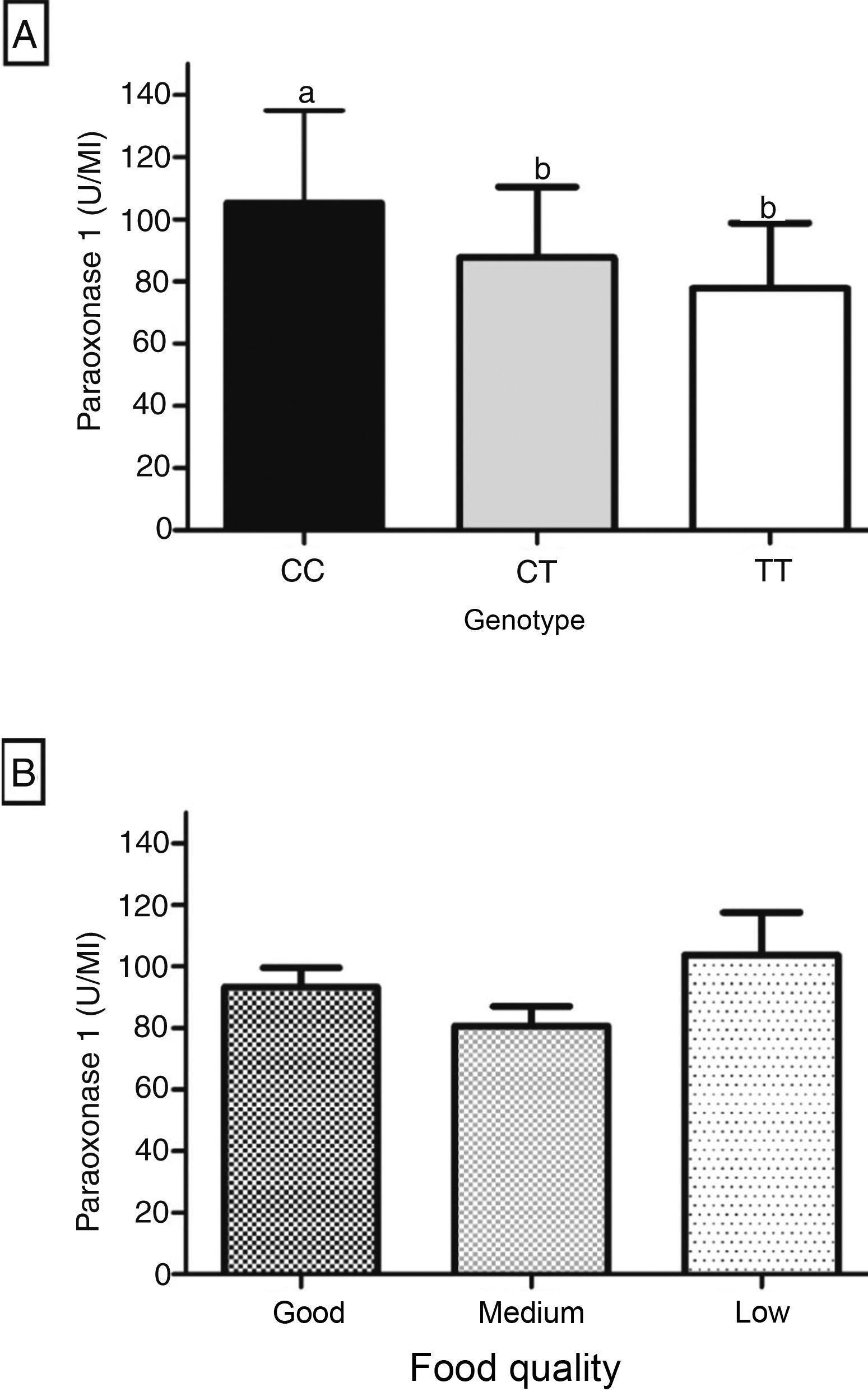
The cardioprotective enzyme paraoxonase-1 (PON1) suffers an important influence from genetic polymorphisms and nutritional factors. The aim of this study was to investigate the influence of diet, nutritional status, and the C(-107)T polymorphism on PON1 arylesterase activity in children.
MethodsThis was a cross-sectional study with 97 children, aged between 5 and 8 years, of both genders, from a pediatric outpatient clinic in southern Brazil. A sociodemographic, behavioral, and food consumption questionnaire was applied, and anthropometric measurements and laboratory blood samples were taken. PON1 arylesterase activity was measured by phenol extinction (U/mL), and DNA extraction and analysis of the PON1 C(-107)T polymorphism were performed. The Hardy–Weinberg equilibrium was tested with the chi-squared test and linear regression was used to estimate PON1 activity according to four adjustment models, with an acceptable error of 5%.
ResultsIn the sample, the male gender accounted for 50.5%, 39.2% were 6 years of age, 54.5% had normal weight, and 51.5% had PON1 activity below the median (90.0, 15–30U/mL). Genotype frequency was 54.6% (53/97), 31.0% (30/97), and 14.4% (14/97), respectively, for CT, CC, and TT, consistent with the Hardy–Weinberg equilibrium (p=0.22). In the regression analysis, the model that included sociodemographic variables as well as frequency of consumption of fruits, vegetables, legumes, dairy products, and beans estimated a variability of 14.8% in PON1 activity combined with the PON1 C(-107)T polymorphism.
ConclusionsDuring childhood, a good-quality diet with greater inclusion of healthy foods was important to predict the activity of the cardioprotective enzyme PON1 combined with the C(-107)T polymorphism of the PON1 gene.
A enzima cardioprotetora Paraoxonase 1 (PON1) sofre importante influência de polimorfismos genéticos e fatores nutricionais. O objetivo deste estudo foi investigar a influência da alimentação, do estado nutricional e do polimorfismo C(-107)T sobre a atividade arilesterase da PON1 em crianças.
MétodosEstudo transversal com 97 crianças entre 5 e 8 anos, de ambos os sexos, de um ambulatório de pediatria no sul do Brasil. Realizou-se questionário sociodemográfico, de comportamento e de consumo alimentar, medidas antropométricas e coleta de sangue em laboratório. A atividade arilesterase da PON1 foi mensurada pela extinção de fenol (U/mL), realizada extração do DNA e análise do polimorfismo PON1 C(-107)T. O equilíbrio de Hardy-Weinberg foi testado com qui-quadrado e usada regressão linear para estimar a atividade da PON1 segundo quatro modelos de ajuste, erro aceitável de 5%.
ResultadosNa amostra o sexo masculino representou 50,5%, 39,2% tinham 6 anos, 54,5% eram eutróficos e 51,5% tinha atividade da PON1 inferior à mediana (90,0;15-30U/ml). A frequência dos genótipos foi 54,6% (53/97), 31,0% (30/97) e 14,4% (14/97), respectivamente, para CT, CC e TT, estiveram em equilíbrio de Hardy-Weinberg (p=0,22). Na análise de regressão o modelo que incluiu variáveis sociodemográficas, de frequência do consumo de frutas, verduras, legumes, laticínios e feijões estimou uma variabilidade de 14,8% na atividade da PON1 combinada ao polimorfismo PON1 C(-107)T.
ConclusõesNa infância uma alimentação de boa qualidade, com maior participação de alimentos saudáveis foi importante para predizer a atividade da enzima cardioprotetora PON1 combinada ao polimorfismo C(-107)T do gene da PON1.
Paraoxonase-1 (PON1) is a 43kDa enzyme expressed in humans, especially in the liver, detected in plasma and linked to the apoproteins A1 and J of high-density lipoprotein (HDL) particles.1 The enzyme prevents oxidative damage to lipoprotein phospholipids, especially low-density lipoprotein (LDL).1,2 Therefore, PON1 prevents the proinflammatory effect of lipoperoxides in the intima layer of blood vessels, and its most important activity results in the reduction in the risk of cardiovascular disease (CVD).2,3 This enzyme hydrolyzes a broad spectrum of substrates, but its arylesterase (phenylacetate) activity is especially important in the reduction of endothelial damage and CVD.1–3 PON1 suffers an important influence from genetic polymorphisms and nutritional factors.1,3,4
The PON1 gene is located on human chromosome 7, has nine exons, and encodes a protein with 354 amino acids.1,5 More than 200 polymorphisms have been described in this gene.1,3 However, the polymorphism characterized in the promoter region PON1 C(-107)T exerts a significant effect on the enzyme, being a strong predictor of its arylesterase activity.5,6 The -107C allele provides PON1 levels up to two-fold higher than those observed with the -107T allele.5,6 There is evidence that this polymorphism in the PON1 gene promoter is located at the binding point of the transcription factor Sp1.5,6 Therefore, the presence of the C allele results in greater expression of the PON1 gene and higher serum enzyme activity.5,6
Among the nutritional factors, monounsaturated fatty acids and antioxidant vitamins, such as vitamins C and E, seem to be responsible for the increased activity of PON1.7–10 Similarly, a higher consumption of fruits and vegetables has been associated with a higher enzyme activity.9,10 On the other hand, studies indicate that excess weight results in lower PON1 activity, including during childhood.11–13 Childhood is a vulnerable period of life but, currently, children are exposed to multiple cardiovascular and metabolic risk factors.14 It is known that diet of Brazilian children is low in fruits and vegetables, and is rich in sugary drinks, cream-filled cookies, hamburgers, snacks, and processed meats.15 Moreover, the significant increase in the prevalence of overweight from the age of 5 years is directly correlated with the increase in associated morbidities in the pediatric population.15,16 Poor diet and excess weight predispose to the accumulation of reactive oxygen species, oxidative damage to biological membranes, and the onset of diseases, particularly CVD.11,12,16
In this context, the aim of this study was to investigate the influence of diet, nutritional status, and the C(-107)T genetic polymorphism on the arylesterase activity of the antiatherogenic PON1 enzyme in childhood.
MethodsSampleA cross-sectional study was performed with children aged between 5 and 8 years of age, of both genders, attending the Pediatric Outpatient Clinic of the Faculdade de Medicina da Universidade Federal de Pelotas (UFPEL), in the city of Pelotas, state of Rio Grande do Sul, Brazil. The research protocol was approved by the Research Ethics Committee of the Faculdade de Medicina da UFPEL (504,362/2013).
Children diagnosed with liver disease, cerebral palsy, bone dysplasia or neoplasms, and those with special needs (physical or motor) and genetic alterations, such as Down syndrome and thalassemia, were excluded from the study. The parents/guardians of the eligible children were duly clarified and invited to participate, and those who authorized their children's participation in the study signed the informed consent. The child's assent was obtained orally before the evaluations started and their decision was respected.
There were 227 children interviewed, of whom 193 met the inclusion criteria; there were five refusals (2.6%). Ninety-one children (49.7%) did not complete the study protocol and were characterized as losses. Non-attendance at the laboratory for blood collection (40.0%) was the main cause of these losses. In the end, 97 children participated in the study.
Sociodemographic, anthropometric, and blood collection dataThe parents/guardians answered a sociodemographic, behavioral, and food consumption questionnaire about the children, anthropometric measurements were obtained, and blood sample collection was requested in the clinical analysis laboratory.
The first two variables categories of the questionnaire were family income (in Brazilian reais (R$), converted into current minimum wages), mother's age (full years), maternal schooling (full years of study), number of people in the household, and screen time (in hours) (at the computer, cell phone, tablet, video games, television). Skin color was observed and classified as white or non-white.
Weight and height were collected using a digital platform scale (Welmy®, Welmy balanças, SP, Brazil), with a capacity of 150kg and accuracy of 100g, with a coupled stadiometer with a capacity of 200cm and accuracy of 0.5cm. To evaluate the nutritional status, the Body Mass Index (BMI) z-score for age was used, according to the World Health Organization's 2007 recommendation,17 using AnthroPlus software (WHO, Anthro Plus, World Health Organization). Children with BMI-for-age>+1SD were classified as overweight. Waist circumference was measured on the waist line, at midpoint between the last rib and the iliac crest. The reference used for comparison was proposed by Freedman et al.18
After a 12-h fast, a 5-mL blood sample was collected, aliquoted and frozen at −80°C for further analysis.
Determination of PON1 arylesterase activityPON1 arylesterase activity was measured in serum samples using phenylacetate as substrate. The enzymatic activity was calculated from the rate of phenol formation by increasing the absorbance at 270nm, at a temperature of 25°C, in a spectrophotometer (FEMTO®, SP, Brazil). The samples were diluted at 1:3 in 20mM of Tris–HCl Buffer (Sigma Chemical Co. MO, USA), pH of 8.0, containing 1mM of CaCl2 (Vetec Chemical Co., RJ, Brazil). The reagent solution consisted of the buffer, to which 1mM of phenylacetate (Sigma Chemical Co., MO, USA) was added. The reaction was determined after 20s of retention and the absorbance was measured for 80s. The authors considered a unit of PON1 arylesterase activity equal to 1μM of phenol/minute and this was expressed in U/mL, based on the phenol extinction coefficient.16 The analyses were performed in duplicate and blank samples containing deionized water were used to correct the non-enzymatic hydrolysis.
DNA extraction and PON1 C(-107)T polymorphism analysisThe DNA was extracted from blood samples containing EDTA, according to the standard procedure previously described by Kanai et al.19 and quantified by a spectrophotometer. DNA samples were diluted to a concentration of 50ng/mL. The genotyping of individuals for the PON1 C(-107)T polymorphism was performed using the restriction fragment length polymorphism (RFLP) technique, as validated by Campo et al.20
Briefly, a 240-bp fragment from the PON1 gene promoter region was amplified by PCR for 35 cycles (5min at 94°C, followed by 45s at 94°C (denaturation), 45s at 67°C (annealing), and 45s at 72°C (extension), followed by 5min at 72°C. For that, the following primers were used: 5′AGCTAGCTGCGGACCCGGCGGGGAGGaG3′ forward and 5′GGCTGCAGCCCTCACCACAACCC3′ reverse. The lowercase letter on the forward primer indicates a pairing error made on purpose introduced in the amplicon to generate a restriction site for the BsrBI enzyme, as there is no specific restriction site for cutting the original DNA sequence. Digestion was performed by incubating the PCR sample with 3IU of the BsrBI enzyme (New England Bio Labs – Cambridge, United Kingdom) for 2h at 37°C. After digestion, the DNA fragments were separated by 3% agarose gel electrophoresis (Kasvi – PR, Brazil) and stained with SYBR Safe (Applied Biosystems, CA, USA). The C allele was identified by the presence of the 28 and 212bp fragments, whereas the T allele resulted in an undigested 240bp fragment.
Index of Food QualityThe Index of Food Quality (IFQ) was created based on the Schoolchildren Diet Index (ALES, from the Portuguese Índice de Alimentação do Escolar).21,22 However, the question about the place where meals were taken replaced the original question, which investigated fish consumption. The IFQ was based on: (1) the identification of children's behavioral components, specifically the habit of having breakfast and having the meals at the table; (2) the frequency of food consumption.
At each specific frequency, a positive or negative score (supplementary material) was assigned. For high-quality nutritional foods such as fruits, vegetables, legumes, beans, and milk, a point was added when they were consumed daily. In the case of consumption less than seven times a week (two to four times, depending on the food), a point was subtracted. For low-nutritional quality foods, such as candy and sweets, cookies, soda, snacks, fried foods, mayonnaise, instant noodles, and hamburgers, a point was added when they were not consumed or were rarely consumed. Additionally, a point was subtracted for the daily frequency of these foods. The IFQ maximum score was 15 points. The distribution of the total scores in tertiles originated three dietary quality categories: low (score ≤3 – p25), medium (score=4–6 – p50), and good (score ≥7 – p75).
Statistical analysisThe data was entered in Microsoft Excel 2013 and analyzed using the STATA software (Stata Statistical Software: Release 13. College Station, TX, USA). The normality of data was tested using the Shapiro–Wilk test; the variables are presented as absolute and relative frequencies, mean and standard deviation (SD), median and interquartile range (IQR) – p25–p75. The Mann–Whitney or Kruskal–Wallis U tests were used to compare scores between two or three variable categories. The chi-squared test was used to test the Hardy–Weinberg equilibrium (HWE), with the observed and expected frequencies. In the unadjusted analysis, simple linear regression was used to estimate PON1 activity according to the genotype. The adjusted analyses were performed using multiple linear regression with four different models: Model 1=included sociodemographic variables of maternal schooling, family income, number of people at the household, skin color, and gender; Model 2=included the absolute frequencies of the consumption of high nutritional quality foods, adjusted to model 1; Model 3=included the frequencies of the consumption of low nutritional quality foods, adjusted to model 2; Model 4=included the BMI z-score, adjusted to model 3. The change in the adjusted coefficient of determination (R2) was evaluated, and the significance level was set at p<0.05.
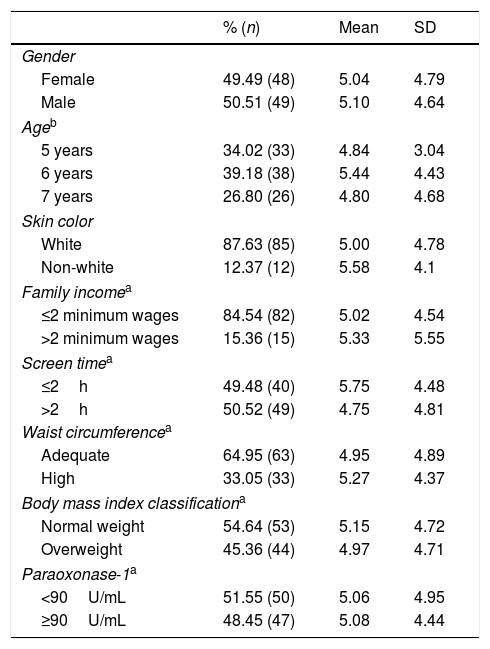
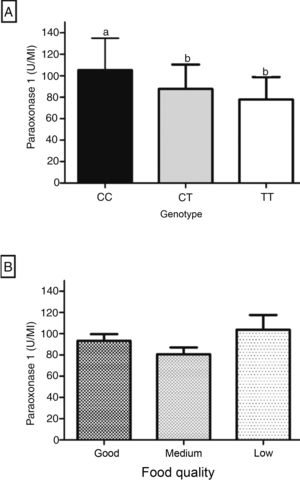
ResultsThe sample characteristics and the IFQ score are shown in Table 1. The male gender represented 50.5% (n=49) of the sample, 39.2% (n=38) were 6 years of age, most individuals – 87.6% (n=85) – were white, family income consisted of two or fewer minimum wages, and 50.5% (n=49) spent more than 2h/day in sedentary activities. Waist circumference was below the risk percentile for 65.0% (n=63) of the sample, and 55.0% (n=53) had normal weight. The PON1 arylesterase activity corresponded to a median of 90.0 (15–30)U/mL, and 51.5% (n=50) of the children had PON1 lower than this value. Regarding the IFQ, the median score was 5.0 (3.0–7.0) points and there was no significant difference between the scores according to the sample's characteristics.
Index of Food Quality score according to sociodemographic, behavioral, and anthropometric characteristics, and the level of PON1 arylesterase activity in children aged between 5 and 8 years (n=97), Pelotas-RS.
| % (n) | Mean | SD | |
|---|---|---|---|
| Gender | |||
| Female | 49.49 (48) | 5.04 | 4.79 |
| Male | 50.51 (49) | 5.10 | 4.64 |
| Ageb | |||
| 5 years | 34.02 (33) | 4.84 | 3.04 |
| 6 years | 39.18 (38) | 5.44 | 4.43 |
| 7 years | 26.80 (26) | 4.80 | 4.68 |
| Skin color | |||
| White | 87.63 (85) | 5.00 | 4.78 |
| Non-white | 12.37 (12) | 5.58 | 4.1 |
| Family incomea | |||
| ≤2 minimum wages | 84.54 (82) | 5.02 | 4.54 |
| >2 minimum wages | 15.36 (15) | 5.33 | 5.55 |
| Screen timea | |||
| ≤2h | 49.48 (40) | 5.75 | 4.48 |
| >2h | 50.52 (49) | 4.75 | 4.81 |
| Waist circumferencea | |||
| Adequate | 64.95 (63) | 4.95 | 4.89 |
| High | 33.05 (33) | 5.27 | 4.37 |
| Body mass index classificationa | |||
| Normal weight | 54.64 (53) | 5.15 | 4.72 |
| Overweight | 45.36 (44) | 4.97 | 4.71 |
| Paraoxonase-1a | |||
| <90U/mL | 51.55 (50) | 5.06 | 4.95 |
| ≥90U/mL | 48.45 (47) | 5.08 | 4.44 |
n, number of individuals; SD, standard deviation.
The genotype frequency for the PON1 C(-107)T polymorphism was 54.6% (53/97) for the CT genotype, 31.0% (30/97) for the CC genotype, and 14.4% (14/97) for the TT genotype. The genotype distribution showed that the population is in Hardy–Weinberg equilibrium (p=0.22). The allelic frequency was 58.0% for the C allele and 42.0% for the T allele.
Fig. 1 shows the influence of genotype and dietary quality on PON1. The arylesterase activity was significantly higher in individuals with the CC genotype, when compared to the CT and TT genotypes (Fig. 1A). PON1 showed an intermediate level of activity in the heterozygous genotype, but there was no significant difference when compared to the TT genotype (Fig. 1A). The enzyme showed no significant difference between the three dietary quality categories, according to the distribution of IFQ scores in tertiles (Fig. 1B).
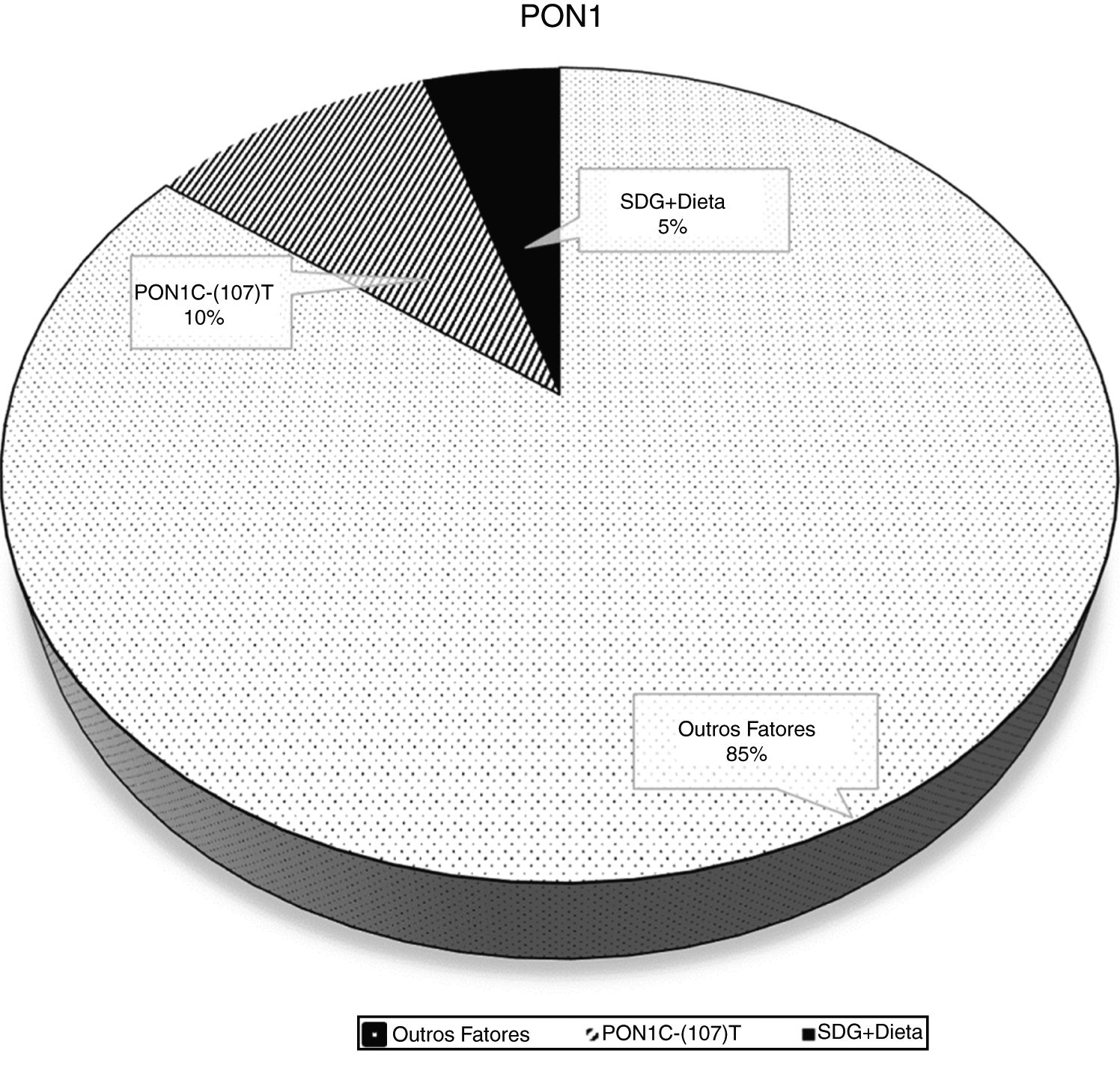
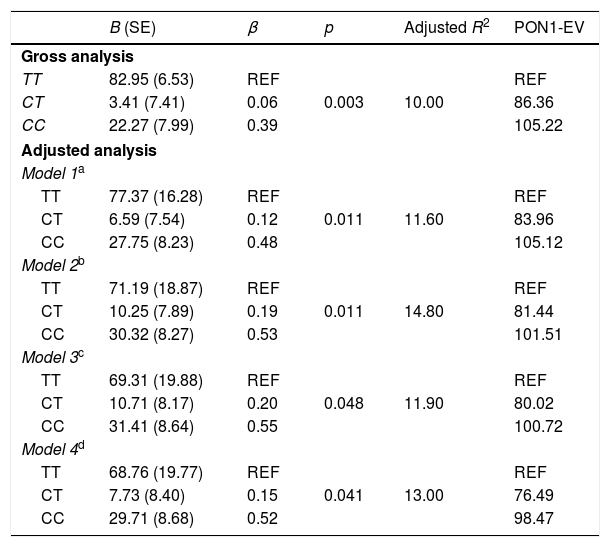
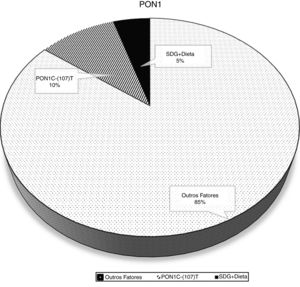
The crude and adjusted analysis of the association between PON1 activity and the PON1 C(-107)T polymorphism is shown in Table 2. The associations were highly significant in the crude analysis. When the analysis was adjusted for sociodemographic variables, food consumption, and nutritional status, the association between the enzyme and the polymorphism remained significant. In model 1 adjustment, the inclusion of sociodemographic variables resulted in an adjusted coefficient of determination of 11.6%. In model 2 adjustment, the inclusion of the frequency of healthy food consumption explained a variability of 14.8% in PON1 activity. The percentage of PON1 variation attributed to genetic and nutritional factors is shown in Fig. 2.
Gross and adjusted analysis of the association between PON1 arylesterase activity and the PON1 C(-107)T polymorphism in children aged between 5 and 8 years (n=97), Pelotas-RS.
| B (SE) | β | p | Adjusted R2 | PON1-EV | |
|---|---|---|---|---|---|
| Gross analysis | |||||
| TT | 82.95 (6.53) | REF | 0.003 | 10.00 | REF |
| CT | 3.41 (7.41) | 0.06 | 86.36 | ||
| CC | 22.27 (7.99) | 0.39 | 105.22 | ||
| Adjusted analysis | |||||
| Model 1a | |||||
| TT | 77.37 (16.28) | REF | 0.011 | 11.60 | REF |
| CT | 6.59 (7.54) | 0.12 | 83.96 | ||
| CC | 27.75 (8.23) | 0.48 | 105.12 | ||
| Model 2b | |||||
| TT | 71.19 (18.87) | REF | 0.011 | 14.80 | REF |
| CT | 10.25 (7.89) | 0.19 | 81.44 | ||
| CC | 30.32 (8.27) | 0.53 | 101.51 | ||
| Model 3c | |||||
| TT | 69.31 (19.88) | REF | 0.048 | 11.90 | REF |
| CT | 10.71 (8.17) | 0.20 | 80.02 | ||
| CC | 31.41 (8.64) | 0.55 | 100.72 | ||
| Model 4d | |||||
| TT | 68.76 (19.77) | REF | 0.041 | 13.00 | REF |
| CT | 7.73 (8.40) | 0.15 | 76.49 | ||
| CC | 29.71 (8.68) | 0.52 | 98.47 | ||
B, regression constant; SE, standard error; PON1-EV, estimated value of Paraoxonase-1; β, regression coefficient; R2, coefficient of determination.
This study showed that nutritional factors such as diet and BMI, when combined with the PON1 C(-107)T polymorphism, are predictors of PON1 arylesterase activity in childhood. In addition, they were significant to explain a 14.8% variation in the enzyme. The CC genotype and healthy foods, with good nutritional quality, were the factors that significantly influenced the higher enzymatic activity.
The Index of Food Quality in schoolchildren is a practical and appropriate tool for the Brazilian reality to monitor the dietary habits of children.21,22 A medium- and good-quality diet, as shown in the current study, does not mean nutritional adequacy. This was demonstrated by the low score achieved in the higher IFQ distribution tertiles. Molina et al.22 evaluated the dietary quality of 1282 schoolchildren and found scores that were similar to the ones that defined the children's diet in this study. Additionally, Molina et al.22 found that most schoolchildren consumed poor-quality food.
Studies suggest a dietary modulation of the enzyme and attribute this to antioxidants, such as vitamins and phenolic compounds, a protective effect on its structure and function.3,4,9 In the present study, the presence of good nutritional-quality foods, combined with the PON1C (-107)T polymorphism, positively altered PON1 activity. This result can be attributed to a higher consumption of fruits and vegetables in the diet of some the children.3,4,9 Specifically, an interventional study showed that the daily consumption of six servings of fruits and vegetables had an effect on the 7% increase in the PON1 arylesterase activity.9 Jarvik et al.10 showed that PON1 activity positively and significantly correlated with the higher intake of antioxidant vitamins, especially vitamins C and E.
Several studies have investigated the association between the enzyme and nutritional status in different phases of the life cycle.11–13,16,23–25 However, few have reported an increase in the enzyme associated with BMI, as observed in the current study.13,24,25 Divergences regarding the BMI effect on the enzyme may be due to the age of the selected individuals, since many samples include both children and adolescents. Obesity in these two phases of life courses with differences that range from the time of exposure to adipose tissue increase, to the level of proinflammatory cytokines, to hormonal status.11,23–25
A longer time of exposure to excess weight and an increase in the inflammatory status, even if it is of a low degree, may result in an inhibitory effect on the enzyme.25,26 However, in childhood, the weight increase and the formation of reactive oxygen species could be compensated by the increase in the enzyme.13 It should be noted that the complex nature of obesity limits speculation, since in addition to the aforementioned aspects, there are also the influence of lifestyle and physical activity.16,27 In particular, Koncsos et al.,27 when evaluating 151 children, found that there was an increase in PON1 after a change in lifestyle.
The PON1 C(-107)T polymorphism influenced PON1, with the CC genotype being associated with higher enzyme activity, TT with lower activity and CT with an intermediate effect. The polymorphisms in the -107C/T region have a greater effect on the arylesterase activity of the enzyme, contributing to 25% of the expression variability in Caucasian adults.5,6 These polymorphisms affect the expression of the protein and the enzymatic concentration, and the enzyme production might be affected in individuals carrying the T allele.5,6 Deakin et al.5 highlight a molecular mechanism in the C(-107)T polymorphism of the PON1 gene involving the Sp1 transcription factor. The presence of the C allele in the promoter region results in greater expression of the PON1 gene and, consequently, a higher level of protein in circulation and an increase in the enzyme activity.6 This is in agreement with the association between the C allele and higher concentration and serum activity of the enzyme, indicating that the transcription factor binds to this promoter region, but with greater affinity for the -107C variant.5,6 The -107T variant interrupts the recognition sequence of the Sp1 transcription factor, resulting in a lower concentration of PON1 for this genotype.5
The effect of the CC genotype on PON1 activity seen in the current study has already been demonstrated in other studies with children in the same age group.13,28 Particularly, Uliano et al.13 showed that children with the CC genotype for the PON1 C(-107)T polymorphism had higher serum enzyme activity than those with the TT genotype. Also, Huen et al.28 found that the PON1 activity in children with the CC genotype was higher than in those with the TT genotype. The genotype frequency observed in this study is in agreement with that of other studies.28–30 For instance, the CHAMACOS (Center for the Health Assessment of Mothers and Children of Salinas) cohort, carried out with children of the same age group, recorded a frequency similar to that found in this study.28,30
The results of the present study should be evaluated considering its strengths and limitations. Among the strengths is the selection of a sample of children at an early stage of development and free from chronic diseases, as well as the identification of a polymorphism associated with the activity of a cardioprotective enzyme. Among the limitations is the dietary assessment method, which estimates only the quality of the diet and does not estimate the amount, and therefore the frequency of consumption may have been underestimated. The number of losses at the follow-up that resulted in a reduced sample, compared to the estimated one, limits the statistical validity of the observed probabilities. However, the adjusted analyses were informative regarding the most relevant influences to predict the enzyme variability in the sample.
It was concluded that in childhood, a good quality diet with a greater participation of healthy foods was important to predict the activity of the cardioprotective enzyme PON1, combined with the C(-107)T polymorphism of the PON1 gene. This result stimulates the advice toward the achievement of food consumption goals, such as fruits and vegetables in childhood, especially regarding the perspective of preventing early CVD.
FundingCAPES.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Sigales TS, Uliano G, Muniz L, Barros C, Schneider A, Valle SC. Influence of nutritional factors and the PON1 C(-107)T polymorphism on paraoxonase-1 activity in childhood. J Pediatr (Rio J). 2020;96:495–502.