To compare serum concentrations of specific IgE and mean papule diameters induced in the immediate skin reactivity test with cow's milk and its fractions with results of the oral challenge test, and to establish cutoff points capable of predicting clinical reactivity to cow's milk in patients treated at a referral service.
MethodsOne hundred and twenty-two children (median of 17 months) with a history of immediate reactions to cow's milk and presence of specific IgE for cow's milk and/or its fractions (positive skin and/or IgE serum tests) were submitted to open oral challenge test with cow's milk.
ResultsThe oral challenge test was positive in 59.8% of the children, 49% of whom were males. Serum levels of specific IgE, as well as mean cow's milk papule diameters, were significantly higher in allergic patients (medians: 3.39kUA/L vs. 1.16kUA/L, 2.5mm vs. 0mm). The optimal cutoff points (Youden's index) of serum IgE specific for cow's milk and its fractions capable of predicting cow's milk reactivity (positive oral challenge test) were: 5.17kUA/L for cow's milk, 0.95kUA/L for α-lactalbumin, 0.82kUA/L for β-lactoglobulin, and 0.72kUA/L for casein, whereas for papule diameters the cutoff points were 3.5mm for cow's milk and 6.5mm, 9.0mm, and 3.0mm for the α-lactalbumin, β-lactoglobulin, and casein fractions, respectively.
ConclusionsThe cutoff points capable of predicting clinical reactivity to cow's milk were: 5.17kUA/L for serum-specific IgE and 3.5mm for papule diameter measurement, values considered discriminatory for the diagnosis of cow's milk allergy.
Comparar concentrações séricas de IgE específica e diâmetros médios das pápulas induzidas no teste cutâneo de leitura imediata com leite de vaca e suas frações com resultados do teste de provocação oral e estabelecer pontos de corte, capazes de predizer reatividade clínica ao leite de vaca em pacientes atendidos em um serviço de referência.
MétodosCento e vinte e duas crianças (mediana 17 meses), com história de reações imediatas ao leite de vaca e presença de IgE espeçíficas para leite de vaca e/ou frações (testes cutâneos e/ou IgE sérica positivos) foram submetidas ao teste de provocac¸ão oral aberto com leite de vaca.
ResultadosO teste de provocac¸ão oral foi positivo em 59,8% das crianças, 49% eram do sexo masculino. Os níveis séricos de IgE específica, assim como os diâmetros médios das pápulas para leite de vaca, foram significantemente maiores nos alérgicos (medianas: 3,39kUA/L vs 1,16 kUA/L; 2,5mm vs 0mm). Os “pontos de corte ótimos” (Índice de Youden) das IgE séricas específicas para o leite de vaca e suas frações capazes de predizer a reatividade ao leite de vaca (teste de provocac¸ão oral positivo) foram: 5,17kUA/L para o leite de vaca, 0,95 kUA/L para α-lactoalbumina, 0,82kUA/L para β-lactoglobulina e 0,72kUA/L para caseína e para os diâmetros de pápulas foram 3,5mm para leite de vaca e 6,5mm, 9,0mm e 3,0mm para as frações α-lactoalbumina, β-lactoglobulina e caseína, respectivamente.
ConclusõesOs níveis de corte capazes de predizer reatividade clínica ao leite de vaca foram: 5,17kUA/L para IgE sérica específica e 3,5mm para a medida do diâmetro da pápula, valores considerados discriminatórios para o diagnóstico da alergia ao leite de vaca.
Cow's milk protein allergy (CMPA) is defined as a reproducible, immunological adverse reaction to one or more cow's milk (CM) proteins.1 It involves the participation of IgE immunoglobulins (IgE), cells (T-lymphocytes), or both.2 It affects 2–3% of children, representing the most common food allergy in childhood.2
For the diagnosis of IgE-mediated CMPA, detailed clinical history and detection of IgE specific for allergens are important tools.3 However, the double-blind, placebo-controlled oral challenge test (DBPCOCT), which consists in offering the food without the physician's or the patient's knowledge, continues to be considered the gold standard for the diagnosis of CMPA.4–7 Nonetheless, it has disadvantages related to the time required for its performance, the costs involved, and the need for an adequate physical structure and a multidisciplinary team, which are limiting factors for its broad use in clinical practice.7
Different values of serum-specific IgE and mean papule diameter in the immediate-reading skin test (IRST), from which the chance of having symptoms at the time of the OCT would be greater than 95% were established; however, the values found were not reproducible in different populations, and differences regarding ethnic-racial characteristics, age, dietary habits, clinical phenotypes, and technical conditions for IRST were presumed.8–15
The desire to minimize OCTs – with their inherent risks – together with the unavailability of an adequate universal cutoff point for all populations, motivated this study, whose objectives are: to compare serum concentrations of specific IgE and mean papule diameter induced by the IRST with CM and its fractions with OCT results, and to establish cutoff points capable of predicting clinical reactivity to CM in patients treated at a referral service.
MethodsThis was a prospective, observational, diagnostic study that included 122 children, aged 5 months to 3 years (median 17 months), with clinical suspicion of IgE-mediated CMPA and evidence of positive IgE specific for CM and/or its fractions, in the first consultation (inclusion criteria).
After approval by the ethics committee, children consecutively admitted to the Food Allergy Service of Universidade Federal de Sergipe (UFS) between November 2008 and May 2014, and who met the inclusion criteria, were selected. Parents and/or guardians answered a standardized questionnaire regarding symptom history, birth conditions, breastfeeding time, time of the first introduction of CM, triggering volume, time interval between ingestion and symptom onset, in addition to the time of the last reaction and medication used.16
Any of the following symptoms described for the skin (hyperemia, urticaria, contact urticaria, and angioedema), respiratory tract (rhinorrhea, sneezing, nasal obstruction, laryngeal stridor, hoarseness, coughing, dyspnea, and cyanosis), gastrointestinal tract (perioral hyperemia, tongue and/or uvular edema, vomiting, and diarrhea), and cardiovascular system (tachycardia, arrhythmias, hypotonia, prostration, and syncope), clearly observed and triggered up to two hours after food contact were considered suspected objective IgE-mediated CMPA symptoms.2,3,6,17 As positive evidence for detection of specific IgE for CM and fractions, IRST results and/or IgE levels brought by the parents/guardians were accepted at the first consultation.18
After being selected, nursing mothers and children were instructed to restrict CM protein and derivatives in their diet for a period of two to four weeks, to not use antihistamines for one week before the OCT, and to have children to fast for two hours before the procedure.7,19,20
Printed leaflets containing instructions on CM elimination diet and careful reading of labels were distributed and a questionnaire on compliance with these requirements was applied prior to the OCT. Guidelines on the risks and benefits of the OCT and the implications of a positive or negative test result were also given to the parents, who, after being instructed, signed the informed consent.21
A recent history (less than two years) of anaphylaxis associated with the presence of specific IgE at admission, symptom persistence after the CM exclusion period, low adherence to OCT preparation guidelines, and the presence of an inflammatory and/or infectious process on the day of the procedure were considered the exclusion criteria.5,7
On the day of the OCT, after verifying compliance with the recommendations, the children underwent a careful physical examination, followed by the following tests: peripheral blood collection to determine serum concentrations of total IgE and IgE specific for CM and its fractions, and IRST.
A peripheral blood sample was collected and centrifuged; the serum was stored and frozen at −20°C until the analysis. The levels of total and specific IgE to total CM, α-lactalbumin, β-lactoglobulin, and casein were determined using the ImmunoCAP® method (Thermo Fisher Scientific, Brazil).22 Serum levels were determined at the Research Laboratory of the Discipline of Nutrology of the Department of Pediatrics of Universidade Federal de São Paulo. The results obtained were expressed in kUA/L and considered positive when greater than 0.35kUA/L.22
Regarding the IRST, the following extracts were used: total CM (10mg/mL), α-lactalbumin (5%), β-lactoglobulin (5%), and casein (5%) (IPI-ASAC®, Spain), in addition to controls: negative (diluent) and positive (histamine 1mg/mL). The appearance of a papule with a mean diameter greater than or equal to 3mm in relation to the negative control, 15min after the IRST by puncture, characterized the result as positive.22
Open OCT, a procedure in which only the physician is aware of what is being offered to the patient, was performed in a hospital environment, under the supervision of a multidisciplinary team prepared to treat any clinical reactions.7 Open OCT was chosen because, regarding the presence of objective symptoms in children under three years of age, it was possible to establish the diagnosis, even without performing the DBPCOCT.5
The children were given 100mL of CM (13% dilution) administered in increasing doses (1, 4, 10, 15, 20, 25, and 25mL) at 15- to 20-minute intervals, equivalent to 0.03, 0.14, 0.34, 0.51, 0.68, 0.85, and 0.85g of CM protein, respectively.7,8,19,20 In case of refusal, a palatable vehicle capable of masking the taste of CM was offered.19
When clinical reactions occurred, the test was considered positive and the child was promptly observed, remaining under observation until the manifestations disappeared.7 The reactions were classified according to severity as mild (skin symptoms and/or upper respiratory tract symptoms only), moderate (gastrointestinal tract symptoms or association of systems excluding lower respiratory tract and/or cardiovascular symptoms), and severe (laryngeal, lower respiratory tract, and/or cardiovascular symptoms).21
If no manifestation occurred after the intake of the full offered volume within two hours, the test was considered negative, and the patient was excluded from the study and discharged.
Analysis of collected dataThe collected data were analyzed using the statistical software SPSS (Released 2008. SPSS Statistics for Windows, Version 17.0. Chicago, USA). Sensitivity (S), specificity (Sp), positive predictive value (PPV), negative predictive value (NPV), and positive (PLR) and negative likelihood ratio (NLR) were calculated to assess the accuracy of cutoff points for serum-specific IgE for total CM and fractions, as well as the mean papule diameter in the IRST when compared to the OCT results (the gold standard).23
The receiver operating characteristic (ROC) curves were obtained from the ratio between S and Sp. Thus, optimal cutoff points were defined, with the objective of defining allergy. For this purpose, two methods were used: Youden's index and Sp maximization.24
PPV and NPV were calculated for a cutoff value of 0.35kUA/L, based on previous positivity references and the “optimal cutoffs” on the ROC curve. The sample power was calculated so that the cutoff points showed specificity equal to or greater than 70%, as described by Champely in 2017.25
To evaluate the agreement between the fractions (α-lactalbumin, β-lactoglobulin, and casein) and total cow's milk, Krippendorff's α was used as the measurement of agreement.26 A value of 1 means there is perfect agreement, 0 indicates absence of agreement, and values lower than 0 indicate systematic disagreement.
The study was approved by the ethics committees of Universidade Federal de Sergipe (UFS) and Universidade Federal de São Paulo (UNIFESP), under the respective numbers CEP/SE N. CAAE – 0087.0.107.174-08 and CEP/SP – 1440/11.
ResultsOf the 122 children submitted to OCT, 60 (49%) were males. The allergic group consisted of 73 patients (59.8%) and the control group (non-allergic), of 49 (40.2%).
The most frequently observed symptoms during open OCT were of the skin, in 86.3% (63/73) of the children, followed by respiratory in 23.3% (17/73) and gastrointestinal symptoms in 13.7% (10/73). There were no cardiovascular symptoms. Urticaria was the most frequent clinical manifestation, affecting 78% of the children.
Serum levels of IgE specific for CM and its fractions (α-lactalbumin, β-lactoglobulin, and casein) were significantly higher in the allergic group compared to non-allergic patients (median 3.39kUA/L vs. 1.16kUA/L; 0.97kUA/L vs. 0.09kUA/L; 1.72kUA/L vs. 0.44kUA/L; and 0.75kUA/L vs. 0.36kUA/L, respectively).
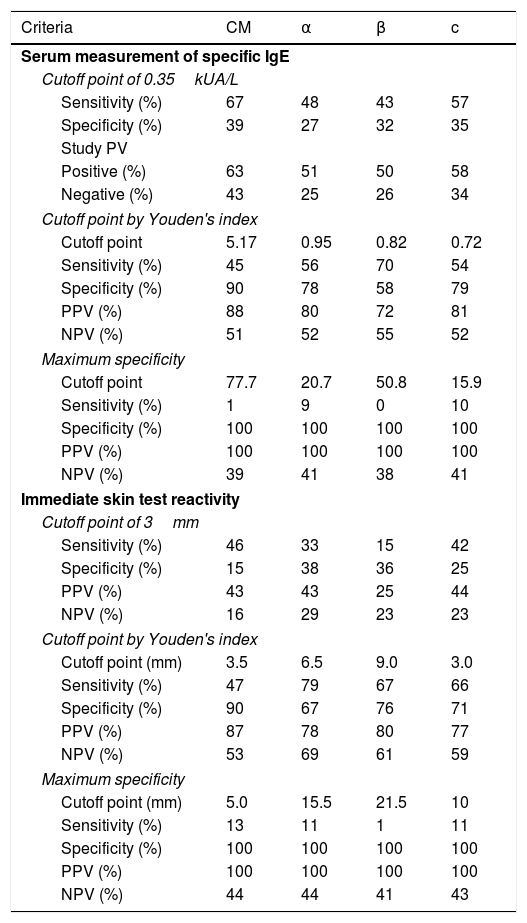
Table 1 describes the accuracy of different concentrations of specific IgE and the mean papule diameter for CM and its fractions, according to the OCT results. For IgE concentrations, when the value of 0.35kUA/L was established as the cutoff point for test positivity, the S for the total CM and its fractions varied from 43% to 67%, the Sp from 27% to 39%, and the NPV from 25% to 43%.
Description of sensitivity (S), specificity (Sp), positive predictive values (PPV), and negative predictive values (NPV) for specific IgE concentrations for cow's milk and its fractions, and for mean papule diameter in the immediate-reading skin reactivity test in the open oral provocation test, by different criteria.
| Criteria | CM | α | β | c |
|---|---|---|---|---|
| Serum measurement of specific IgE | ||||
| Cutoff point of 0.35kUA/L | ||||
| Sensitivity (%) | 67 | 48 | 43 | 57 |
| Specificity (%) | 39 | 27 | 32 | 35 |
| Study PV | ||||
| Positive (%) | 63 | 51 | 50 | 58 |
| Negative (%) | 43 | 25 | 26 | 34 |
| Cutoff point by Youden's index | ||||
| Cutoff point | 5.17 | 0.95 | 0.82 | 0.72 |
| Sensitivity (%) | 45 | 56 | 70 | 54 |
| Specificity (%) | 90 | 78 | 58 | 79 |
| PPV (%) | 88 | 80 | 72 | 81 |
| NPV (%) | 51 | 52 | 55 | 52 |
| Maximum specificity | ||||
| Cutoff point | 77.7 | 20.7 | 50.8 | 15.9 |
| Sensitivity (%) | 1 | 9 | 0 | 10 |
| Specificity (%) | 100 | 100 | 100 | 100 |
| PPV (%) | 100 | 100 | 100 | 100 |
| NPV (%) | 39 | 41 | 38 | 41 |
| Immediate skin test reactivity | ||||
| Cutoff point of 3mm | ||||
| Sensitivity (%) | 46 | 33 | 15 | 42 |
| Specificity (%) | 15 | 38 | 36 | 25 |
| PPV (%) | 43 | 43 | 25 | 44 |
| NPV (%) | 16 | 29 | 23 | 23 |
| Cutoff point by Youden's index | ||||
| Cutoff point (mm) | 3.5 | 6.5 | 9.0 | 3.0 |
| Sensitivity (%) | 47 | 79 | 67 | 66 |
| Specificity (%) | 90 | 67 | 76 | 71 |
| PPV (%) | 87 | 78 | 80 | 77 |
| NPV (%) | 53 | 69 | 61 | 59 |
| Maximum specificity | ||||
| Cutoff point (mm) | 5.0 | 15.5 | 21.5 | 10 |
| Sensitivity (%) | 13 | 11 | 1 | 11 |
| Specificity (%) | 100 | 100 | 100 | 100 |
| PPV (%) | 100 | 100 | 100 | 100 |
| NPV (%) | 44 | 44 | 41 | 43 |
CM, cow's milk; α, α-lactalbumin; β, β-lactoglobulin; c, casein.
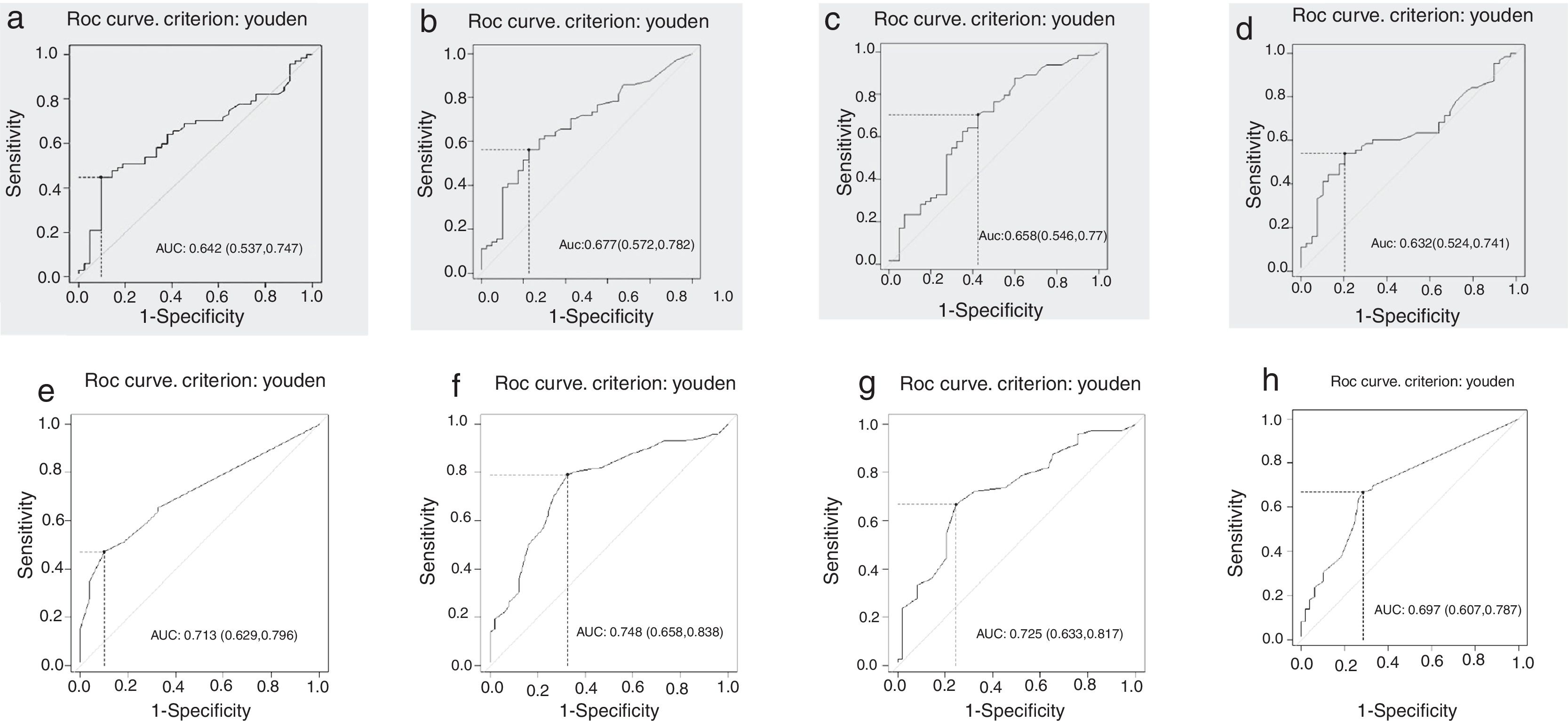
The ROC curves constructed from the ratio between S and Sp of the specific IgE levels were discriminatory, with an area under the curve greater than 0.6 for CM and its fractions (Fig. 1). According to the proposed criterion for establishing an optimal cutoff point, Youden's index, the specific serum IgE concentration considered optimal for CM was 5.17kUA/L, quite different from that obtained with the Sp maximization criterion (PPV=100%), with values of 77.7kUA/L (Table 1).
Receiver operating characteristic (ROC) curves to obtain optimum levels of specific serum IgE for: (a) total cow's milk, (b) α-lactalbumin, (c) β-lactoglobulin, (d) casein; and for the mean papule diameter of the immediate reading skin test for: (e) total cow's milk (f), α-lactalbumin, (g) β-lactoglobulin, and (h) casein in the open oral challenge test for cow's milk and its fractions.
As for the fractions, according to Youden's index, the following values were considered: 0.95kUA/L for α-lactalbumin, 0.82kUA/L for β-lactoglobulin, and 0.72kUA/L for casein (Table 1). When evaluated alone, the PLR, which represents how much a positive result increases the chance of an individual of being ill, showed low diagnostic accuracy (values between 2 and 5), being null for β-lactoglobulin (value between 1 and 2) and higher for total CM. The evaluation of the different possible combinations showed that any association has better accuracy than total CM alone, mainly if associated with casein (PLR=9.53).
The mean papule diameters were significantly higher in the allergic group: CM: median 2.5mm vs. 0mm; α-lactalbumin: 8.75mm vs. 5.5mm; β-lactoglobulin: 9.75mm vs. 8.0mm; and casein: 3.5mm vs. 0mm. When evaluating the performance of different mean papule diameters, when the 3-mm value was established as a cutoff for IRST positivity, the S for total CM and its fractions varied from 15% to 46%, whereas Sp ranged from 15% to 38%, and NPV from 16% to 29% (Table 1).
The ROC curves were adequate, with area under the curve greater than 0.7 for CM and its fractions, except for casein, with an area of 0.697 (0.607–0.787), being only discriminatory (Fig. 1). Using Youden's index, the optimal cutoff point for the mean papule diameter for CM was 3.5mm. With the maximization of Sp (PPV=100%) the cutoff point for CM was 5.0mm, 15.5mm for α-lactalbumin, 21.5mm for β-lactoglobulin, and 10mm for casein (Table 1). The use of the components contributed to improve the diagnosis of those with a negative IRST for total CM (NLR: 0.74).
The power of the established cutoff points was calculated to have a specificity equal to or greater than 70%. The power of the specific serum IgE cutoff point and the skin test for cow's milk, calculated using Youden's index, was 99.9% in both, thus considered as having high power.
DiscussionIn clinical practice, new diagnostic tests are used for different reasons: for screening, to obtain better information, due to lower costs, or as a complement to existing exams.23 Perfect tests do not exist, but it is necessary to know how well a test can differentiate ill individuals from healthy ones, i.e., the diagnostic test accuracy.24
The present study has some advantages, as it is a prospective, cross-sectional study of diagnostic accuracy that evaluated children up to 3 years of age with objective symptoms of CMPA, which allowed the use of open OCT as a diagnostic method, eliminating the need for DBPCOCT, a less feasible procedure.5,6 For the most part, the studies included patients in different age ranges, and some of them included adults.8–15
All children were submitted to open OCT regardless of IgE levels and mean papule diameters, minimizing selection bias. The time of the exclusion diet before OCT did not interfere with the results. OCT with symptoms was considered positive (allergic) and, without symptoms, negative (non-allergic or tolerant). The solely discriminatory performance of the ROC curves is due to the uncertainty regarding the predictive power for allergy and/or tolerance by specific IgE serum levels and mean papule diameter for patients in the assessed age range, non-anaphylactic, and in distinct locations.
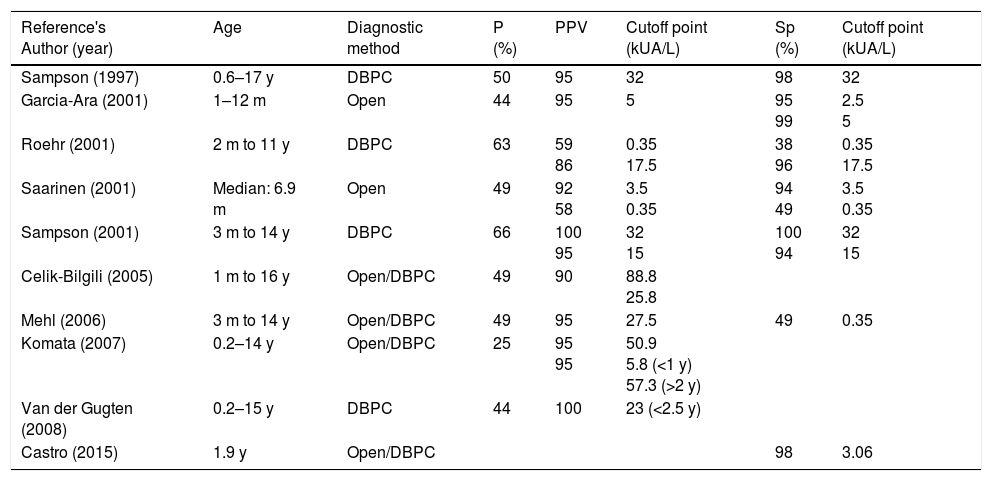
The use of ImmunoCAP®, a technique of quantitative serum-specific IgE detection, allowed the researchers to establish a relationship between IgE levels and a food allergy diagnosis.3 Published studies that proposed and discussed cutoff point values for CMPA diagnosis described different results between studied populations, pointing to the need to establish values for each specific population, which motivated this study (Table 2).8–15
Specific serum IgE values (ImmunoCAP®) predictive for the diagnosis of cow's milk allergy (reproduced and modified from Luyt et al.).2
| Reference's Author (year) | Age | Diagnostic method | P (%) | PPV | Cutoff point (kUA/L) | Sp (%) | Cutoff point (kUA/L) |
|---|---|---|---|---|---|---|---|
| Sampson (1997) | 0.6–17 y | DBPC | 50 | 95 | 32 | 98 | 32 |
| Garcia-Ara (2001) | 1–12 m | Open | 44 | 95 | 5 | 95 99 | 2.5 5 |
| Roehr (2001) | 2 m to 11 y | DBPC | 63 | 59 86 | 0.35 17.5 | 38 96 | 0.35 17.5 |
| Saarinen (2001) | Median: 6.9 m | Open | 49 | 92 58 | 3.5 0.35 | 94 49 | 3.5 0.35 |
| Sampson (2001) | 3 m to 14 y | DBPC | 66 | 100 95 | 32 15 | 100 94 | 32 15 |
| Celik-Bilgili (2005) | 1 m to 16 y | Open/DBPC | 49 | 90 | 88.8 25.8 | ||
| Mehl (2006) | 3 m to 14 y | Open/DBPC | 49 | 95 | 27.5 | 49 | 0.35 |
| Komata (2007) | 0.2–14 y | Open/DBPC | 25 | 95 95 | 50.9 5.8 (<1 y) 57.3 (>2 y) | ||
| Van der Gugten (2008) | 0.2–15 y | DBPC | 44 | 100 | 23 (<2.5 y) | ||
| Castro (2015) | 1.9 y | Open/DBPC | 98 | 3.06 |
P, percentage; Sp, specificity; PPV, positive predictive values; DBPC, double-blinded placebo-controlled.
In Brazil, cutoff points for CM-specific IgE serum concentrations were established for phenotypically distinct populations in São Paulo, with different results: 3.06kUA/L for anaphylactic27 and 11kUA/L for non-anaphylactic cases.28
According to the definition, the cutoff point is considered good when it combines high PPV and Sp. In the present study, the ROC curve showed that the optimal cutoff point for CM (i.e., the point at which the weight of S is equal to that of Sp) using Youden's index was 5.17kUA/L (Sp: 90% and PPV: 88%). When trying to find a better cutoff point, i.e., with a higher PPV, establishing the PPV at 90% or 95%, the value provided by the curve was 77.7kUA/L (Sp and PPV of 100%; Table 1).
In clinical practice, the most appropriate level of specific IgE to indicate whether or not to perform OCT depends on the clinical context. If the challenge may be dangerous to the patient, a low cutoff point would be of interest, even at the cost of misidentifying the patient as allergic. Although the value of 5.17kUA/L did not show such adequate Sp and PPV, accepting 77kUA/L would subject all children to OCT, thus exposing many of them to risk situations (Table 1).
Some studies have also documented the usefulness of IRST as a predictor of OCT positivity for CMPA diagnosis.13–15 The optimal cutoff point established for CM in IRST was 3.5mm (Sp=90% and PPV=87%). When trying to find a better cutoff point, establishing the PPV at 90% or 95%, the value provided by the ROC curve was 5.0mm (specificity and PPV of 100%; Table 1).
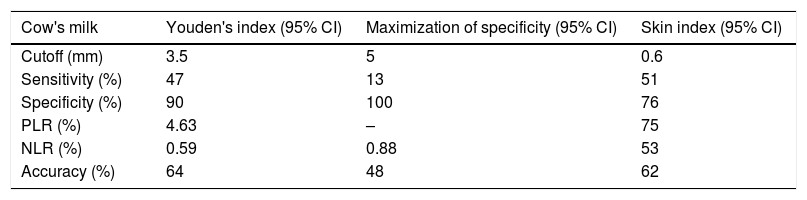
Although there is no age restriction for IRST, it is assumed that patients younger than 2 years of age may have smaller diameters, because the skin reactivity acquired during childhood reaches its peak from 15 to 25 years of age and progressively declines.29 For this reason, the skin IRST index, the ratio between papule size and the positive control, can be used to predict results of oral provocation tests.30 When establishing a previously determined positive value of 0.6,30 and comparing the accuracy obtained with those of the cutoff points established by Youden's index and the maximization of Sp, the best measure obtained was the value of 3.5mm (Table 3). Some authors correlated their skin index results with those of OCT; however, no usefulness was found in this study.13,14,29
Accuracy of the different cutoff points of the mean papule diameter for total cow's milk in the open rral provocation tests.
| Cow's milk | Youden's index (95% CI) | Maximization of specificity (95% CI) | Skin index (95% CI) |
|---|---|---|---|
| Cutoff (mm) | 3.5 | 5 | 0.6 |
| Sensitivity (%) | 47 | 13 | 51 |
| Specificity (%) | 90 | 100 | 76 |
| PLR (%) | 4.63 | – | 75 |
| NLR (%) | 0.59 | 0.88 | 53 |
| Accuracy (%) | 64 | 48 | 62 |
PLR, positive likelihood ratio; NLR, negative likelihood ratio; CI, confidence interval.
If the disease prevalence is high among the population, the PPV and NPV results are influenced. A prevalence adjustment would be impossible due to the lack of knowledge about the prevalence of CMPA in this region and in Brazil and, thus, alternative measures of performance such as PLR and NLR were performed.
In the diagnostic evaluation of CM-associated component use in the evaluation of children with suspected CMPA, these results would agree with those published by Castro et al., who demonstrated that there was no increase in the diagnostic evaluation when using the components in addition to CM, if they had been analyzed one by one.27 However, when the different possible combinations are evaluated, it is shown that any association has better accuracy than total CM alone. Garcia-Ara et al. also observed that, when validating their results with OCT, there was an advantage in the diagnosis when the protein fractions were associated. Additionally, the negativity of the fractions excluded CMPA in many cases due to its high NPV.8
To evaluate if the results obtained were associated with the agreement between the fractions (α-lactalbumin, β-lactoglobulin, and casein) and CM, since fractions should be included in the total component of CM, Krippendorf's α was used as the measure of agreement.26 The observation of levels that were discretely superior to zero allowed us to state that the use of fractions, both for IgE measurements and for papule diameter in the IRST, helps in the diagnosis of CMPA in children under 3 years of age.
In conclusion, the cutoff level of the specific IgE for CM in children treated at a specialized service showed to be discriminatory for the diagnosis of CMPA, with a value of 5.17kUA/L, as well as a mean papule diameter for CM of 3.5mm.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Franco JM, Pinheiro AP, Vieira SC, Barreto ÍD, Gurgel RQ, Cocco RR, et al. Accuracy of serum IgE concentrations and papule diameter in the diagnosis of cow's milk allergy. J Pediatr (Rio J). 2018;94:279–85.
Study carried out at Universidade Federal de São Paulo (UNIFESP), Escola Paulista de Medicina, São Paulo, SP; and Universidade Federal de Sergipe, Aracaju, SE, Brazil.