Several studies have been performed concerning pathologies of the stomach and esophagus in the pediatric age group. However, there have been very few studies of duodenal pathologies in children. The authors aimed to examine the clinical, endoscopic, and histopathological characteristics, as well as the etiology of duodenal pathologies in children.
MethodPatients aged between 1 and 17 years undergoing esophagogastroduodenoscopy during two years at this unit, were investigated retrospectively. Demographic, clinical, endoscopic data, and the presence of duodenal pathologies, gastritis, and esophagitis were recorded in all of the children.
ResultsOut of 747 children who underwent endoscopy, duodenal pathology was observed in 226 (30.3%) patients. Pathology was also present in the esophagus in 31.6% of patients and in the stomach in 58.4%. The level of chronic diarrhea was higher in patients with duodenal pathology when compared with those without duodenal pathology (p=0.002, OR: 3.91, 95% CI: 1.59–9.57). Helicobacter pylori infection was more common in patients with pathology in the duodenum (59.3%).
ConclusionDuodenal pathology was detected in 30.3% of the present patients. A significantly higher level of chronic diarrhea was observed in subjects with duodenal pathologies compared to those with no such pathology. The rate of Helicobacter pylori infection was considerably higher than that in previous studies. In addition, there is a weak correlation between endoscopic appearance and histology of duodenitis.
Foram realizados vários estudos com relação a patologias do estômago e esôfago na faixa etária pediátrica. Contudo, poucos estudos das patologias duodenais em crianças. Visamos examinar as características clínicas, endoscópicas e histopatológicas juntamente com a etiologia das patologias duodenais em crianças.
MétodoForam investigados retrospectivamente pacientes com idades entre 1 e 17 anos submetidos a esofagogastroduodenoscopia durante dois anos em nossa unidade. Os dados demográficos, clínicos e endoscópicos e a presença de patologias duodenais, gastrite e esofagite foram registrados com relação a todas as crianças.
ResultadosDas 747 crianças submetidas a endoscopia, 226 (30,3%) pacientes apresentaram patologia duodenal. A patologia também esteve presente no esôfago de 31,6% dos pacientes e no estômago de 58,4% deles. O nível de diarreia crônica foi maior nos pacientes com patologia duodenal, em comparação aos pacientes sem patologia duodenal (p=0,002, RC: 3,91, IC de 95%: 1,59-9,57). Infecção por Helicobacter pylori foi mais comum em pacientes com patologia no duodeno (59,3%).
ConclusãoFoi detectada patologia duodenal em 30,3% de nossos pacientes. Um nível significativamente maior de diarreia crônica foi observado em indivíduos com patologias duodenais, em comparação aos sem nenhuma patologia. A infecção por Helicobacter pylori esteve presente consideravelmente maior que em estudos anteriores. Além disso, há uma fraca correlação entre a imagem endoscópica e a histologia de duodenite.
Esophagogastroduodenoscopy (EGD) is a reliable technique widely used in the diagnosis of diseases of the esophagus, stomach, and proximal duodenum. The advantages of this method are that the internal surface of the organ undergoing endoscopy can be observed directly, pathological sampling from lesions can be performed, and treatment can be administered when necessary. Since its introduction in pediatric patients in 1970, the use of endoscopic procedures has increased dramatically.1 Franciosi et al.2 reported that the number of upper endoscopic procedures increased by 12-fold between 1985 and 2005. As a consequence of this trend, new disorders of the GI tract and new pathologies were described in children.
In parallel to the increased use of EGD among children, numerous studies evaluating the pathologies of the stomach and esophagus in children have been performed.3,4 However, there have been very few studies investigating the association between duodenal endoscopic findings and histologic diagnosis in the pediatric population. Alper et al.5 reported that the prevalence of duodenitis in children undergoing endoscopy was 12.7%, and the correlation between endoscopic appearance and histology was considered to be poor. In another study, Alabd Alrazzak et al.6 observed an 11% rate of duodenitis prevalence, while in a study of 1000 children undergoing EGD, Sheiko et al.7 observed endoscopic disorder in the duodenum at a rate of 9.9% and histopathologic disorder at a rate of 10.7%.
The aim of the study was to assess the endoscopic and histopathologic features of duodenal disorders with respect to age groups in children undergoing diagnostic EGD for the first time.
MethodsThe authors retrospectively reviewed all upper endoscopic charts of children between the ages of 1 and 17 years who underwent diagnostic EGD at Kanuni Training and Research Hospital Pediatric Gastroenterology Department, Turkey, between September 2014 and September 2016. Clinical data, demographic characteristics, and histopathology findings were also reviewed. Patients with known gastrointestinal disease (such as celiac disease, Crohn's disease, and ulcerative colitis), with neurodevelopmental delay (such as cerebral palsy), and those undergoing endoscopy for therapeutic purposes were excluded. In children undergoing endoscopy more than once, the data of the first EGD was recorded. This study was performed in agreement with the Declaration of Helsinki and following the approval of the Local Ethics Committee. Symptoms were grouped into growth retardation, chronic anemia, chronic diarrhea, chronic abdominal pain, dyspepsia, and reflux symptoms (vomiting, chest pain, regurgitation, and eructation).8 Chronic abdominal pain was defined as three or more bouts in at least a three-month period.9 Chronic diarrhea was defined as that lasting more than 14 days.10 The patients were stratified into four groups with respect to the growth stages; infants (0–2 years), preschoolers (3–5 years), school-aged children (6–11 years), and adolescents (>12 years).
All procedures were performed by the same experienced gastroenterologist (U.E.A.) on an video gastroscopy device (Olympus, GIF-H180, PA, USA). Mucosal breaks of >5mm observed in endoscopy were defined as ulcers.11 In addition, gross endoscopic findings such as scalloping, nodularity, loss of mucosal folds, or mosaic pattern in the duodenum were regarded as pathological findings.12–14 Non-specific findings such as hyperemia, increased or decreased vascularity, and pallor were not regarded as pathological.15
At least two biopsies were taken from all patients from the esophagus, stomach (antrum), and the second part of the duodenum. All the initial examinations were performed by experienced gastrointestinal pathologists blinded to the patients’ clinical status and endoscopic findings. For cases with a diagnosis of duodenum pathology, all microscopic sections were retrieved and reviewed by two experienced pathologists, and the diagnosis of duodenum pathology was confirmed in all patients. Increased intraepithelial lymphocytes in the duodenum (>30 lymphocytes) and chronic changes involving neutrophilic infiltration or villous/crypt structural changes were regarded as duodenitis.16 Patients at >stage 2 according to the modified Marsh criteria and with positive serology were diagnosed with celiac disease.17 The diagnosis of eosinophilic gastroenteritis (EG) was established upon observation of ≥25 eosinophils in one high-power field in the duodenum following the exclusion of parasitic infections, Helicobacter pylori infection, celiac disease, inflammatory bowel disease, hypereosinophilic syndrome, malignity, and connective tissue diseases (such as Churg–Strauss syndrome).18 Endoscopic and/or histopathological abnormalities in the duodenum were considered as duodenal pathology. The antral biopsy specimens were fixed in 10% formalin and stained with hematoxylin and eosin (H&E). Giemsa staining was used to confirm the presence of H. pylori. Microscopic detection of gastric non-Helicobacter pylori helicobacter (NHPH), either in histologic sections or in smears, is the most widely used diagnostic method and usually allows differentiation between H. pylori and NHPH infections.19 For example, Helicobacter felis is characterized by the presence of periplasmic fibrils. Helicobacter salomonis is a less tightly coiled bacteria usually with a one periplasmic fibril, whereas long spiral shape is the morphological distinction of Helicobacter heilmannii.19 Findings such as papillary elongation in the esophagus, basal cell hyperplasia, and an increase in intraepithelial neutrophils were evaluated in favor of reflux esophagitis.20 Eosinophilia in the esophagus was defined as ≥30 eosinophils in one high-power field and/or observation of eosinophilic microabscesses.4 In these cases, EGD was repeated subsequent to the administration of high-dose PPI (lansoprazole 1mg/kg per dose, twice daily) for eight weeks. Persisting eosinophilia in the esophagus was regarded as eosinophilic esophagitis.4 Reflux esophagitis and eosinophilic esophagitis were evaluated under the group of esophagitis. Gastritis is diagnosed by the presence of mixed inflammatory infiltrate.5
Data were analyzed using SPSS software (SPSS for Windows, version 13.0, Chicago, USA). Descriptive data were expressed as mean±standard deviation (SD), with the Student's t-test for independent paired samples was used to compare normally distributed variables between two groups, and the Mann–Whitney U test, for non-normally distributed variables. The chi-squared test was used to compare categoric variables. Statistical significance was set at p<0.05.
ResultsDuring the two-year study period, 870 EGDs were performed in total. A total of 123 EGDs were excluded, including repeated EGD procedures (n=67), EGD of patients with previously known gastrointestinal disease (n=18), retarded neuromotor development (n=14), or undergoing procedures for therapeutic purposes (n=24). Finally, 747 patients were enrolled in the study. The patients were aged between 1 and 17 years and the majority was female (n=458, 61.3%). One patient (0.1%) was an infant, 41 (5.5%) were preschoolers, 242 (32.3%) were school-aged children, and 463 patients (62.1%) were adolescents. Duodenal pathology was observed in 226 (30.3%) patients. Pathology was also present in the esophagus in 31.6% of patients and in the stomach in 58.4%. Of the 236 esophageal pathologies, 232 were reflux esophagitis and four were eosinophilic esophagitis.
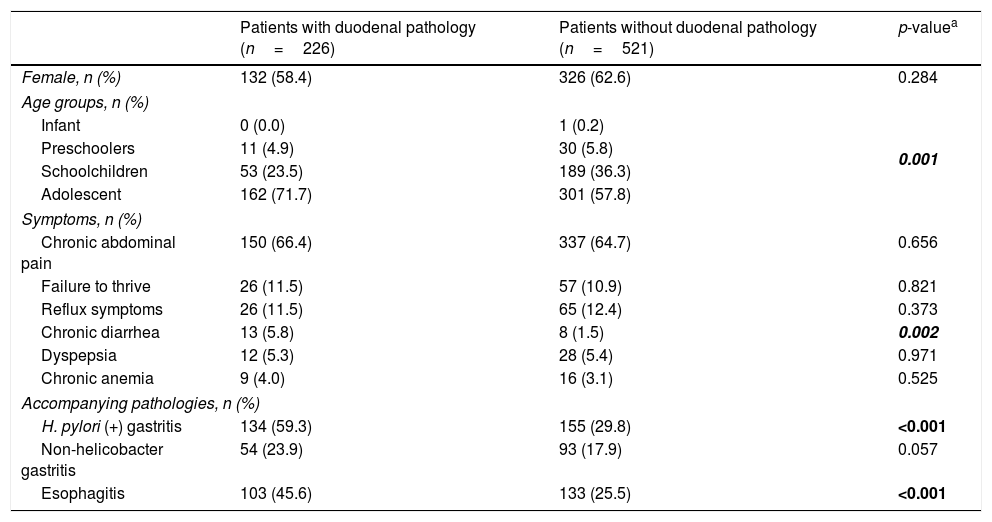
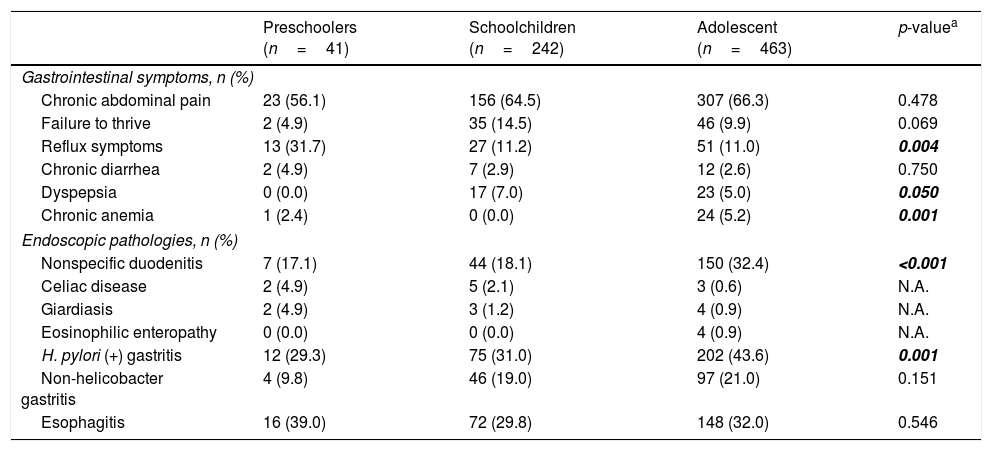
The mean age of the patients with pathology in the duodenum was 12.9 years (range 4–17 years) and the majority was female (58.4%). The demographic characteristics of patients with gastrointestinal symptoms and accompanying pathologies are shown in Table 1. The level of chronic diarrhea was higher in patients with duodenal pathology compared to those without duodenal pathology (p=0.002, OR: 3.91, 95% CI: 1.59–9.57). It was observed that reflux symptoms were more common in preschoolers; dyspeptic complaints, in school-aged children; and chronic anemia, in adolescents (p<0.05; Table 2). H. pylori gastritis and esophagitis were more common in patients with pathology in the duodenum compared to those without pathology (p<0.001, OR: 3.43, 95% CI: 2.48–4.76 vs. p<0.001, OR: 0.40, 95% CI: 0.29–0.56; Table 1).
Demographic characteristics of patients with gastrointestinal symptoms and accompanying pathologies.
| Patients with duodenal pathology (n=226) | Patients without duodenal pathology (n=521) | p-valuea | |
|---|---|---|---|
| Female, n (%) | 132 (58.4) | 326 (62.6) | 0.284 |
| Age groups, n (%) | |||
| Infant | 0 (0.0) | 1 (0.2) | 0.001 |
| Preschoolers | 11 (4.9) | 30 (5.8) | |
| Schoolchildren | 53 (23.5) | 189 (36.3) | |
| Adolescent | 162 (71.7) | 301 (57.8) | |
| Symptoms, n (%) | |||
| Chronic abdominal pain | 150 (66.4) | 337 (64.7) | 0.656 |
| Failure to thrive | 26 (11.5) | 57 (10.9) | 0.821 |
| Reflux symptoms | 26 (11.5) | 65 (12.4) | 0.373 |
| Chronic diarrhea | 13 (5.8) | 8 (1.5) | 0.002 |
| Dyspepsia | 12 (5.3) | 28 (5.4) | 0.971 |
| Chronic anemia | 9 (4.0) | 16 (3.1) | 0.525 |
| Accompanying pathologies, n (%) | |||
| H. pylori (+) gastritis | 134 (59.3) | 155 (29.8) | <0.001 |
| Non-helicobacter gastritis | 54 (23.9) | 93 (17.9) | 0.057 |
| Esophagitis | 103 (45.6) | 133 (25.5) | <0.001 |
Comparison of gastrointestinal symptoms according to age groups.
| Preschoolers (n=41) | Schoolchildren (n=242) | Adolescent (n=463) | p-valuea | |
|---|---|---|---|---|
| Gastrointestinal symptoms, n (%) | ||||
| Chronic abdominal pain | 23 (56.1) | 156 (64.5) | 307 (66.3) | 0.478 |
| Failure to thrive | 2 (4.9) | 35 (14.5) | 46 (9.9) | 0.069 |
| Reflux symptoms | 13 (31.7) | 27 (11.2) | 51 (11.0) | 0.004 |
| Chronic diarrhea | 2 (4.9) | 7 (2.9) | 12 (2.6) | 0.750 |
| Dyspepsia | 0 (0.0) | 17 (7.0) | 23 (5.0) | 0.050 |
| Chronic anemia | 1 (2.4) | 0 (0.0) | 24 (5.2) | 0.001 |
| Endoscopic pathologies, n (%) | ||||
| Nonspecific duodenitis | 7 (17.1) | 44 (18.1) | 150 (32.4) | <0.001 |
| Celiac disease | 2 (4.9) | 5 (2.1) | 3 (0.6) | N.A. |
| Giardiasis | 2 (4.9) | 3 (1.2) | 4 (0.9) | N.A. |
| Eosinophilic enteropathy | 0 (0.0) | 0 (0.0) | 4 (0.9) | N.A. |
| H. pylori (+) gastritis | 12 (29.3) | 75 (31.0) | 202 (43.6) | 0.001 |
| Non-helicobacter gastritis | 4 (9.8) | 46 (19.0) | 97 (21.0) | 0.151 |
| Esophagitis | 16 (39.0) | 72 (29.8) | 148 (32.0) | 0.546 |
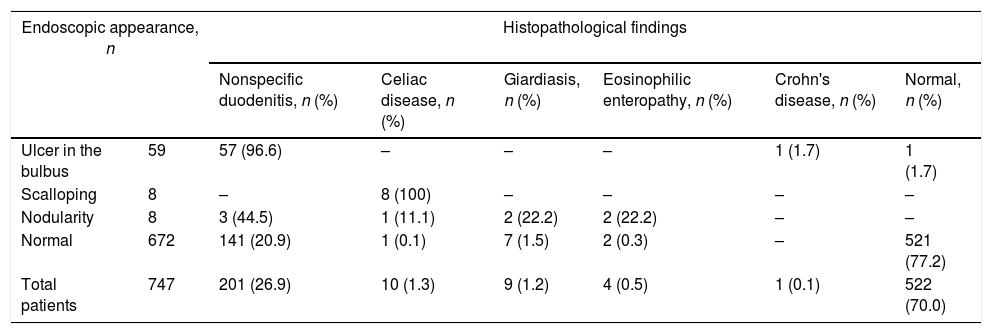
Endoscopy determined ulcer in the bulbus in 59 patients (7.8%), scalloping in eight (1.0%), and nodularity in eight (1.0%). The endoscopic and histopathological characteristics of the duodenum are shown in Table 3. Histopathological examination of the duodenum in patients with ulcer in the bulbus revealed nonspecific duodenitis in 96.6%. Normal histopathological findings were observed in one patient. Abdominal pain persisting for 6 months, weight loss and sedimentation elevation were present in a 16-year-old girl with ulcer in the bulbus, in whom granuloma was determined on histopathological examination; this patient was diagnosed with Crohn's disease. H. pylori gastritis was present in 51 (86.4%) of the patients with ulcer in the bulbus. Anti-tissue transglutaminase IgA positivity was present in all patients with scalloping in the duodenum, and these were diagnosed with celiac disease following histopathological examination. Among the patients with nodularity in the duodenum, eosinophilic enteropathy was diagnosed in two (22.2%), giardiasis in two (22.2%), and celiac disease in one (11.1%). Duodenal disorders were more common in the adolescent group when compared with the other groups (Table 2).
Endoscopic and histopathological characteristics of the duodenum.
| Endoscopic appearance, n | Histopathological findings | ||||||
|---|---|---|---|---|---|---|---|
| Nonspecific duodenitis, n (%) | Celiac disease, n (%) | Giardiasis, n (%) | Eosinophilic enteropathy, n (%) | Crohn's disease, n (%) | Normal, n (%) | ||
| Ulcer in the bulbus | 59 | 57 (96.6) | – | – | – | 1 (1.7) | 1 (1.7) |
| Scalloping | 8 | – | 8 (100) | – | – | – | – |
| Nodularity | 8 | 3 (44.5) | 1 (11.1) | 2 (22.2) | 2 (22.2) | – | – |
| Normal | 672 | 141 (20.9) | 1 (0.1) | 7 (1.5) | 2 (0.3) | – | 521 (77.2) |
| Total patients | 747 | 201 (26.9) | 10 (1.3) | 9 (1.2) | 4 (0.5) | 1 (0.1) | 522 (70.0) |
Pathology was identified with histopathological examination in 151 (22.4%) of the 672 patients in whom pathology in the duodenum was not determined with endoscopy. Nonspecific duodenitis was present in 141 patients (93.3%), giardiasis in seven (4.6%), eosinophilic enteritis in two (1.4%), and celiac disease in one (0.7%). Abnormality was identified histopathologically in 74 (98.6%) of the 75 patients in whom pathology was determined at endoscopy (Table 3).
DiscussionDiagnostic sensitivity for the diagnosis of GIS disorders has increased remarkably with the advances in imaging technology and the increase in the use of EGD. Previous studies have reported an incidence of duodenitis in children undergoing endoscopy of 10.7–12.7%.5,6 Alabd Alrazzak et al.6 observed a prevalence of duodenitis of 11.0%, while in a study of 1000 children undergoing EGD, Sheiko et al.7 diagnosed duodenal pathology in 9.9% of their sample when using endoscopy and in 10.7% when using histopathological methods. Recently, Alper et al.5 investigated 2772 children undergoing EGD and diagnosed duodenitis in 12.7%. The incidence of overall duodenal disorders in the present cohort was 30.3%.
In agreement with Alper et al.,5 the most common indication for EGD in this study was Chronic abdominal pain (CAP). Those authors reported a higher level of CAP in subjects with duodenal pathology than in those without. In the current study, however, no difference was observed between subjects with or without duodenal pathology in terms of the prevalence of CAP. A significantly higher level of chronic diarrhea was observed in subjects with duodenal pathologies compared to those with no such pathology. The American Society for Gastrointestinal Endoscopy recommends EGD in cases of chronic diarrhea, particularly if absorption disorder is suspected.21
H. pylori infection was present in 59.3% of the current study patients with duodenal pathology. This level is considerably higher than that observed in previous studies. For example, while Alper et al.5 made specific diagnosis in 64% of patients with duodenitis (the majority consisting of celiac disease and inflammatory bowel disease, while H. pylori was observed at the low level of 6%), 36% of patients were diagnosed with nonspecific duodenitis. However, although the prevalence of H. pylori infection ranges from 6.5% to 31.0% in Europe, the reported prevalence is 53.0% to 66.3% in Turkey.22,23 In a study of 751 children undergoing EGD in Israel, a country with a high prevalence of H. pylori infection alike Turkey, Egbaria et al.11 identified duodenal ulcer in 26 (3.5%) cases and H. pylori in 14 (∼54%) of these. A prospective study of 303 children in Saudi Arabia indicated the presence of H. pylori in 14 (56%) of 25 children with duodenal ulcer.24 These results show that H. pylori infection bears a high level of responsibility for duodenal pathologies in communities in which this agent is widespread.
Several studies have shown that the prevalence of H. pylori infection increases with age.25,26 It has been observed at younger ages in developing countries, whereas the infection is more common in older ages in developed countries.24 In a recent pediatric study from Turkey, Ozbey et al. reported that the prevalence of H. pylori infection among children reaches its peak between the ages of 13 and 18 years.27 Similar to that study, in the present study H. pylori infection was more common in the adolescent age group. Parallel to the increase in the incidence of H. pylori infection with age, duodenal pathologies were also observed at a higher rate in the adolescent age group.
In a multicenter study conducted in Europe, the incidence of duodenal ulcer in children undergoing EGD was reported as 2.4%.28 In Israel, this rate was 3.5%.11 In the present study, the incidence of ulcer in duodenum was 7.8%. The difference in the duodenal ulcer incidence between the studies may be due to the differences in the dietary habits and socio-cultural levels of the communities and use of gastro-protective drugs before endoscopy.
A weak correlation has been observed between endoscopic findings and histopathological results in previous studies.5,29,30 In a study of 75 children, Long et al.29 reported that endoscopic appearance was 54% sensitive in estimating duodenitis. In a study by Alper et al.,5 in which a biopsy was performed in all patients, as in the present research, sensitivity was determined at 37%. Studies advocating the performance of routine duodenal biopsies in pediatric populations are lacking, and the practice among pediatric gastroenterologists is diverse. Kori et al.30 observed histopathological abnormalities in 35 (17.4%) of 201 patients with a normal endoscopic duodenal appearance. Several studies reported that the diagnosis of celiac disease was made and even granulomas, cryptitis and villus structure disorders were detected by histopathological evaluation of duodenal biopsies of some patients with normal duodenum appearance at endoscopy.5,30 Despite the high consensus of pathologists to identify histologic anomalies, there is considerable variability in the identification and classification of endoscopic abnormalities among endoscopists.7 In the current study, histopathological abnormality was also observed in a significant proportion (22.4%) of patients with a normal endoscopic duodenal appearance. The present findings suggest that a routine biopsy should be performed during EGD regardless of endoscopic appearance and endoscopy indications in children. This may minimize the overlooking of certain GIS disorders that may be clinically significant and can be remedied by appropriate treatment. Moreover, by performing a routine biopsy, the risks related to unjustifiable treatment or those associated with sedation or intervention during a repeat endoscopy procedure could be eliminated; the cost increase would also be avoided.
The present study has some limitations. It was retrospective in design. The number of patients in the infant group was low, therefore the duodenal disorders in that age group could not be evaluated. In addition, it was carried out in a single center; therefore, it may not fully reflect the duodenal disorders in different populations.
In conclusion, a higher level of duodenal pathologies was observed in this study involving both endoscopic and histopathological examination of duodenal pathologies compared to previous studies, and a high level of H. pylori infection was determined to accompany such pathologies. This variation among studies may be due to the fact that they were performed in different societies and patient populations. Further studies involving different communities and larger patient numbers are therefore needed to determine the prevalence, etiology, and clinical characteristics of duodenal pathologies. Another finding of this study was that histopathological abnormalities were observed at a considerable rate even in patients with normal endoscopic appearance. It can be suggested that the inclusion of routine duodenal biopsies as part of upper endoscopy in pediatric patients to be considered favorably. This practice may yield additional pathologic findings that might otherwise have been missed and may have clinical significance. It should be performed regardless of the indication for endoscopy or the general appearance of the mucosa.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Akbulut UE, Fidan S, Emeksiz HC, Ors OP. Duodenal pathologies in children: a single-center experience. J Pediatr (Rio J). 2018;94:273–8.