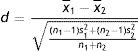Duchenne muscular dystrophy, an X-linked genetic disease, leads to progressive muscle weakness mainly in the lower limbs. Motor function tests help to monitor disease progression. Can low-cost, simple assessments help in the diagnostic suspicion of Duchenne muscular dystrophy? The authors aim to define the sensitivity of time to rise from the floor, time to walk 10meters, and time to run 10meters, evaluating them as eventual diagnostic screening tools.
MethodsThis is an analytical, observational, retrospective (1998–2015), and prospective study (2015–2018). Cases were recruited from the database of the pediatric neurology department and the healthy, from child care consultations, with normal gait development (up to 15 months) and without other comorbidities (neuromuscular, pulmonary, heart diseases) from the same university hospital.
Results128 Duchenne muscular dystrophy patients and 344 healthy children were analyzed, equally distributed in age groups. In Duchenne muscular dystrophy, there is a progressive increase in the means of the times to perform the motor tests according to the age group, which accelerates very abruptly after 7 years of age. Healthy children acquire maximum motor capacity at 6 years and stabilize their times. The time to rise showed a p-value <0.05 and a strong association (effect size [ES] >0.8) in all age groups (except at 12 years), with time to walk 10 meters from 9 years, and with time to run 10 meters , from 5 years. The 100% sensitivity points were defined as follows: time to rise, at 2s; time to walk 10 meters, 5s; time to run 10 meters, 4s.
ConclusionsTime to rise is a useful and simple tool in the screening of neuromuscular disorders such as Duchenne muscular dystrophy, a previously incurable disease with new perspectives for treatment.
A distrofia muscular de Duchenne, doença genética ligada ao X, determina fraqueza muscular progressiva principalmente em membros inferiores. Os testes de função motora ajudam a monitorar a progressão da doença. Avaliações simples de baixo custo podem ajudar na suspeita diagnóstica da distrofia muscular de Duchenne? Objetivamos definir a sensibilidade do tempo levantar, tempo andar 10 metros e tempo correr 10 metros, avaliando-os como eventuais ferramentas de triagem diagnóstica.
MétodosEstudo analítico, observacional, retrospectivo (1998 até 2015) e prospectivo (2015 até 2018). Os casos foram recrutados do banco de dados do serviço de neurologia infantil e os saudáveis, de consultas de puericultura, com desenvolvimento de marcha normal (até os 15 meses) e sem outras comorbidades (neuromusculares, pneumopatias, cardiopatias), do mesmo hospital universitário.
ResultadosForam analisados 128 pacientes com distrofia muscular de Duchenne e 344 saudáveis, distribuídos igualmente em faixas etárias. Na distrofia muscular de Duchenne ocorre aumento progressivo das médias dos tempos para realizar as provas motoras, de forma acentuada a partir dos 7 anos. Os saudáveis estabilizam os tempos a partir dos 6 anos, adquirindo capacidade motora máxima. O tempo de levantar apresentou p-valor <0,05 e forte associação (TE >0,8) em todas as faixas etárias (exceto aos 12 anos), tempo de andar 10 metros a partir de 9 anos e o tempo de correr 10 metros, dos 5 anos. Os pontos de 100% sensibilidade foram definidos: tempo de levantar aos 2 segundos; tempo de andar, 5 segundos e tempo de correr 10 metros, 4 segundos.
ConclusõesO tempo de levantar é útil e simples na triagem de doenças neuromusculares como a distrofia muscular de Duchenne, doença antes incurável com novas perspectivas de tratamento.
Duchenne muscular dystrophy (DMD) is the second most common genetic disease1 with an incidence of 1 for every 3500–5000 male newborns.2 The gene at the Xp21.2 locus encodes the dystrophin protein3 and defines its X-linked recessive inheritance.
The first clinical manifestations of DMD are neuropsychomotor developmental delay and/or motor difficulties after the second year of life (stage 1).4–6 Pelvic girdle weakness appears between 2 and 4 years of age, as does difficulty in running, climbing stairs, jumping, walking on tiptoe, and later, as frequent falls (stage 2). Early on, the myopathic rise (Gowers’ sign), calf hypertrophy, and digitigrade gait with pelvic tilt are observed. As the disease progresses, other muscle groups are gradually involved, with increased motor limitations (stage 3–5).6
An early diagnosis is essential to institute measures that can attenuate the natural course of the disease and prevent new cases through genetic counseling. Early diagnosis is only possible through symptom recognition, screening test request (serum creatine phosphokinase measurement, commonly above 10,000IU/L), and referral to specialists for definitive diagnosis through molecular analysis and/or muscle biopsy with immunohistochemistry.7
Motor measurements have been used to follow the same individual over time, obtaining objective evolution parameters, aiding in the perception of progression or stability of their disorders, helping to reassess clinical conducts and being used as outcome measures in clinical trials. Standardized measures of time while performing motor functions are recommended in the follow-up of these patients in the national and international consensus: the 6-minute walk test (6MWT), time to walk/run 10meters (10MWT and 10MRT), time to rise (TR) from the floor, stand up from a chair test, and time to climb 4-steps test.6–11
Few studies have evaluated the TR separately, but its role as a predictor of disease progression has gained prominence.12 The 10MWT test is easily performed, as it requires less distance to be covered and has less environmental influence when compared to the 6MWT. Moreover, it can be performed both in more advanced neuromuscular disease, closer to gait loss, and in the earlier stages of disease, with less motor impairment.13 However, there are few data by age group for the 10MWT test; sometimes, only follow-up data from longitudinal studies for 36 months14 up to 48 months,15 or individual measures by age, are available.13 The 10MRT evaluates the maximum motor capacity of the timed tests and may be used as an auxiliary tool.
The authors aimed to verify the motor performance of children with DMD, stratified by age, based on the values of the standard motor tests of typical Brazilian children (TR, 10MWT, and 10MRT, previously published16). This study also defined the sensitivity and specificity of these tests, evaluating them as eventual tools for DMD diagnostic screening.
MethodsAn analytical, observational, retrospective (data collected between 1998 and 2015) and prospective study (April 2015–2018) was conducted of the motor function of children with DMD treated at the Child Neurology Service of Instituto de Puericultura e Pediatria Martagão Gesteira (IPPMG) of Universidade Federal do Rio de Janeiro (UFRJ). The motor function was compared to that of typical children (without neuromuscular disease), stratified by age, from 2 to 12 years of age. The study was approved by the Research Ethics Committee (CEP) of IPPMG.
The database of patients with DMD included 137 patients who were followed or were being followed at the service until the beginning of the prospective study. There were 19 new patients with confirmed diagnosis included, totaling a sample of 156 patients.
It is important to emphasize that the routine of the neuropediatric service includes the performance of the standardized motor tests in the evaluation of patients with neuromuscular disease, which is carried out during the consultations by a trained and skilled team. All patients are advised to follow the current recommendations for DMD (corticotherapy and physical therapy).
When information was discordant, the medical records were reviewed for ratification. A prospective study with a serial definition of time of follow-up was not performed. The aim was to obtain motor data during outpatient visits (which follow the annual or semi-annual scheduling). In case of extra consultations due to complications or other demands, the motor data were also included.
The sample consisted of 345 children treated at the IPPMG general pediatric outpatient clinic, aged between 2 and 12 years, collected from July 2011 to 2012.16
The initial age of 2 years was chosen for the evaluation, which is reasonable and early enough for the diagnosis of possible neuromuscular disease and a few months after the development of this important motor milestone.17 An age limit of 12 years was established, which is the maximum age for being treated in the IPPMG service.
The inclusion criteria for the group with DMD were children who were followed and had been diagnosed with DMD (molecular test or muscle biopsy and/or positive family history). The group of typical children included those who were seen at the general pediatrics consultation from July 2011 to 2012, aged 2–12 years, and with normal gait development (beginning at up to 15 months).
The exclusion criteria in the DMD group included diagnosis of other neuromuscular diseases, acute pneumopathy or heart disease in the last six months, and refusal to participate in the study. In the typical group, in addition to delayed gait, orthopedic and rheumatological diseases; moderate/severe cognitive deficit; other chronic diseases, and those already listed above.
Description of study variablesTime to rise (TR) from the floor: how long the child took to stand up was timed, from the sitting position with crossed legs (the lotus position) until the orthostatic position was attained, without lateral support or any other impulse help.
Time to walk 10meters (10MWT): how long the child took to walk a pre-established distance of 10meters using normal steps in the upright position on flat ground, without lateral support or any other help, was timed.
Time to run 10meters (10MRT): how long the child took to run a pre-established distance of 10meters was timed.
The motor tests were explained and demonstrated prior to test performance. The children performed the tests barefoot, wearing light clothing, in a quiet environment with adequate temperature and using a stopwatch. Simple non-standard, stimulating verbal commands were used to encourage better motor response.
When an error occurred regarding the understanding or performance of the task, such values were disregarded, and the motor test was repeated. Sometimes and for several different reasons (difficulties with collaboration, performance, and willingness), DMD patients were not able to perform all the motor tests, but rather used motor data from the tests that were performed and reported those that were not performed. The authors sought to consider all the collected data aiming to assist in the evaluation of disease progression.
Statistical analysisThe data were recorded in the Microsoft Office Excel software (Microsoft®, Office Excel, Version 2007, WA, USA). For DMD patients, most of the times, there was more than one motor data recording per age group, for the same motor test. It was decided to use the mean value for the corresponding age group, in comparison with the healthy ones. After the databases were completed, the data were analyzed using the IBM SPSS Statistics program (IBM SPSS Statistics for Windows, Version 21.0, NY, USA), utilizing measures of central tendency (mean, median) and dispersion (standard deviation and minimum-maximum range).
The Mann–Whitney non-parametric t-test was used to compare means of distinct population subgroups (two independent samples with small sample numbers). Student's t-test p-values <0.05 (5%) were considered statistically significant. Aiming to aggregate information to the concept of statistical significance, it was decided to calculate the effect size (ES), capable of quantifying the existing difference. Calculation of Cohen's d was then performed, according to the equation:
The criterion used in the comparative analysis was as follows: values ≥0.20 and <0.50 indicate low responsiveness; values ≥0.50 and <0.80, moderate responsiveness, and values ≥0.80, high responsiveness.
Next, the first motor function assessment of DMD patients were considered as suspected cases of the disease and compared with the typical, age-stratified children. A ROC curve was constructed for each motor test and age group. Due to the potential application of motor function tests in neuromuscular disease screening, the ideal sensitivity was defined as 100%, with the highest possible corresponding specificity. Thus, cutoff values for DMD motor tests and respective age groups were defined. All suspected cases were confirmed as cases of DMD patients, except for four patients who had other diagnoses.
ResultsOverall resultsOf the initial sample of 137 DMD files, 20 patients already showed gait loss in their first evaluation, four patients did not have confirmation of the main hypothesis of DMD, with diagnosis of other neuromuscular diseases (false positives), and two patients had duplicate records, i.e., there was a total of 111 patients in the prospective study. Of the 19 new patients, one was excluded due to severe intellectual disability and behavioral disorder, another due to age of first evaluation above 12 years. Therefore, 17 patients were included in the prospective study. The final sample consisted of 128 patients with DMD.
Moreover, since most of the times the same DMD patient contributed data to more than one age group and in the subsequent motor tests, there were 935 motor tests of patients with DMD, distributed as 405 TR, 459 10MWT, and 71 10MRT. The typical group sample comprised 344 children; a single motor evaluation was performed, with a total number of 1026 motor tests distributed as 339 TR, 344 10MWT, and 343 10MRT.
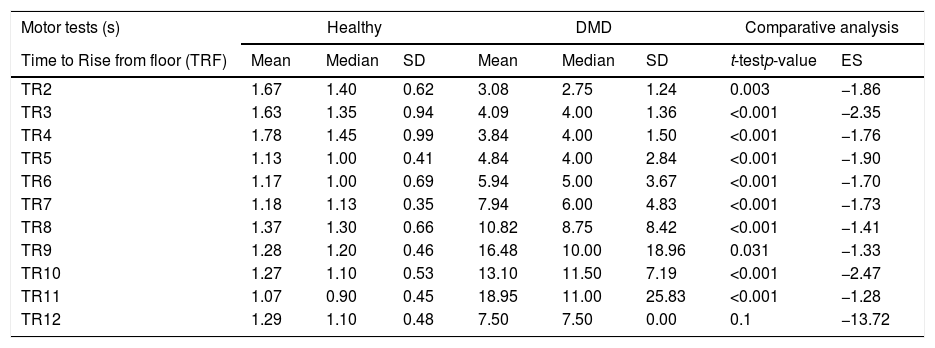
Results of motor function testsThe mean, median, and SD values of the motor tests in patients with DMD and healthy ones, in addition to the results of the comparative analysis (p-value and ES), are shown in Table 1.
Comparison of time to rise, 10meter walk test, and 10meter run test in DMD and healthy children by age range.
| Motor tests (s) | Healthy | DMD | Comparative analysis | |||||
|---|---|---|---|---|---|---|---|---|
| Time to Rise from floor (TRF) | Mean | Median | SD | Mean | Median | SD | t-testp-value | ES |
| TR2 | 1.67 | 1.40 | 0.62 | 3.08 | 2.75 | 1.24 | 0.003 | −1.86 |
| TR3 | 1.63 | 1.35 | 0.94 | 4.09 | 4.00 | 1.36 | <0.001 | −2.35 |
| TR4 | 1.78 | 1.45 | 0.99 | 3.84 | 4.00 | 1.50 | <0.001 | −1.76 |
| TR5 | 1.13 | 1.00 | 0.41 | 4.84 | 4.00 | 2.84 | <0.001 | −1.90 |
| TR6 | 1.17 | 1.00 | 0.69 | 5.94 | 5.00 | 3.67 | <0.001 | −1.70 |
| TR7 | 1.18 | 1.13 | 0.35 | 7.94 | 6.00 | 4.83 | <0.001 | −1.73 |
| TR8 | 1.37 | 1.30 | 0.66 | 10.82 | 8.75 | 8.42 | <0.001 | −1.41 |
| TR9 | 1.28 | 1.20 | 0.46 | 16.48 | 10.00 | 18.96 | 0.031 | −1.33 |
| TR10 | 1.27 | 1.10 | 0.53 | 13.10 | 11.50 | 7.19 | <0.001 | −2.47 |
| TR11 | 1.07 | 0.90 | 0.45 | 18.95 | 11.00 | 25.83 | <0.001 | −1.28 |
| TR12 | 1.29 | 1.10 | 0.48 | 7.50 | 7.50 | 0.00 | 0.1 | −13.72 |
| 10Meter walk test (10MWT) | Mean | Median | SD | Mean | Median | SD | t-testp-value | ES |
|---|---|---|---|---|---|---|---|---|
| WT2 | 13.14 | 13.60 | 2.06 | 7.25 | 7.50 | 0.96 | 0.002 | 3.12 |
| WT3 | 12.05 | 12.00 | 1.86 | 7.98 | 7.99 | 1.56 | <0.001 | 2.32 |
| WT4 | 11.07 | 10.70 | 2.39 | 9.46 | 9.43 | 2.48 | 0.016 | 0.68 |
| WT5 | 10.35 | 10.30 | 1.77 | 9.34 | 9.50 | 2.94 | 0.046 | 0.43 |
| WT6 | 10.11 | 9.75 | 2.33 | 10.05 | 9.00 | 3.21 | 0.732 | 0.02 |
| WT7 | 9.85 | 9.95 | 2.05 | 11.61 | 10.00 | 4.94 | 0.776 | −0.42 |
| WT8 | 9.95 | 9.80 | 1.84 | 10.98 | 10.00 | 3.59 | 0.302 | −0.34 |
| WT9 | 9.91 | 9.60 | 2.12 | 13.53 | 12.00 | 5.75 | <0.001 | −0.86 |
| WT10 | 10.49 | 10.30 | 1.86 | 13.68 | 12.00 | 4.64 | 0.001 | −0.86 |
| WT11 | 9.32 | 9.30 | 1.51 | 13.54 | 12.00 | 4.11 | 0.001 | −1.46 |
| WT12 | 9.42 | 9.00 | 1.63 | 16.64 | 13.00 | 8.47 | 0.002 | −1.48 |
| 10Meter run test (10MRT) | Mean | Median | SD | Mean | Median | SD | t-testp-value | ES |
|---|---|---|---|---|---|---|---|---|
| RT2 | 7.95 | 7.75 | 1.48 | 5.00 | 5.00 | 0.00 | 0.096 | 1.50 |
| RT3 | 6.89 | 7.00 | 1.10 | 6.25 | 6.25 | 1.06 | 0.313 | −0.61 |
| RT4 | 6.18 | 5.90 | 1.20 | 6.01 | 5.65 | 1.46 | 0.648 | 0.14 |
| RT5 | 5.38 | 5.20 | 0.92 | 8.38 | 8.25 | 3.20 | 0.05 | −2.40 |
| RT6 | 4.99 | 5.00 | 0.63 | 6.92 | 6.34 | 1.60 | <0.001 | −2.09 |
| RT7 | 4.86 | 4.65 | 0.61 | 8.66 | 7.81 | 3.60 | <0.001 | −2.03 |
| RT8 | 4.88 | 4.80 | 0.65 | 7.36 | 6.70 | 2.97 | 0.001 | −1.54 |
| RT9 | 4.78 | 4.70 | 0.70 | 7.63 | 8.70 | 2.75 | 0.031 | −2.80 |
| RT10 | 4.72 | 4.70 | 0.67 | 8.70 | 9.00 | 1.79 | 0.001 | −4.55 |
| RT11 | 4.63 | 4.80 | 0.81 | 8.60 | 10.00 | 2.42 | 0.008 | −3.90 |
| RT12 | 4.89 | 4.90 | 0.71 | 9.67 | 9.67 | 0.00 | 0.099 | −7.14 |
The table represents the mean of the motor tests (TR – rime to rise, TW10M – time to walk 10meters, and TR10M – time to run 10meters) of patients with DMD and healthy ones, in addition to the comparative analysis of p-value (p-value <0.05) and effect size (ES).
TR2, time to rise at 2 years; TR3, time to rise at 3 years; TR4, time to rise at 4 years; TR5, time to rise at 5 years; TR6, time to rise at 6 Years; TR7, time to rise at 7 years; TR8, time to rise at 8 years; TR9, time to rise at 9 years; TR10, time to rise at 10 years; TR11, time to rise at 11 years; TR12, time to rise at 12 years.
TW2, time to walk at 2 years; TW3, time to walk at 3 years; TW4, time to walk at 4 years; TW5, time to walk at 5 years; TW6, time to walk at 6 Years; TW7, time to walk at 7 years; TW8, time to walk at 8 years; TW9, time to walk at 9 years; TW10, time to walk at 10 years; TW11, time to walk at 11 years; TW12, time to walk at 12 years.
TR2, time to run at 2 years; TR3, time to run at 3 years; TR4, time to run at 4 years; TR5, time to run at 5 years; TR6, time to run at 6 Years; TR7, time to run at 7 years; TR8, time to run at 8 years; TR9, time to run at 9 years; TR10, time to run at 10 years; TR11, time to run at 11 years; TR12, time to run at 12 years.
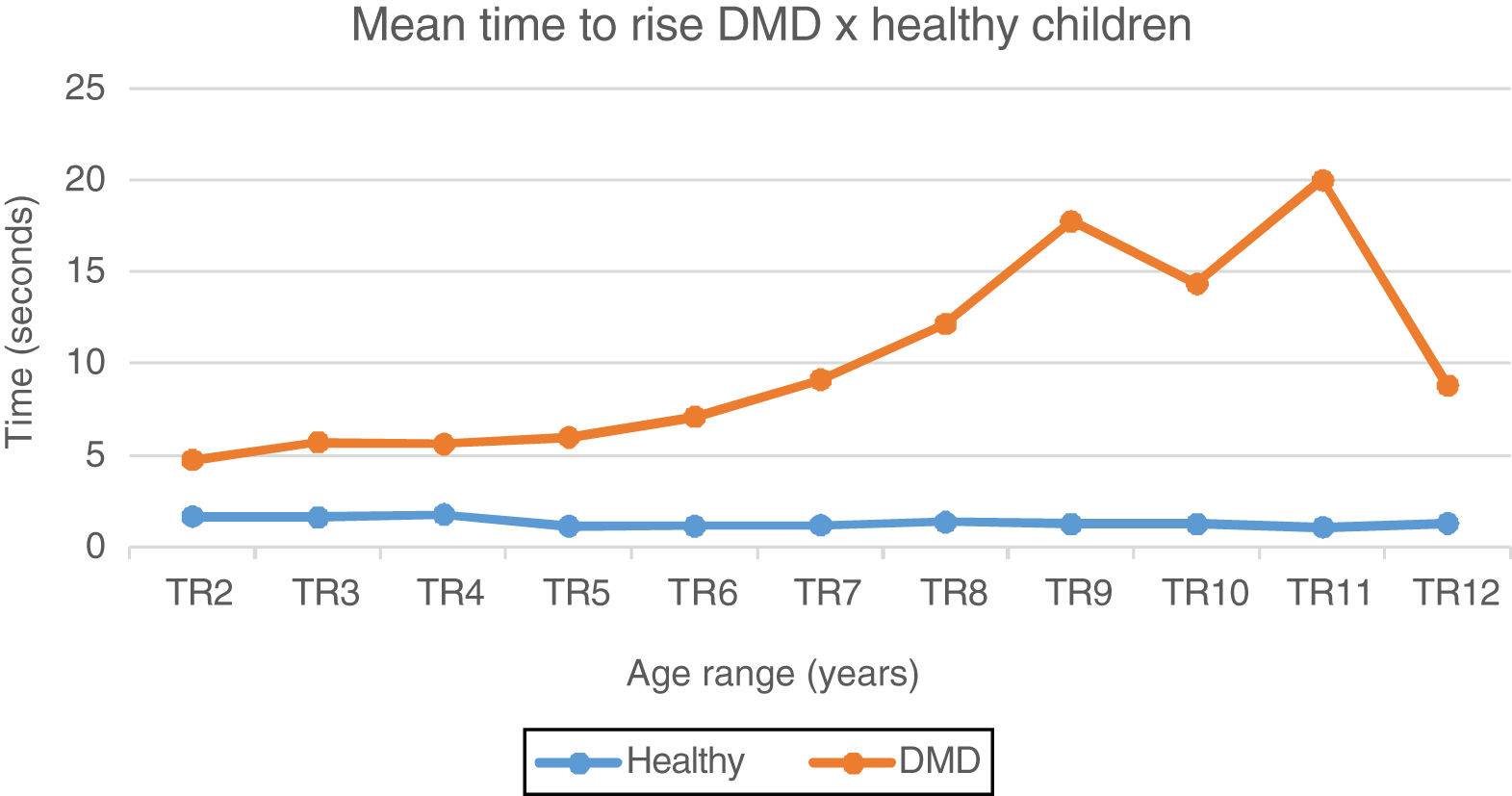
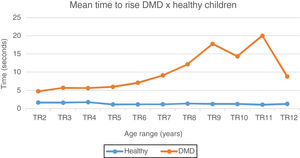
In the TR test, mean and median values of DMD patients are higher than those observed in healthy patients in all age groups (from 1.8 times at 2 years to 17.7 times at 11 years), as shown in Fig. 1.
Mean time to rise chart: DMD and healthy. Chart representation of the mean of time to rise from the ground in patients with DMD and healthy ones. TR2, time to rise at 2 years; TR3, rime to rise at 3 years; TR4, time to rise at 4 years; TR5, time to rise at 5 years; TR6, time to rise at 6 Years; TR7, time to rise at 7 years; TR8, time to rise at 8 years; TR9, time to rise at 9 years; TR10, time to rise at 10 years; TR11, time to rise at 11 years; TR12, time to rise at 12 years.
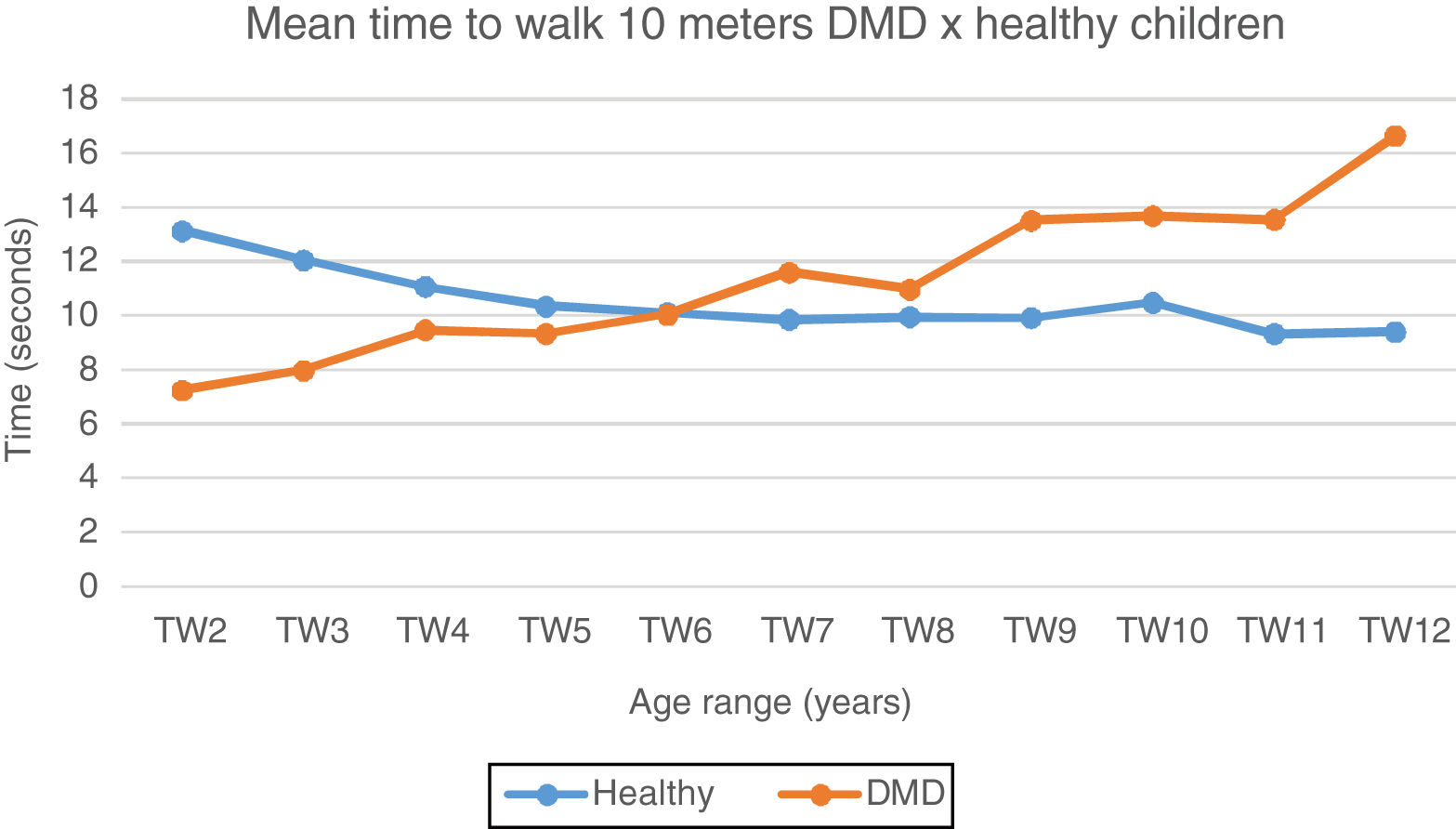
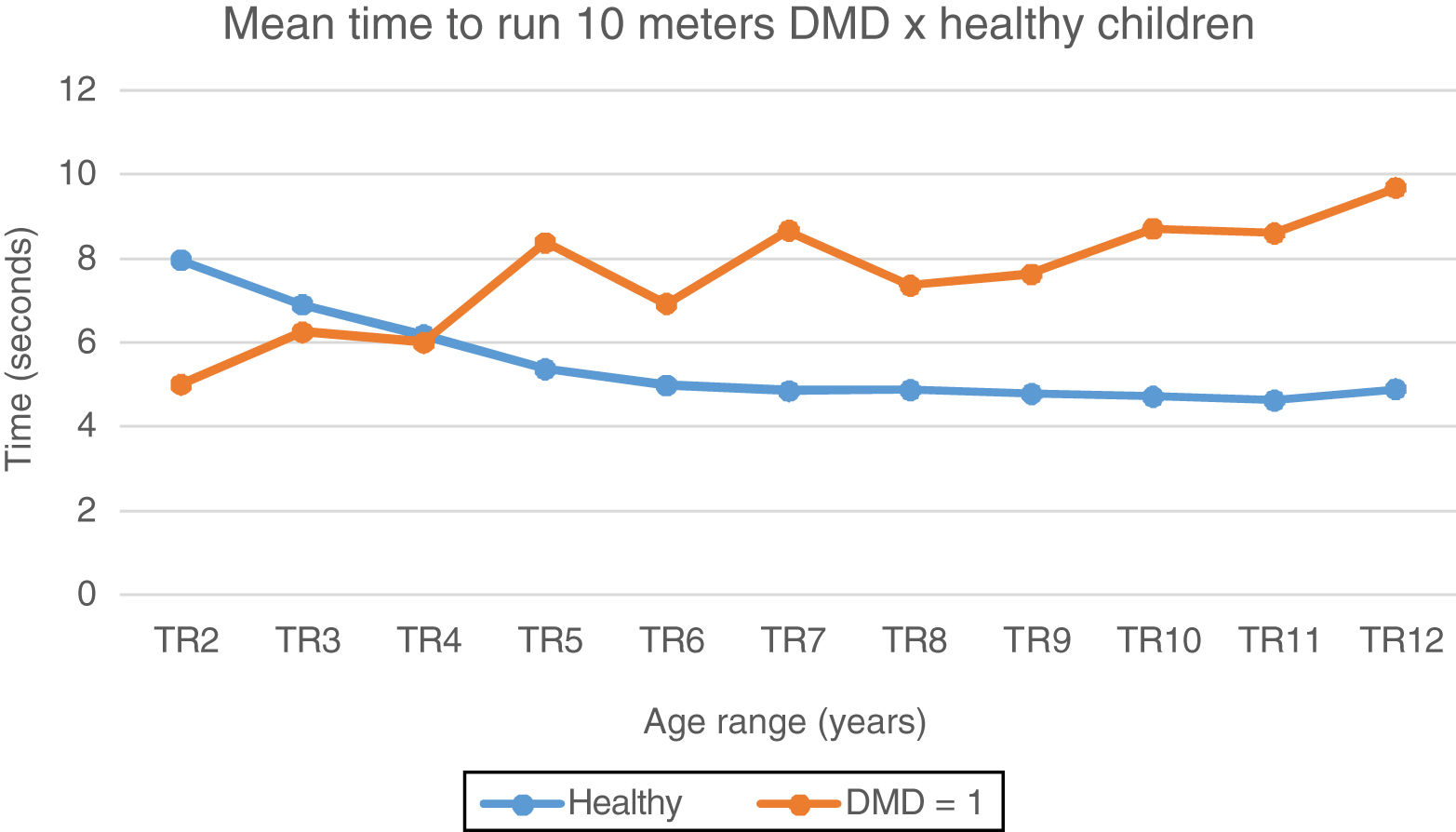
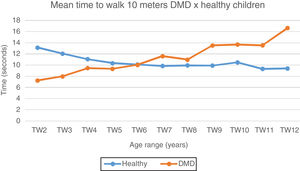
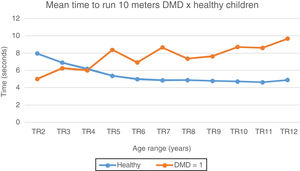
In the 10MWT, it can be observed that the mean and median values at 6 years of age show a gradual increase with subsequent gait loss, when compared to the typical children, who stabilize mean 10MWT values at around 10s (Fig. 2). In the 10MRT, this study observed a progressive pattern of worsening of the times in DMD patients from the age of four years, when compared to the typical ones (Fig. 3).
Mean time to walk 10meters: DMD and healthy. Chart representation of the mean of time to walk 10meters in patients with DMD and healthy ones. TW2, time to walk at 2 years; TW3, time to walk at 3 years; TW4, time to walk at 4 years; TW5, time to walk at 5 years; TW6, time to walk at 6 years; TW7, time to walk at 7 years; TW8, time to walk at 8 years; TW9, time to walk at 9 years; TW10, time to walk at 10 years; TW11, time to walk at 11 years; TW12, time to walk at 12 years.
Mean time to run 10meters: DMD and healthy. Chart representation of the mean of time to run 10meters in patients with DMD and healthy ones. TR2, time to run at 2 years; TR3, time to run at 3 years; TR4, time to run at 4 years; TR5, time to run at 5 years; TR6, time to run at 6 years; TR7, time to run at 7 years; TR8, time to run at 8 years; TR9, time to run at 9 years; TR10, time to run at 10 years; TR11, time to run at 11 years; TR12, time to run at 12 years.
Sensitivity was 100% for all motor function tests evaluated in all age groups, while the specificity ranged from 39% to 100% in the TR, 0 to 3% in the 10MWT, and 0 to 100% in the 10MRT.
In relation to TR, in preschool children up to the beginning of school age, the values are close to 2s (for the cutoff point for suspected DMD). For the 10MWT, cut-off values of 5s were observed in preschool and school-aged children, and in the 10MRT, the minimum cutoff value was 4s.
DiscussionThis study demonstrates that patients with DMD show worse and progressive performance in the TR, 10MWT, and 10MRT tests, which gradually worsen at the beginning of school age, culminating in the inability to perform them in early adolescence, while typical children stabilize their performance at age 6 when they acquire their maximal motor function.
The evaluated motor function tests differentiated very well the typical children from those with DMD, which constitutes an unprecedented study in the Brazilian population. This study shows a rectilinear curve in the TR from the floor test in preschoolers with DMD, without gains or losses, corresponding to stage 1 (pre-symptomatic).6 From the age of 5, the increase in time is gradual, corresponding to stage 2 of DMD,6 when Gowers’ sign appears. From six to nine years, it corresponds to a faster disease progression stage, which coincides with stage 3 (transition),6 a phase when the loss of the ability to rise from the floor and climb stairs occurs. In early adolescence, as of age 11, patients are already in stage 4,6 when a small number of individuals are still able to perform TR (n=10), with increasing test performance times and, at 12 years, only one patient was able to perform the test.
In 10MWT, from the age of six years, the performance of DMD patients worsens. From the age of nine, differences in the means regarding 10MWT performance become more and more significant, which coincides and can be justified by the end of the early ambulation stage to the beginning of the late stage, a transition period to lower-limb function loss progression.6 There is a stabilization of the values at the age of 9–11 years, which may be due to slower and stable evolutions, but culminated at 12 years with double the time to perform the 10MWT, with most patients already showing lack of ambulation. Therefore, at the end of school age and early adolescence, many have already lost the ability to perform the TR and a percentage of them starts having difficulty or is already unable to walk.
In the 10MRT, from the age of four, patients with DMD are slower than typical children. There were two peaks of increase in well-defined age groups (5 and 7 years), compatible with the pattern of the transition from stage 2 of early ambulation to a stage 3 late ambulation phase, and, according to the age limit of seven years, we can still observe improvements in the performance, as already reported for the 6MWT.15 Since this test requires greater speed and explosive muscle use, making compensatory mechanisms less impossible, this performance variability between individuals is more noticeable, especially in the 5- to 7-year age groups. Afterwards, progression shows a linear worsening, which follows the evolution pattern of disease worsening corresponding to the loss of the ability to run, even before losing the ability to walk, i.e., in stage 4.
Differences found in the means of TR were more significant than those of the other walking tests (10MWT and 10MRT). The TR, which is a fast and easy applicable test, is also the earliest to guide the screening of suspected cases of DMD. Additionally, it may play a role in predicting disease progression, as shown in the 2016 Italian study.12
The worsening of motor tests (6MWT and TR) along the years had already been described, as observed the present data, in a longitudinal study of 24 months,18 with more evident values for boys over 7 years of age. The same authors demonstrated a high correlation of TR with the 6MWT (r=0.6, p<0.01), confirming that TR is the earliest prognostic factor for the progression of DMD and, consequently, gait loss.12
Moreover, in a 12-month longitudinal study,19 progressive worsening was demonstrated starting at the age of 6 years. The current study also shows a gradual increase in the 10MWT values, but with a faster progression in the Brazilian patients.
In previous studies evaluating the 6MWT and in the North Star Ambulatory Assessment (NSAA) in patients with DMD, the progression described (12, 24, and 36 months)14,18,19 is similar to that of the present study.
A group of United States researchers15 analyzed the 6MWT and other timed motor function tests in a 48-week multicenter study. In the TR, although the rising posture was different, values were close to those observed in the present study. However, the Brazilian 10MWT/10MRT of the present study showed worse performance than the American ones.
The rate of progression is not linear. This can be observed in the 10MWT data, where the highest rate of progression occurs at the beginning of the second decade of life. Other authors, using data from the patients of previous studies,15,19–21 have developed models of DMD evolution, with potential improvement of the 6MWT up to 10 years of age, but with a subsequent rapid decline in performance.
There have been few studies that described 10MWT or TR by age group. A database22 of 240 boys with DMD, aged 4–12 years, describes that TR remains in the range of 4–6s in the first six years and then reaches 6–8s, similar to the current data. Regarding the means of the 10MWT/10MRT, the abovementioned study showed mean values of 6–8s until 7 years of age, with a few oscillations and followed by a subsequent increase up to 8–10s. In the present patients, from the age of 4 years, mean 10MWT times over 8s were observed, i.e., worse performance in the preschool stage. It must be noted that the present study is a pioneer in assessing TR at preschool age.
There have been few comparative studies prior to present work, and the pre-existing studies had smaller samples. However, they suggested a worse performance of DMD patients when compared to typical children, in schoolchildren for TR and 10MWT,22 as well as for 6MWT.23
A limitation of the present study was the number of data for each motor test and the lower number of 10MRT data in DMD. This is justified by the greater technical difficulty for patients with muscle weakness in performing a test of their maximal motor function, which requires physical effort and predisposes to fatigue, but replicates what occurs in the follow-up of these patients. In the group of typical children, the number of data was more homogeneous according to the age groups and motor tests, with a reduction in the extremes of age (2 and 12 years), which also occurs even more broadly in DMD, due to the worsening progression in adolescence. The division by age group forms subgroups that, although allowing a better analysis, reduce the distribution of the sample number by age group, which was nevertheless satisfactory in the present study.
In fact, the absolute times of Brazilian patients with DMD are higher when compared to American and European studies. The late onset of corticosteroid therapy that would be directly related to the disease progression stage and muscle destruction, with replacement of the muscle by adipose tissue and fibrosis,24 factors of inter-individual variability and genetic modifiers,25 may justify this finding.
For early diagnostic screening, the following cut-off points for motor function tests were defined: for TR, 2s; for 10MWT, 5s; and for 10MRT, 4s. These parameters (emphasis on TR of 2s, as it is the earliest discriminator) are easy-to-perform tests, performed by the pediatrician in an outpatient and home environment, with very low costs, which may help in the suspected new cases of DMD at the initial stages. The recommendation of complementary tests for the families that have an index case can be performed at birth. The TR test can be performed during the routine child-care consultation from the age of two, with a mandatory screening test for neuromuscular diseases.
It is only possible to reduce the time to diagnosis if we increase the suspicion of DMD, with strategies that can effectively act in the pre-symptomatic stages or in the detection of early symptoms. Unfortunately, the mean age of a definitive diagnosis in Brazil is 7.5 years,4 very close to the mean age of gait loss, whereas in most countries in the world, it is around 4–5 years.26 In short, the proposal is to use tools that can help shorten the time to diagnosis and, additionally, provide better follow-up of patients with DMD.
In normal children, there is an improvement in motor function performance up to 6 years, with subsequent stabilization of the tests until adolescence. In boys with DMD, the mean times of these tests increase, particularly from the age of 7, culminating with the inability to perform motor tests at the end of the school age and start of adolescence.
The fast performance, low cost, and simple technique show the assessed motor tests can be used as diagnostic tools for the screening of neuromuscular diseases when used in motor development monitoring in childcare. The TR is an excellent tool, capable of shortening the diagnosis of DMD, a fact of utmost importance for the new perspectives of specific therapies.
Conflicts of interestThe authors declare no conflicts of interest.
To the statistician Ronir Raggio Luiz and his assistant Jéssica Pronestino. To the students of the PINC (Scientific Initiation Project): Bruna Maciel, Luisa Vieira, Monique Minini Lima, and Yuri Devaud. To all the children and their parents/guardians who participated in this study for their helpfulness and solidarity, by making themselves available to aid this scientific research, knowing that its main objective was to shorten the diagnosis of Duchenne muscular dystrophy. To all family members and children with DMD, as an example of perseverance and optimism.
Please cite this article as: Pereira AC, Araújo AP, Ribeiro MG. Can simple and low-cost motor function assessments help in the diagnostic suspicion of Duchenne muscular dystrophy? J Pediatr (Rio J). 2020;96:503–10.
Study conducted at Universidade Federal do Rio de Janeiro (UFRJ), Instituto de Pediatria e Puericultura Martagão Gesteira (IPPMG), Departamento de Pediatria, Ambulatório de Neurologia Infantil, Rio de Janeiro, RJ, Brazil.