the age groups, with greater acceleration in boys than in girls. This event is closely related to the increase in muscle mass, in a way that when considering the VO2max fixed by indicators of muscle mass, there is no increase with age in male children and adolescents (VO2max/kg – body weight remains constant), while there is a progressive decline in girls (decrease in VO2max/kg-body weight).24–26 Thus, the anaerobic power does not differ between sexes in prepubescent individuals, but it increase proportionally in males from puberty onwards. All of that is due to the development of muscle mass and effect of hormonal maturation on the functional musculoskeletal characteristics.27–30
Although the CF group presented reduction of the alveolar ventilation and change in the V/Q ratio, as evidenced by the curve of slope 3, the patients in this group were able to perform the physical exercise protocol without complications.
VCap is not widely used in clinical practice mainly due to little understanding of the data obtained, as well as the scarcity of published studies. This study described the behavior of slope 2 and slope 3 during submaximal physical exercise in CF patients; it was observed that patients show functional alterations from the baseline period, which are accentuated during exercise. In addition, although no ventilation homogeneity was observed in the pulmonary periphery in the CF group, patients were able to perform submaximal exercises for short periods of time. Data from slope 2 and slope 3 showed that even asymptomatic patients presented changes in lung function, which indicates the need for earlier intervention to minimize chronic lung degradation.
In conclusion, the VCap parameters analysis demonstrated that CF subjects present alterations in respiratory dynamics during physical exercise and that these alterations are related to the distal airspaces with heterogeneity of emptying gases.
FundingFundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for research support and scholarships (#2011/12939-4, #2011/18845-1, #2015/12183-8 and #2015/12858-5 to FALM and JDR); Fundo de Apoio à Pesquisa, ao Ensino e à Extensão da Universidade Estadual de Campinas for research support (#0648/2015 to FALM). Conselho Nacional de Desenvolvimento Científico e Tecnológico for scholarship to PLFP.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank Adyléia Aparecida Contrera Dalbo Toro, Arthur Kmit, Roberto José Negrão Nogueira, Kátia Cristina Alberto Aguiar, Aléthea Guimarães Farias, Taís Daiene Russo Hortêncio, Aline Gonçalves, Gabriel Hessel, Renan Marrichi Mauch, Andressa de Oliveira Peixoto, Luciana Cardoso Bonadia, Carmen Sílvia Bertuzzo, Stéphanie Villa-Nova Pereira, Antônio Fernando Ribeiro, Carla Cristina de Souza Gomez, and Silvana Dalge Severino, who contributed to the studies on cystic fibrosis in this reference center. They would also like to thank the Espaço da Escrita project/General Coordination of Unicamp for the English translation of this article.
Please cite this article as: Parazzi PL, Marson FA, Ribeiro MA, Schivinski CI, Ribeiro JD. Evaluation of respiratory dynamics by volumetric capnography during submaximal exercise protocol of six minutes on treadmill in cystic fibrosis patients. J Pediatr (Rio J). 2019;95:76–86.