To describe the results obtained in a neonatal screening program after its implementation and to assess the clinical and molecular profiles of confirmed and suspicious congenital adrenal hyperplasia cases.
MethodsA cross-sectional study was conducted. Newborns with suspected disease due to high 17-hydroxyprogesterone levels and adjusted for birth weight were selected. Classical congenital adrenal hyperplasia (salt-wasting and simple virilizing forms) was diagnosed by an increase in 17-hydroxyprogesterone levels as confirmed in the retest, clinical evaluation, and genotype determined by SNaPshot and multiplex ligation-dependent probe amplification.
ResultsAfter 24 months, 15 classic congenital adrenal hyperplasia cases were diagnosed in a total of 217,965 newborns, with an estimated incidence of 1:14,531. From 132 patients, seven non-classical and 14 heterozygous patients were screened for CYP21A2 mutations, and 96 patients presented false positives with wild type CYP21A2. On retest, increased 17-hydroxyprogesterone levels were found in classical congenital adrenal hyperplasia patients and showed significant correlation with genotype-related classical genital adrenal hyperplasia. The most frequent mutations were IVS2-13A/C>G followed by gene deletion or rearrangement events in the classical form. In non-classical and heterozygous diseases, p.Val282Leu was the most common mutation.
ConclusionsThe results underscore the effectiveness of congenital adrenal hyperplasia neonatal screening in the public health system and indicate that the adopted strategy was appropriate. The second sample collection along with genotyping of suspected cases helped to properly diagnose both severe and milder cases and delineate them from false positive patients.
Descrever os resultados obtidos em um programa de triagem neonatal após sua implantação e avaliar os perfis clínicos e moleculares de casos confirmados e suspeitos de hiperplasia adrenal congênita.
MétodosFoi feito um estudo transversal. Recém-nascidos com suspeita da doença devido aos altos níveis de 17-alfa-hidroxiprogesterona e ajustados pelo peso ao nascer foram selecionados. A hiperplasia adrenal congênita clássica (forma perdedora de sal e forma virilizante simples) foi diagnosticada por um aumento nos níveis de 17-alfa-hidroxiprogesterona confirmado no reteste, avaliação clínica e genótipo determinado com o uso do ensaio SNaPshot e amplificação multiplex de sondas dependente de ligação.
ResultadosApós 24 meses, 15 casos clássicos de hiperplasia adrenal congênita foram diagnosticados em 217.965 recém-nascidos, com uma incidência estimada de 1: 14.531. De 132 pacientes, sete não-clássicos e 14 heterozigotos foram submetidos à triagem para mutações no gene CYP21A2 e 96 pacientes apresentaram resultados falso-positivos com CYP21A2 do tipo selvagem. No reteste, níveis aumentados de 17-alfa-hidroxiprogesterona foram encontrados em pacientes com hiperplasia adrenal congênita clássica e mostraram correlação significativa com HAC clássica relacionada ao genótipo. As mutações mais frequentes foram IVS2-13A/C>G, seguidas de deleção gênica ou eventos de rearranjo na forma clássica. Em casos doenças não clássicas e heterozigose, a mutação p.Val282Leu foi a mais comum.
ConclusõesOs resultados ressaltam a eficácia da triagem neonatal para a hiperplasia adrenal congênita no sistema público de saúde e indicam que a estratégia adotada foi adequada. A segunda coleta de amostras, juntamente com a genotipagem dos casos suspeitos, ajudou a diagnosticar adequadamente os casos graves e mais leves e diferenciá-los de pacientes com resultado falso-positivo.
Congenital adrenal hyperplasia (CAH), an autosomal recessive disease, is characterized by impairment of metabolic cortisol and aldosterone synthesis.1 It is caused by mutations in the CYP21A2 gene in approximately 90% of all cases, leading to 21-hydroxylase (21-OH) deficiency and causing accumulation of androgen precursors.2 The main disease marker is 17-hydroxyprogesterone (17-OHP).2 There are three recognized clinical forms of CAH: classical salt-wasting (SW); simple virilizing (SV); and late-onset, termed non-classical (NC) CAH.1,3 The main purpose of neonatal screening is to identify newborns at risk of being affected by the two classical forms.4,5 However, asymptomatic cases with persistently elevated 17-OHP levels in neonatal screening are suspected for NC-CAH and may be further identified by molecular diagnosis.6,7
The overall incidence for CAH described in the literature is 1:15,000 in live newborns.4 The prevalence of NC-CAH is estimated at 1:1000 individuals.1 In 2/3 cases of the classical forms, salt loss affects both genders, and symptoms begin in the second week of life, thus reinforcing the importance of an early diagnosis.5 In girls, intrauterine androgen excess exposure causes different degrees of virilization and genital ambiguity, as defined by the Prader Scale.8 Increases in 17-OHP have been observed in newborns without CAH (false positives) due to stressful situations and preterm birth.9,10 In turn, false negative cases can occur as a result of maternal use of corticosteroids at the end of pregnancy.11,12 In order to minimize the number of false positives, cutoffs stratified by both Brazilian and international birth weight categories have been established.9–11
Molecular analysis of the CYP21A2 gene can improve neonatal screening specificity, and may also help in the clinical management of the disease.7,13 The main CYP21A2 mutations have been categorized into four groups (Null, A, B, and C) according to residual 21-OH enzymatic activity, which allows the determination of the expected phenotype.2,6,14,15 Both Null and A are associated with SW-CAH; group B is associated with SV-CAH, and group C, with NC-CAH.2,6,13–15
In Brazil, the states of Goiás, Santa Catarina, and São Paulo have accumulated over ten years experience in neonatal CAH screening.9,14,16,17 Therefore, the aims of this study were: (1) to describe the results 24 months after a CAH neonatal screening in a public health program in Southern Brazil was implemented and (2) to assess the clinical profiles of confirmed and suspicious CAH cases in addition to molecular genotyping of these cases as a complementary tool to improve CAH diagnosis.
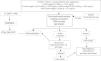
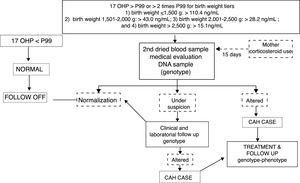
MethodsA cross-sectional study was conducted with newborns included in the first two years of the public CAH screening program implementation in the state of Rio Grande do Sul, Brazil. The database of babies with suspected CAH, which was based on altered 17-OHP values from a 2014 to 2016 screening, were reviewed. Classical CAH (C-CAH), SW, and SV were diagnosed by increased 17-OHP levels detected at screening, confirmed by retest and/or clinical evaluation, followed by genotype studies. Affected girls presented virilized external genitalia, while boys did not necessarily present genital changes, but penis enlargement could be observed. False positives were characterized by lower 17-OHP levels on retest and by the wild type (WT) genotype, as shown in Fig. 1.18 Data related to genotype distribution was recently reported.19
Flowchart of CAH genotype-phenotype evaluation. 17 OHP, 17-hydroxyprogesterone; CAH, congenital adrenal hyperplasia. Adapted from Kopacek et al.18
The study protocol was approved by the Research Ethics Committee at Hospital Materno Infantil Presidente Vargas and by Ethics Committee of Fundação Estadual de Produção e Pesquisa em Saúde (No. 341.289/June 8, 2013), and all parents gave their written informed consent.
Dried blood spots were obtained using filter paper (S&S 903) from the 3rd to 40th postnatal days. The GSP solid phase (time-resolved) immunofluorescence assay (Neonatal 17-OHP kit; PerkinElmer, Turku, Finland) was used to measure 17-OHP. Fig. 1 describes the study strategy.9,11,18 Newborns whose mothers used corticosteroids at the end of pregnancy, as recorded on filter paper, were called for a second collection after 15 days of life.11,12,18
Genotyping was performed as previously described by Prado et al.19 in 132 subjects. The SNaPshot assay was used to all subjects. This assay analyzed 12 point mutations in CYP21A2 gene (p.Gln319Ter, p.Arg357Trp, p.Leu308Phefs, p.Val237Glu, IVS2-13A/C>G, p.Ile173Asn, p.Pro31Leu, p.Pro454Ser, p.Val282Leu, p.Gly111Valfs, p.Arg409Cys, and p.His63Leu).19–21 For cases with clinical and/or biochemical suspicion of classical CAH, but no mutation identified by previous technique, a commercial multiplex ligation-dependent probe amplification (MLPA) assay with SALSA MLPA probemix P050-C1 CAH kit (MRCHolland, Amsterdam, The Netherlands) was performed to detect rearrangements events.
Results were expressed as mean+standard deviation (SD) or frequencies (%). Comparisons among groups were analyzed by univariate analysis of variance (ANOVA) followed by Tukey test. Covariance ANOVA (ANCOVA) was used for 17-OHP adjustments by birth weight, and data was expressed as mean+standard error of the mean (SEM). A partial correlation was estimated between 17-OHP and electrolytes. Analyses were performed using SPSS version 18.0 (SPSS Inc, Chicago, United States). Data was considered to be significant when p<0.05.
ResultsFrom a total of 217,965 samples obtained at the two-year screening, 15 C-CAH cases were diagnosed with an estimated incidence of 1:14,531. Nine newborns were females and six were males; 80% (n=12) were clinically characterized as SW-CAH patients and the remainder as SV patients. According to previously published data by this group,18 around 70–80% of the first sample were collected at age 3–7 days, and this finding was similar for C-CAH (median 5 [2.0–38.0] days). Most retest samples were collected around the second or third week of life (median 17 [14.0–21.0] days), similar to C-CAH (median 19 [8.0–51.0] days). Table 1 describes clinical data, 17-OHP, and electrolyte values, as well as the phenotype-genotype evaluation of these cases. Three girls (cases 3, 5, and 11) had mild virilization (Prader I classification) and were not clinically characterized as presenting with clitoromegaly previous to CAH screening. Another girl (case 2) was sex assigned as a boy, and the other one (case 10) was registered with a neutral name, suitable for either sex due to genital ambiguity. In the other four girls (cases 4, 12, 13, and 14) genital ambiguity was identified, but CAH was not diagnosed prior to screening. Among the males, case 6 and 15 presented with penis enlargement and the other four had normal male genitalia. Ten of the 15 cases (66.6%) were born at birth weight>2500g and no cases weighed<2000g. Mothers of three cases (5, 8, and 10) were using glucocorticoids at the end of their pregnancies. In two of them, a net increase in 17-OHP levels in the second sample was observed. In a third infant (case 8), the onset of corticotherapy due to salt loss preceded the possibility of obtaining a second sample. Four infants (26.6%) had consanguineous parents.
Clinical aspects and genotypes in classical, non-classical, and heterozygous CAH patients.
| Patient No. | Sex | Weight (g) | Maternal corticoid use | Consanguinity | 17 OHP (ng/mL)Sample 1 | 17 OHP (ng/mL)Sample 2 | [Na+]e (nmol/L) | [K+]e (nmol/L) | Virilization/Prader Scale | CAHPhenotype | Genotype | Mutation groups | Phenotype/genotype correlation | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Classical CAH | 1 | M | 3640 | No | Yes | 105.0 | 283.0 | 133 | 5.91 | Normal | SW | IVS2-13A/C>G/IVS2-13A/C>G | A/A | Yes |
| 2 | F | 3325 | No | Yes | 432.0 | 759.0 | 108 | 5.4 | Prader IV | SW | Del CYP21A2/LGC | Null/null | Yes | |
| 3 | F | 2490 | No | No | 44.9 | 94.9 | 132 | 5.58 | Prader I | SV | IVS2-13A/C>G/IVS2-13A/C>G | A/A | No | |
| 4 | F | 2040 | No | No | 512.0 | 521.0 | 126 | 5.34 | Prader III | SW | Del 30Kb/IVS-2-13A/C>G | Null/A | Yes | |
| 5 | F | 3200 | Yes | No | 61.8 | 209.0 | 132 | 5.0 | Prader I | SV | Del CYP21A2/p.Ile173Asn | Null/B | Yes | |
| 6 | M | 3450 | No | No | 733.0 | – | 120 | 5.7 | Penis enlargement | SW | Del CYP21A2/Cluster E6 | Null/null | Yes | |
| 7 | M | 2980 | No | No | 461.0 | 489.0 | 109 | 6.54 | Normal | SW | Del CYP21A2/p.Arg357Trp | Null/null | Yes | |
| 8 | M | 3295 | Yes | Yes | 382.0 | – | 126 | 6.6 | Normal | SW | IVS2-13A/C>G/IVS2-13A/C>G | A/A | Yes | |
| 9 | M | 2395 | No | No | 469.0 | 435.0 | 118 | 8.9 | Normal | SW | IVS2-13A/C>G/LGC | Null/A | Yes | |
| 10 | F | 2270 | Yes | No | 37.0 | 354.0 | 129 | 5.9 | Prader IV | SW | IVS2-13A/C>G/IVS2-13A/C>G | A/A | Yes | |
| 11 | F | 2890 | No | No | 34.2 | 133.0 | 130 | 5.3 | Prader I | SW | LGC/p.Ile172Asn | Null/B | No | |
| 12 | F | 3580 | No | No | 459.0 | – | 131 | 6.0 | Prader IV | SW | IVS2-13A/C>G/IVS2-13A/C>G | A/A | Yes | |
| 13 | F | 3730 | No | Yes | 515.0 | – | – | – | Prader IV | SW | p.Gln319Ter/p.Gln319Ter | Null/null | Yes | |
| 14 | F | 3500 | No | No | 65.2 | 313.0 | 125 | 7.4 | Prader III | SW | p.Leu308Phefs/IVS2-13A/C>G | Null/A | Yes | |
| 15 | M | 2340 | No | No | 32.5 | 58.0 | 131 | 5.85 | Penis enlargement | SV | p.Ile173Asn/p.Ile173Asn | B/B | Yes | |
| Mean±SD/% positive | 30083 (±0.588) | 20% | 27% | 289.57d (±240.13) | 331.72d (±210.37) | 125±8.30d | 6.10±1.01d | 86.6% | ||||||
| Non classical CAH | 16 | M | 3465 | Yes | No | 2.8 | 46.30 | 135 | 5.11 | Normala | NC | p.Val282Leu/p.Val281Leu | C | Yes |
| 17 | M | 3340 | Yes | No | 4.16 | 15.1 | 136 | 5.25 | Normal | NC | p.Val282Leu/p.Val282Leu | C | Yes | |
| 18 | M | 2508 | No | No | 31.2 | 5.00 | 135 | 4.84 | Normal | NC | p.Gln319Ter/p.Val282Leu | C | Yes | |
| 19 | F | 2660 | No | No | 38.2 | 47.2 | 135 | 5.3 | Normal | NC | p.Pro454Ser/p.Pro454Ser | C | Yes | |
| 20 | F | 2988 | No | No | 15.2 | 18.3 | 131 | 5.82 | Normal | NC | p.Gln319Ter+p.Arg357Trp+p.Leu308Phefs/p.Val282Leu | C | Yes | |
| 21 | M | 2830 | No | No | 25.8 | 15.1 | 129b | 4.67 | Normal | NC | IVS2-13A/C>G/p.Val282Leu | C | Yes | |
| 22 | F | 2630 | Yes | No | 15.1 | 22.1 | 135 | 5.4c | Normal | NC | p.Arg357Trp/p.Val282Leu | C | Yes | |
| Mean±SD/% positive | 29173 (±.366) | 43% | 0% | 18.92 (±13.39) | 24.16 (±16.28) | 134.5±1.76 | 5.16+0.40 | 100% | ||||||
| Heterozygous | 23 | F | 3680 | Yes | No | 14.5 | 15.4 | 133 | 5.6 | Normal | None | p.Gln319Ter/WT | Null/WT | Yes |
| 24 | M | 2750 | No | No | 18.3 | 16.9 | 135 | 5.96 | Normal | None | p.Arg357Trp/WT | Null/WT | Yes | |
| 25 | F | 2605 | Yes | No | 24.5 | 16.9 | 134 | – | Normal | None | p.Gln319Ter/WT | Null/WT | Yes | |
| 26 | M | 2345 | Yes | No | 6.1 | 17.7 | 135 | 5.3 | Normal | None | p.Val282Leu/WT | C/WT | Yes | |
| 27 | M | 2570 | No | Yes | 31.0 | 11.5 | 133 | 6.34c | Normal | None | p.Val282Leu/WT | C/WT | Yes | |
| 28 | M | 2535 | Yes | No | 36.8 | 9.0 | 134 | 5.11 | Normal | None | p.Gln319Ter/WT | Null/WT | Yes | |
| 29 | M | 2600 | No | No | 50.9 | 10.9 | 137 | 5.4 | Normal | None | p.Arg357Trp/WT | Null/WT | Yes | |
| 30 | M | 2550 | Yes | Yes | 25.8 | 16.2 | 131 | 5.18 | Normal | None | p.Val282Leu/WT | C/WT | Yes | |
| 31 | M | 1215 | No | No | 30.1 | 127.0h | – | – | Normal | None | p.Val282Leu/WT | C/WT | Yes | |
| 32 | F | 2504g | No | No | 35.3 | 10.4 | 134 | 4.95 | Transient clitoromegaly | Suspected CAHf | p.Val282Leu/WT | C/WT | No | |
| 33 | F | 3150 | No | No | 15.4 | 16.10 | 135 | 4.3 | Normal | None | p.Gln319Ter/WT | Null/WT | Yes | |
| 34 | F | 2560g | Yes | No | 22.4 | 16.7 | 137 | 5.15 | Transient clitoromegalya | Suspected CAHf | p.Val282Leu/WT | C/WT | No | |
| 35 | M | 2750 | No | No | 25.3 | 15.9 | 136 | 5.29 | Normal | None | IVS2-13A/C>T/WT | nd/WT | Yes | |
| 36 | F | 2905g | No | No | 18.20 | 35.0 | 139 | 4.8 | Transient clitoromegaly | suspected CAHf | IVS2-13A/C>T/WT | nd/WT | No | |
| Mean±SD/% positive | 2623 (±0.535) | 43% | 14% | 25.33 (±11.21) | 23.97 (±30.28) | 134.85±2.07 | 5.18±0.43 | 79% |
F, female; M, male; NC, non-classical; HT, heterozygous; CAH, congenital adrenal hiperplasia; LGC, large gene conversion; nd, not defined.
Table 1 also describes the clinical profile, 17-OHP values, and phenotype-genotype evaluation of NC-CAH and heterozygous cases that were detected during screening. Only one heterozygous patient (case 31) presented low birth weight related to extreme prematurity and had an increased 17-OHP value in the second sample that was associated with clinical worsening in the intensive neonatal unit. This patient was followed-up in a tertiary hospital, far from the screening center. Normal electrolyte values and no other clinical signs of CAH were reported by this hospital. A third sample was collected after clinical improvement and showed a considerable decrease in 17-OHP (17ng/mL). In this patient, genotyping was crucial for diagnosis elucidation. Another three patients with NC-CAH (16, 17, and 22) presented higher 17-OHP values in the second sample and a history of maternal corticosteroid use. Hyponatremia and hyperkalemia were not observed in NC-CAH and heterozygous cases. Among initial suspected cases of CAH, one patient with transient genital hyperpigmentation (patient 16) was diagnosed as NC-CAH, and three girls with transient clitoromegaly were later identified as heterozygous cases. In these cases, spontaneous regression of clitoromegaly was observed during the follow-up through the first six months of life. Parental consanguinity was found in two (14.3%) of heterozygous patients.
Table 1 shows the genotype distribution according to the enzyme activity groups (Null, A, B, and C) and heterozygosity in the newborn population. Genotype-phenotype agreement was observed in 13 C-CAH cases (86.6%). The IVS2-13A/C>G was the most frequent mutation (33% in homozygosity and 20% in compound heterozygosity), followed by p.Ile173Asn, deletion, and gene conversion, each observed in 20% of the C-CAH cases. Seven patients presented with non-classical mutations (group C), and the other 14 heterozygous patients had the WT allele detected during neonatal screening. Among the entire sample, p.Val282Leu was the most frequent mutation in NC-CAH patients (57% heterozygous and 28.5% homozygous); it was also observed in six heterozygous patients (43%; Table 1). A new allele, IVS2-13A/C>T, was described by Prado et al. in a heterozygous girl born at term with birth weight of 2905g (sample 1: 17 OHP 18.2ng/mL and sample 2: 35.0ng/mL) and in a boy born late-preterm with birth weight of 2540g (sample 1: 17-OHP 25.3ng/mL and sample 2: 15.9ng/mL) with positive screening.19 Surprisingly, the clitoromegaly identified in the girl during the first examination resolved spontaneously during the follow-up period. Both had normal electrolyte profiles and did not show any other symptoms associated with CAH during the six-month follow-up.
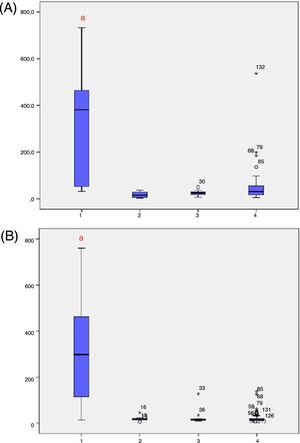
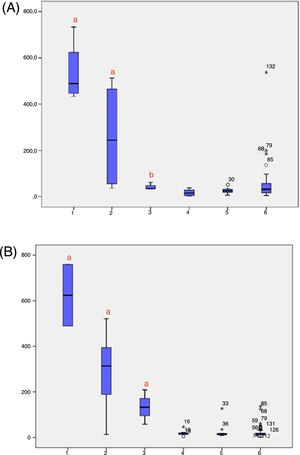
Levels of 17-OHP, adjusted for birth weight, were analyzed according to the groups (1=C-CAH; 2=NC-CAH; 3=heterozygous; 4=WT) in samples 1 and 2 (Fig. 2A and B, respectively). Significantly higher 17-OHP levels were observed in C-CAH when compared with other groups in samples 1 and 2. Fig. 3 shows 17-OHP levels, adjusted for birth weight, in samples 1 and 2, classified according to the mutation groups. In sample 1, individuals with mutation groups null and A had significantly higher levels of 17-OHP and in sample 2, mutation groups null, A, and B maintained higher 17-OHP levels, which were statistically different from group C (NC-CAH), heterozygous, and WT patients.
17 OHP levels in the four groups (classical CAH, NC CAH, heterozygous, and wild type). Four groups (1=classical CAH; 2=NC CAH; 3=heterozygous; 4=wild type). (A) 17 OHP levels in sample 1; (B) 17 OHP levels in sample 2. CAH, congenital adrenal hyperplasia; NC, non-classical. ap<0.05 versus all other groups.
17-OHP levels according to genotype (mutation severity group, heterozygous, and wild type); (A) 17 OHP levels in sample 1; (B) 17 OHP levels in sample 2. Genotype (1=group null; 2=group A; 3=group B; 4=group C; 5=heterozygous; 6=wild type). ap<0.05 versus all other groups. bp<0.05 versus group 1 and 2. CAH, congenital adrenal hyperplasia; 17-OHP, 17 hydroxyprogesterone; SW, salt-wasting; SV, simple virilizing; C-CAH, classic congenital adrenal hyperplasia; NC, non-classic, late onset; WT, wild-type.
Table 1 also shows electrolyte values in the different groups. Classical CAH cases had significantly lower Na+ and higher K+ levels. A strong inverse correlation between adjusted-for-birth weight 17-OHP and Na+ (r=−0.795 for 17-OHP sample 1 and r=−0.740, for sample 2, p<0.05) and a positive correlation with K+ were observed (r=0.494 for 17-OHP sample 1 and r=0.531 for sample 2, p<0.05).
DiscussionIn the present study, the two-year CAH neonatal screening program using significantly higher levels of the marker (17-OHP) properly allowed the diagnosis of 15 classical CAH newborns. Furthermore, the retest successfully discriminated between cases and disease severity in agreement with the corresponding genotype investigation.
The screening program was highly effective in detecting some cases that would not be clinically recognized before the screening. In this sense, mild genital atypia (cases 3, 4, and 11) and increased penis size in boys (cases 6 and 15) were not detected before specialized evaluation. Even in those with ambiguous genitalia, CAH was not diagnosed before screening and incorrect sex assignment was made in two cases (2 and 10). Indeed, it was observed that >50% of the females had an incorrect clinical evaluation of the virilization features, which was similarly identified in previous studies.9,15,22 This reinforces the importance of universal newborn screening for hyperplasia in Brazil.
Concerning the large difference observed in the 17-OHP cutoff values used for the birth weight categories,9,11 it is important to note that infants in the lowest weight categories are commonly hospitalized in intensive care units and continuously monitored, including for electrolytes disturbances. In fact, as previously observed during the first year of screening, most of the false positives were newborns<2000g.18 A second sample showing lower 17-OHP levels in asymptomatic cases is usually sufficient to elucidate false positive cases, especially in preterm and low birth weight infants. Furthermore, as previously reported, consanguinity rates are higher among CAH cases.18,22 Thus, this additional information can be useful in distinguishing between actual cases and false positives. Similarly, information about maternal corticosteroid use at the end of gestation is relevant. In this case, the collection of a second sample at 15 days of age helps to diagnose cases such as that of patient 11, who presented with mild virilization not previously recognized and further SW-CAH development.23
High 17-OHP levels may be present in preterm and critically ill newborns, reinforcing the screening stratified by birth weight.9–11 In addition, the genotype has been recommended to improve the diagnosis of neonatal screening accuracy. It helps to discriminate between actual cases and false positives, to clarify borderline cases, and eventually allow carriers of the NC-CAH to be diagnosed.7,13,15 In confirmed cases, high 17-OHP values corresponded to the severity of each genotype group.14,15,23 Interestingly, when 17-OHP levels were stratified according to mutation group severity in the second sample, a better differentiation of SV forms (group B mutations) from NC-CAH (group C mutations), heterozygous, and WT was observed (Figs. 2 and 3). These last three groups had significantly lower 17-OHP levels, justifying the strategy of retesting these patients and when there is still doubt, performing genotype analysis.23
The efficacy of the screening program can also be demonstrated when severe forms such as carriers of group null mutations are identified.15 Deletion and large gene conversions characterizing group null were observed in 20% of the present sample, which is in agreement to a recent, large genotype-phenotype correlation study.24 The mutations group null in homozygosis was observed in 26.67% of the present sample (4/15 patients) and null and A groups in compound heterozygosis characterizing SW-CAH in another three patients. In patient 11, no clear phenotype-genotype correlations were observed, as a result of the manifestation of a mild SW-CAH phenotype. When null and B groups mutations are in compound heterozygosis, including p.Ile173Asn in one allelle, SV-CAH is expected in most cases.6,15 This observation has been reported by other groups,24,25 including a very rare association of p.Ile173Asn mutation with a NC-CAH.24 The SW-CAH associated with p.Ile173Asn is more common when a second more severe mutation is associated in heterozygosity.25,26
In Brazil, a study by Bachega et al. determined the frequency of point mutations in 130 patients with C-CAH and NC-CAH and established genotype-phenotype correlation.27 The most frequent mutations were IVS2-13A/C>G in 55% of the alleles of the patients with the SW form, p.Ile173Asn in 42% of the SV-CAH, and p.Val282Leu in 70% of NC-CAH individuals. The frequency of IVS2-13A/C>G mutation in the present sample was, therefore, not surprising for the Brazilian population (53% in homo- and/or heterozygosity in C-CAH). In a Brazilian sample from São Paulo14 and in a larger Argentine cohort study, IVS2-13A/C>G corresponded to 21% in allele frequency and 20% of mutations in homozygosity,28 respectively. Brazil and Argentina show very similar rates of this mutation.14,28 The state of Rio Grande do Sul is near Argentina, and their populations share common ethnic origins. This group also describes the association of IVS2-13A/C>G alleles to SV forms,8 which may explain the non-concordant phenotype-genotype in case 32.
The new variant, IVS2-13A/C>T, was identified in two patients. The T allele was observed in heterozygous cases with the benign C allele, as previously described.19 Because the female patient presented clitoromegaly at birth, even in heterozygous cases, this finding cannot be overlooked and requires further genetic studies. The description of three cases with transient clitoromegaly in the present sample is worthy of additional study. It has been reported that some heterozygous patients had higher androgen levels, especially carriers of p.Val282Leu mutation with premature adrenarche.1,29 It is expected that NC-CAH and heterozygous patients are asymptomatic at birth, but the authors hypothesized that possible transient androgen elevations may occur. Further studies to clarify this phenomenon may use expanded genotyping by sequencing the entire CYP21A2 gene in order to exclude other mutations not detected by SNaPshot and MLPA assays. In the present sample, a better understanding of this new variant could provide more specific information.
One of the strengths of this study was that diagnoses and follow-up examinations were performed by the same pediatric endocrinologist. Therefore, the adequate clinical assessment of patients made in this study was suitable to clinical and molecular characterize this sample in CAH neonatal screening. Another strength was the fact that data was collected from a population screening program, which favored the evaluation of a large number of individuals. In this context, this study was the first neonatal screening program/genotyping using the SNaPshot methodology.19 This strategy allowed simultaneous detection of the 12 mutations most commonly associated to CAH in a fast and agile way, adding quality to the neonatal screening program. This group of point mutations represents 89% of the mutations detected in this cohort from Southern Brazil.19
In conclusion, the results of this study underscore the effectiveness of the screening program in detecting CAH cases and excluding suspicious cases based on 17-OHP level cutoffs linked to birth weight stratification. In addition, the second sample collection, followed by genotyping of suspected samples, helped to properly diagnose severe SW/SV-CAH cases and milder SV cases, as well as to differentiate between C-CAH cases and false positive patients. The present results also indicated that genotyping is a valuable and complementary diagnostic tool for neonatal screening, which provides information on disease severity, allows genetic counseling in severe cases, and avoids over-treating late onset NC-CAH and false positive patients.
FundingThis work was supported by grants from Brazilian National Institute of Hormones and Womens Health, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq INCT 573747/2008-3), Brazilian National Public Health System (PPSUS) and FAPERGS, Porto Alegre, Brazil.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Kopacek C, Prado MJ, da Silva CM, de Castro SM, Beltrão LA, Vargas PR, et al. Clinical and molecular profile of newborns with confirmed or suspicious congenital adrenal hyperplasia detected after a public screening program implementation. J Pediatr (Rio J). 2019;95:282–90.
Study conducted at the Serviço de Referência em Triagem Neonatal (reference center for neonatal screening from the Public Health System) at the Hospital Materno Infantil Presidente Vargas and at the Centro de Desenvolvimento Científico e Tecnológico (CDCT), Porto Alegre, RS, Brazil.