There is a scarcity of studies that assessed the association between adherence to combination therapy and asthma control in pediatric patients. The authors investigated the association between adherence to fluticasone propionate/salmeterol xinafoate combination-metered aerosol and the level of asthma control in children.
MethodsThis was a prospective observational study of 84 patients aged 5–16 years with moderate persistent asthma, who remained uncontrolled despite the use of 1000μg/day of inhaled nonextrafine-hydrofluoric alkane-beclomethasone dipropionate in the three months prior to study enrollment. Participants were prescribed two daily doses of FP (125μg)/salmeterol xinafoate (25μg) combination by metered aerosol/spacer for six months. Adherence rates were assessed using the device's dose counter after the 2nd, 4th, and 6th months of follow up. Asthma control was assessed using a simplified Global Initiative for Asthma 2014 Report classification.
ResultsMean adherence rates after the second, fourth, and sixth months were 87.8%, 74.9%, and 62.1% respectively, for controlled asthma, and 71.7%, 56.0%, and 47.6% respectively, for uncontrolled asthma (all p-values≤0.03). The proportion of children achieving asthma control increased to 42.9%, 67.9% and 89.3% after the 2nd, 4th and 6th months of follow-up, respectively (p≤0.001).
ConclusionAdherence rates between 87.8% in the 2nd month and 62.1% in the 6th month were strong determinants of asthma control.
São escassos os estudos que avaliaram a relação entre a taxa de adesão à combinação de proprionato de fluticasona/xinafoato de salmeterol e o nível de controle da asma na infância. O presente estudo teve como objetivo avaliar essa relação.
MétodosEstudo prospectivo observacional com 84 participantes, de 5 a 16 anos, todos eles com asma persistente moderada que permaneceram não controlados apesar do uso de 1.000μg/dia de dipropionato de beclometasona em partículas não extrafinas nos três meses que antecederam a admissão no estudo. Os participantes receberam prescrição de 125μg de propionato de fluticasona e 25μg xinafoato de salmeterol através de inalador pressurizado, duas vezes ao dia, e foram avaliados após o 2°, 4° e 6° meses de tratamento. A taxa de adesão foi obtida por meio do contador analógico de doses incorporado ao inalador. A classificação do nível de controle da asma foi baseada numa simplificação das recomendações da Global Initiative for Asthma.
ResultadosAs taxas de adesão aos 2, 4 e 6 meses para a asma controlada foram 87,8%, 74,9% e 62,1% e para a asma não controlada de 71,7%, 56,0% e 47,6% (p ≤ 0,03), respectivamente. A proporção de pacientes com asma controlada elevou- se para 42,9%, 67,9% e 89,3% nas três avaliações subsequentes (p ≤ 0,001).
ConclusõesTaxas de adesão entre 87,8% no 2° mês e de 62,1% no 6°. mês foram determinantes para o nível de controle da asma.
The first-line treatment of asthma is the use of inhaled corticosteroids (IC), but disease control is not always attained with the use of these drugs alone.1
Adherence to a given therapeutic regimen is the process by which the patient strictly follows a regimen of care and, regarding inhaled medications, the most accurate and reliable methods of evaluating adherence are the electronic and analog dose-counting monitors. Both are more reliable than the reports by patients or family members, clinical judgment, drug dispensing by the pharmacy, and weighing of dose-counting inhalers.2,3 Assessing adherence through the analog counter in the pressurized inhaler is similar to the electronic method, thus allowing the obtaining of a reliable adherence rate.4 In turn, both have the drawback of intentional emptying of the pressurized inhaler.5
Asthma treatment adherence rates are generally lower than prescribed, and studies have shown that a low adherence rate is one of the most common causes of lack of clinical and functional disease control.6 In the case of children, when parents have a negative perception of asthma, they may question the need for IC use at the same time as they are concerned about the adverse effects of medication. Moreover, when they do not have the necessary information to understand the disease, the adherence rate may be even lower.7 Adherence rates greater than 60% are associated with a better level of asthma control.8 However, great variability can be found in studies carried out in the last two decades. Bender, for instance, reported rates of 19–58% in ten studies that assessed adherence through electronic dose-counting,9 whereas other researchers reported a variation of 30% to 70%. Rates of less than 50% are insufficient to control the disease and, consequently, are associated with a greater frequency of disease exacerbations, hospitalizations, and the need for emergency care.10
In turn, it is known that a clinical response to the isolated use of IC is observed only after the first weeks of treatment, whereas the association with prolonged-action beta-2 agonist could bring faster clinical benefits, due to its bronchodilator action. Studies have shown that this association may help the attainment of control in patients with moderate and severe persistent asthma through its synergistic effect.11,12
A literature search in the Pubmed/MedLine, Lilacs, Scielo, Ebsco, and Cochrane Collaboration databases including the past 15 years identified few studies that assessed the adherence rate to the fluticasone propionate-salmeterol xinafoate association and the level of asthma control in children in developing countries.12 The present study aimed to evaluate the association between asthma treatment adherence and control rates through this therapeutic combination.
MethodsStudy site and designThis was a prospective observational study with a six-month follow-up, for which patients with moderate persistent asthma were recruited and were followed at a secondary pediatric referral service linked to the Municipal Health Secretariat of the city of Belo Horizonte, state of Minas Gerais, Brazil. The number of children treated at that service during the period covered by the study was 127. All 84 children who met the inclusion criteria were consecutively admitted and completed the follow-up period predefined in the research protocol. Data collection lasted one year and three months.
Inclusion and exclusion criteriaChildren and adolescents aged 5–16 years of age, with moderate persistent asthma without clinical control at admission despite adequate adherence (verified through medication dispensing at the pharmacy) to the prescription of 1000μg/day of beclomethasone dipropionate with hydrofluoroalkane propellant (hydrofluoric alkane) in non-extrafine particles. The diagnosis of asthma was based on the clinical criteria proposed by the Global Initiative for Asthma (GINA).13 The parents or guardians, all of whom were literate, signed the informed consent form to authorize the participation of their children in the study; those older than 12 years also signed the term of assent. Children and adolescents who were passive smokers and those with comorbidities were excluded.
Asthma control levelAs the main objective of asthma treatment is to achieve full disease control, only two categories were adopted on the two-, four-, and six-month follow-up visits: controlled asthma and uncontrolled asthma, the latter encompassing partially and totally uncontrolled disease presentations, in accordance with the GINA classification.13 Thus, controlled asthma was defined by the following criteria: (1) absence of daytime symptoms, maintenance of normal physical activities, absence of nocturnal symptoms, absence of nocturnal arousal with symptoms, no need to use rescue medication, and no exacerbation in the four weeks preceding the two-, four-, and six-month evaluations. The presence of one of the above criteria was considered as uncontrolled asthma. Spirometry, albeit not part of the GINA control criteria,13 leads to a more reliable asthma monitoring and was performed in those patients capable of undergoing the test. A Koko® spirometer (PDS Instrumentation, Colorado, USA), Pulmonary Function Tests (PFT) version 4.12 used was and the reliability and reproducibility criteria recommended by the American Thoracic Society and European Respiratory Society were used,14 whereas the reference values used were obtained from Polgar and Promadhat.15
Therapeutic regimenAll participants received the medications free of charge: salbutamol for treatment of exacerbations, 100ug/jet Nebulizer (Aerolin® – GlaxoSmithKleine, Brazil) and the combination of 125μ/fluticasone propionate and 25μ/salmeterol xinafoate jet nebulizer (Seretide® – GlaxoSmithKleine, SP, Brazil), the latter with a dose counter, prescribed in two daily doses (morning and evening). The number of doses consumed in the two previous months corresponded to that recorded by the counter during each of the three bimonthly evaluations performed during follow-up. A large-volume valve spacer (Flumax® – Inside Materiais Avançados, SP, Brazil) was also supplied. All children used the same type of spacer, the only one provided free of charge, regardless of age. This conservative measure was taken due to the lack of resources by the participating families, which often lacked good understanding and educational level, despite the fact that parents were literate; this aimed to ensure the adequate quality of the inhalation technique, a crucial condition for the evaluation of both adherence and level of disease control. Regarding the guidelines for the inhalation route in each consultation, the following parameters were observed: spacer hygiene, agitation of the pressurized inhaler before use, and number of inspirations after each nebulization.
Adherence ratesThe adherence rate was expressed by calculating the percentage of the number of used doses divided by the number of prescribed doses ×100, considering the day of medication dispensation (the inhaler contains 120 doses) and the number of remaining doses verified on the date of the three control visits (two, four, and six months after admission). The service where the medication was dispensed was the same where the study was carried out. The expected adequate adherence was 100%.
Statistical analysisThe dependent variable was the asthma control level, i.e., adequate control or lack of control. The independent variables were time of follow-up, adherence rate, gender, maternal age and level of schooling, and mean monthly family income. When assessing the patients’ profile at the first consultation, absolute and relative frequencies were used for the categorical variables, whereas the means and standard deviations were used for the quantitative variables.
The chi-squared test was used to compare the group of patients who had controlled and uncontrolled asthma. Non-parametric tests were used to compare the rate of adherence among participants who had controlled asthma and those with uncontrolled asthma, stratified by consultation (Mann–Whitney test), and to compare the rate of adherence between consultations, stratified by controlled and uncontrolled asthma (Kruskal–Wallis test; the Nemenyi test was used for multiple comparisons).
Marginal logistic regression was used to verify whether the independent variables had an influence on asthma control and, for this purpose, the backward method was used, which is the procedure of consecutively removing the variables with greater p-value; this procedure is repeated until only significant variables remain in the model. A conventional significance level of 5% was used for the backward method.
The generalized estimating equations (GEEs) method was used to evaluate the individual correlation of adherence measurements with the asthma control level.16
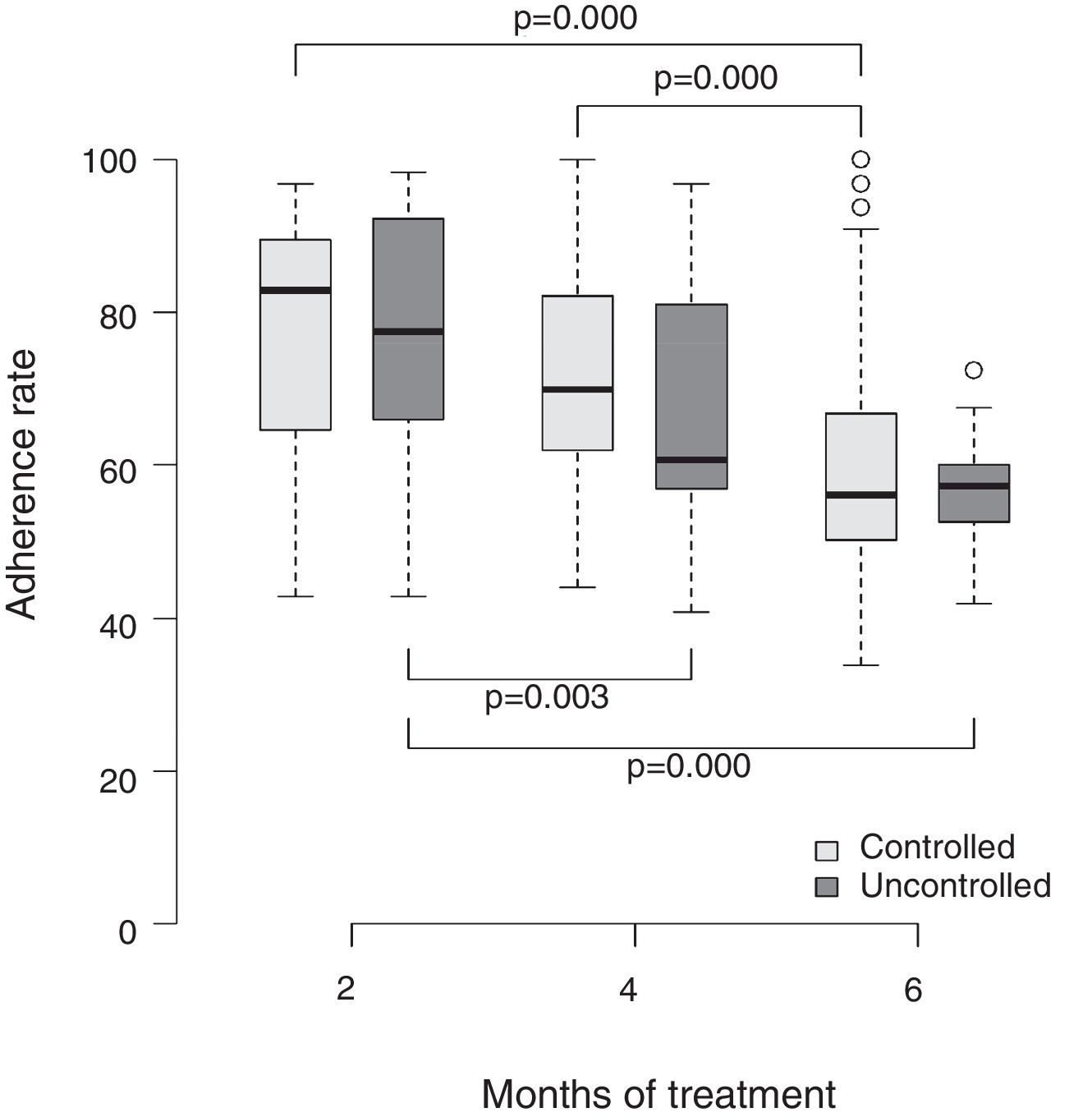
The mean rate of drug adherence was compared with the asthma control level and expressed in box-plot charts in the second, fourth, and sixth months. The means (and respective standard deviation), medians, minimum and maximum variations, and first and third quartiles of the adherence rates of the respective months were also calculated. The boxplot is adequate to represent the non-parametric tests, since it provides clinically important information, such as the medians and quartiles of each group/time. The software used in the analysis was R® version 3.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
Ethical aspectsThe study protocol, as well as the terms of consent and assent, were approved by the Research Ethics Committee of Faculdade de Ciências Médicas de Minas Gerais, under number 0117.0.418.000-11.
ResultsEighty-four patients were recruited for the study with the following admission characteristics: the mean age was 7.4 years, 54.8% of them were aged between 5 and 7 years, with a predominance of females (56.0%). The mean maternal age was 32.5 years, of whom 46.4% were older than 30 years, and most mothers (72.6%) reported at least eight years of elementary school education. The mean monthly family income was R$ 700.00 in 67.9% of the families.
As expected, 47 of the 84 patients (56.0%) underwent spirometry, of whom 38 were children older than 6 years and nine were aged 5 years. The mean initial and final forced expiratory flow in the first second (FEV1) were 76.3% and 80.3% of the predicted values, respectively.
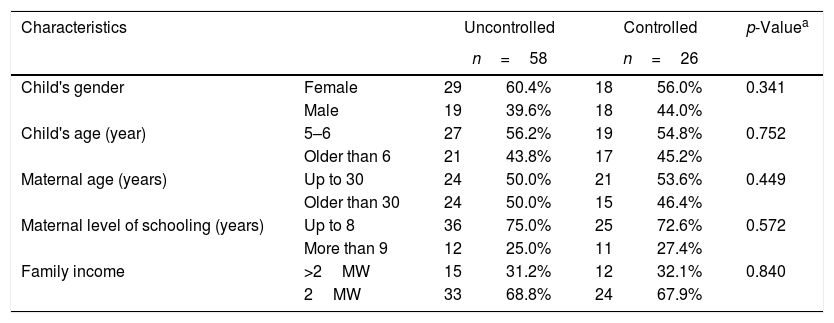
Table 1 shows the results of the evaluation of the second month of treatment; no statistical difference was observed between the two groups.
Characteristics of the patients in each group at the first consultation (2 months of treatment).
| Characteristics | Uncontrolled | Controlled | p-Valuea | |||
|---|---|---|---|---|---|---|
| n=58 | n=26 | |||||
| Child's gender | Female | 29 | 60.4% | 18 | 56.0% | 0.341 |
| Male | 19 | 39.6% | 18 | 44.0% | ||
| Child's age (year) | 5–6 | 27 | 56.2% | 19 | 54.8% | 0.752 |
| Older than 6 | 21 | 43.8% | 17 | 45.2% | ||
| Maternal age (years) | Up to 30 | 24 | 50.0% | 21 | 53.6% | 0.449 |
| Older than 30 | 24 | 50.0% | 15 | 46.4% | ||
| Maternal level of schooling (years) | Up to 8 | 36 | 75.0% | 25 | 72.6% | 0.572 |
| More than 9 | 12 | 25.0% | 11 | 27.4% | ||
| Family income | >2MW | 15 | 31.2% | 12 | 32.1% | 0.840 |
| 2MW | 33 | 68.8% | 24 | 67.9% | ||
MWs, Brazilian minimum wages.
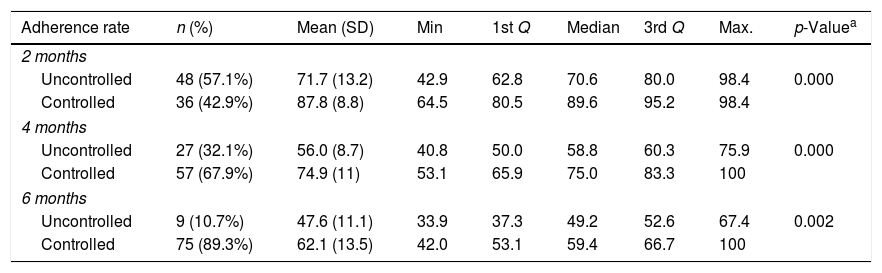
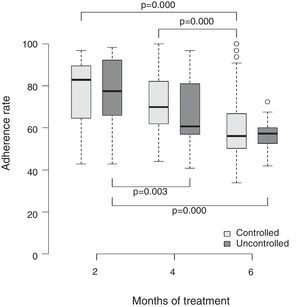
Table 2 shows the distribution of the asthma control level according to the adherence rate verified in the three bimonthly consultations. It was observed that in the group where asthma was not controlled, the mean adherence rates in the second, fourth, and sixth months were 71.7%, 56.0%, and 47.6%, whereas the group with controlled asthma presented significantly higher rates, 87.8%, 74.9%, and 62.1%, respectively, and this difference was statistically significant (p-value=0.002).
Comparison of adherence rates between groups stratified by time of treatment.
| Adherence rate | n (%) | Mean (SD) | Min | 1st Q | Median | 3rd Q | Max. | p-Valuea |
|---|---|---|---|---|---|---|---|---|
| 2 months | ||||||||
| Uncontrolled | 48 (57.1%) | 71.7 (13.2) | 42.9 | 62.8 | 70.6 | 80.0 | 98.4 | 0.000 |
| Controlled | 36 (42.9%) | 87.8 (8.8) | 64.5 | 80.5 | 89.6 | 95.2 | 98.4 | |
| 4 months | ||||||||
| Uncontrolled | 27 (32.1%) | 56.0 (8.7) | 40.8 | 50.0 | 58.8 | 60.3 | 75.9 | 0.000 |
| Controlled | 57 (67.9%) | 74.9 (11) | 53.1 | 65.9 | 75.0 | 83.3 | 100 | |
| 6 months | ||||||||
| Uncontrolled | 9 (10.7%) | 47.6 (11.1) | 33.9 | 37.3 | 49.2 | 52.6 | 67.4 | 0.002 |
| Controlled | 75 (89.3%) | 62.1 (13.5) | 42.0 | 53.1 | 59.4 | 66.7 | 100 | |
SD, standard deviation; Q, quartile; Min., minimum; Max., maximum.
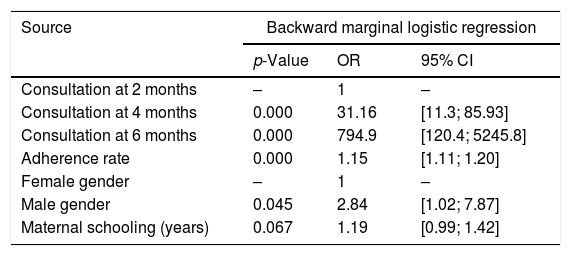
Table 3 shows the results of the multivariate analysis, demonstrating the influence of time of treatment, participant's gender and age, maternal age and level of schooling, and monthly family income on the level of asthma control.
Factors associated with the level of asthma control.
| Source | Backward marginal logistic regression | ||
|---|---|---|---|
| p-Value | OR | 95% CI | |
| Consultation at 2 months | – | 1 | – |
| Consultation at 4 months | 0.000 | 31.16 | [11.3; 85.93] |
| Consultation at 6 months | 0.000 | 794.9 | [120.4; 5245.8] |
| Adherence rate | 0.000 | 1.15 | [1.11; 1.20] |
| Female gender | – | 1 | – |
| Male gender | 0.045 | 2.84 | [1.02; 7.87] |
| Maternal schooling (years) | 0.067 | 1.19 | [0.99; 1.42] |
OR, odds ratio; 95% CI, 95% confidence interval.
Adherence rate, gender, and maternal level of schooling were significantly associated with controlled asthma. Boys were 2.84 times (95% CI: 1.02–7.87) more likely to attain asthma control than girls, and each additional year of maternal schooling increased the likelihood of achieving asthma control by 1.19 times (95% CI: 0.99–1.42).
Fig. 1 shows the comparison of the adherence rate in the second, fourth, and sixth months of treatment and the level of asthma control. Once again, higher adherence rates were associated with a better level of disease control. A consistent dose–response gradient was observed for adherence rate and asthma control level at the three moments of the clinical–functional evaluation.
DiscussionIn this observational study carried out exclusively in the pediatric population, it was observed that adherence greater than 60% led to better control of moderate persistent asthma. This is the main clinical implication of the present results.
Studies on asthma treatment adherence in developing countries through reliable methods such as dose counters are rare, and those evaluating the rate of adherence to combination therapy (i.e., inhaled corticosteroid and long-acting beta-agonist) and the level of asthma control are even rarer. This is the second message of the present study, which aimed to contribute to better management of asthma in children.
The factors that influenced asthma control were the decrease in the adherence rate during follow-up, male gender, and maternal level of schooling. Studies on the influence of gender are controversial, but the decrease in adherence rates during the course of treatment is well known in all chronic diseases, such as asthma.16
The influence of maternal schooling observed in the present study is corroborated by a study carried out by Urrutia-Pereira et al., in an asthma prevention program in Rio Grande do Sul, where 35.6% of the participating mothers had an educational level of less than eight years of schooling.17
Bender reports that there has been little change in the IC adherence rate in the last 25 years; in ten studies included in his review, the lack of asthma control was associated with adherence rates ranging from 19% to 58%. It was concluded that better understanding of the management by the caregivers and use of the correct inhalation technique were determinant for better disease control.9
One limitation of the present study is that the dose counter coupled to the inhaler does not exclude deliberate emptying of medication.18 However, Given et al. demonstrated the reliability of this method.4 The adherence rate measurement should be performed by reliable methods, such as dose counters, to differentiate between patients adhering to the therapeutic regimen and those who do not, both in clinical practice and in research.19–21
In clinical practice, if an optimal level of asthma control is not achieved, the treatment adherence rate, among other factors, should be verified before prescribing a higher dose of IC, combined or not with another drug.
In meta-analysis of ten studies that aimed to measure adherence to IC treatment through electronic monitors in pediatric patients,22 individuals who experienced exacerbations had a mean rate of isolated IC adherence of 14%, whereas in those with controlled asthma, the mean adherence rate was 68%, which is very close to that found in the present study, i.e., sufficient for asthma control.
Pedersen et al. followed-up 19 children aged 6–15 years with moderate and severe asthma who used budesonide for four weeks. The use of half (i.e., 100mcg/day of budesonide) of the prescribed dose – assessed by weighing the inhalers – was enough to control the symptoms. The very short period of study follow-up is noteworthy.23
Klok et al., in a review of 14 studies on asthma treatment adherence using an electronic monitor in children, regardless of disease severity, found a time of follow-up from 13 weeks to 18 months and variation in adherence to IC treatment of 34–92%; in the research that lasted six months (similar to the present study) an adherence rate of 50% was observed.24
The final model of the multivariate analysis showed that male gender, maternal schooling, and duration of follow-up were associated with adequate asthma control.
The present study has further clinical implications in addition to those previously mentioned. The results suggest that both the rate of adherence to inhaled medication and the level of asthma control should be systematically verified in any clinical evaluation of the asthmatic patient, whether or not those evaluations were programmed. Moreover, they indicate that, to attain clinical and functional control of moderate persistent asthma in children and adolescents who are using the therapeutic regimen adopted in the present study, the rate of adherence could, in fact, be lower than the ideal of 100%, since a minimum of 60% adherence to the therapeutic regimen adopted was sufficient to achieve asthma control. These adherence rates were lower than those reported in the literature.1,24
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Jentzsch NS, Silva GC, Mendes GM, Brand PL, Camargos P. Treatment adherence and level of control in moderate persistent asthma in children and adolescents treated with fluticasone and salmeterol. J Pediatr (Rio J). 2019;95:69–75.
The medications and spacers were provided free of charge through the Brazilian Unified Health System (SUS) of Belo Horizonte City Hall.