To assess clinical and laboratory data, and acute kidney injury (AKI) in HIV-infected children using and not using highly active antiretroviral therapy (HAART) prior to admission.
MethodsA retrospective study was conducted with HIV-infected pediatric patients (<16 years). Children who were using and not using HAART prior to admission were compared.
ResultsSixty-three patients were included. Mean age was 5.3±4.27 years; 55.6% were females. AKI was observed in 33 (52.3%) children. Patients on HAART presented lower levels of potassium (3.9±0.8 vs. 4.5±0.7mEq/L, p=0.019) and bicarbonate (19.1±4.9 vs. 23.5±2.2mEq/L, p=0.013) and had a higher estimated glomerular filtration rate (102.2±36.7 vs. 77.0±32.8mL/min/1.73m2, p=0.011) than those not on HAART. In the multivariate analysis, the use of HAART prior to the admission was a protective factor for AKI (p=0.036; OR=0.30; 95% CI=0.097–0.926).
ConclusionAKI is a common complication of pediatric HIV infection. Use of HAART prior to the admission preserved glomerular filtration and was a protective factor for AKI, but increased medication side effects, such as hypokalemia and renal metabolic acidosis.
Avaliar dados clínicos e laboratoriais, bem como ocorrência de lesão renal aguda (LRA) em crianças HIV positivas com e sem uso de terapia antirretroviral altamente ativa (TARV) antes da admissão.
MétodoFoi realizado estudo retrospectivo em pacientes pediátricos HIV positivos (<16 anos). Foram comparadas as crianças que estavam em uso com aquelas sem uso de TARV prévia à internação.
ResultadosForam incluídos 63 pacientes, com média de idade de 5,3±4,27 anos, sendo 55,6% do sexo feminino. LRA foi encontrada em 33 casos (52,3%). Os pacientes usando TARV apresentaram menores níveis de potássio (3,9±0,8 vs. 4,5±0,7 mEq/L, p=0,019) e bicarbonato (19,1±4,9 vs. 23,5±2,2 mEq/L, p=0,013), bem como maior taxa de filtração glomerular estimada (102,2±36,7 vs. 77,0±32,8mL/min/1,73m2, p=0,011) que o pacientes sem TARV prévia. Na análise multivariada o uso de TARV prévia à internação foi fator protetor contra LRA (p=0,036; RC=0,30; IC de 95%=0,097-0,926).
ConclusãoA LRA é uma complicação comum da infecção pediátrica pelo HIV. O uso de TARV antes da internação foi associado a melhor taxa de filtração glomerular e foi fator de proteção contra LRA, porém desencadeou efeitos colaterais como hipocalemia e acidose metabólica.
According to the United Nations Program on HIV/AIDS (UNAIDS), it was estimated that approximately 35 million people worldwide were living with HIV or acquired immune deficiency syndrome (AIDS) in 2012. Furthermore, the 2012 annual number of new HIV infections was calculated to be nearly 2.5 million. Over 12% of them (330,000 cases worldwide) occurred in the pediatric population younger than 15 years. In 2011, 230,000 children died due to HIV/AIDS worldwide.1
The prevalence of renal diseases in HIV-infected pediatric patients varies substantially among regions and periods. In the pre-Highly Active Antiretroviral Therapy (HAART) era, most of the infected children in the United States died of non-renal AIDS complications, such as opportunistic infections (OIs). This phenomenon is still common in some developing countries, where this therapy is not available to all patients. However, after the establishment of HAART in developed countries, the number of HIV-infected children who require renal replacement therapy has increased considerably.2 Kidney disease complicating HIV infection is now among the 10 most common non-infectious conditions occurring in perinatally HIV-infected children and adolescents in the HAART era, with an incidence rate of 2.6 per 100 patients.3
HIV patients are at high risk of developing both acute kidney injury (AKI) and chronic kidney disease (CKD) due to several factors.4 In this context, the spectrum of kidney diseases in HIV-infected pediatric population is different from adults, including chronic glomerular disorders, such as HIV-associated nephropathy; HIV immune complex kidney disease; some types of thrombotic microangiopathies, such as atypical forms of hemolytic uremic syndrome and thrombocytopenic purpura; tubular disorders; and AKI.5
The aim of this study was to evaluate clinical and laboratory data, as well as the occurrence of AKI, using the modified pediatric RIFLE (pRIFLE) criteria in HIV-infected children, by comparing groups according to the use of HAART prior to hospital admission.
Patients and methodsSetting and patient selectionThis was a retrospective study performed with HIV-infected children consecutively admitted to São José Infectious Diseases Hospital, Northeast Brazil, from January 2007 to December 2012. All children younger than 16 years with confirmed serology for HIV infection were included. This is the same age range for which the Schwartz equation was validated.6 Patients with history of previous renal diseases, arterial hypertension, diabetes mellitus, nephrolithiasis, use of nephrotoxic drugs (except for HAART), and other co-morbidities that could affect renal function were excluded.
Studied parametersDemographic characteristics such as age and gender were analyzed, as well as duration of hospital stay, co-infections, use of HAART, and clinical manifestations on admission, laboratory data, dialysis requirement, and mortality. Clinical investigations included a record of all clinical signs and symptoms presented by patients at admission and during hospital stay.
Laboratory data included assessments of serum creatinine (Cr), urea (Ur), sodium (Na), potassium (K), albumin (Alb), alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), hemoglobin (Hb), hematocrit (Ht), platelets, leukocytes, lymphocytes, serum pH (pH), CO2 partial pressure (pCO2), bicarbonate (HCO3), urine pH, and urine density. Mean viral load (VL) and CD4 count were also evaluated. VL=100,000copies/mm3 and CD4 count=200/mm3 were established as cut off points in order to compare HAART and non-HAART patients, since these values were previously associated with development of AKI and poor outcomes.7
DefinitionsAcute Kidney Injury was defined according to pediatric RIFLE (pRIFLE) criteria, using Schwartz equation to estimate glomerular filtration rate (eGFR).6 pRIFLE categories include:8,9
- -
Risk: eGFR reduction by 25% or urine output<0.5mL/kg/h for 8h.
- -
Injury: eGFR reduction by 50% or urine output<0.5mL/kg/h for 16h.
- -
Failure: eGFR reduction by 75% or eGFR<35mL/min/1.73m2 or urine output<0.3mL/kg/h for 24h or anuric for 12h.
- -
Loss: persistent failure>four weeks.
- -
End-stage renal disease: persistent failure>three months.
A baseline eGFR of 120mL/min/m2 was assigned to all children as previously reported, since none of them had available serum creatinine levels measured within three months before admission.8,9 eGFR was calculated by using serum creatinine on hospital admission. The percentage of eGFR reduction [100×(baseline eGFR−admission eGFR)/baseline eGFR] was assessed to determine pRIFLE category. Patients were classified according pRIFLE category on admission. Due to the absence of anthropometric data in charts, mean height for each age and gender was obtained from World Health Organization (WHO) Growth data, in order to calculate eGFR.10,11
Oliguria was defined as urine output <1mL/kg/h in infants (0–12 months) and <0.5mL/kg/h in children who had been effectively hydrated. Dialysis was indicated in those patients that remained oliguric after effective hydration, in those cases where uremia was associated with hemorrhagic or severe respiratory failure, and those with hyperkalemia or metabolic acidosis refractory to clinical treatment. Dialysis was indicated in those patients who remained oliguric after effective hydration, in rapid elevation of blood urea nitrogen (hypercatabolic state), in those cases where uremia was associated to hemorrhagic or severe respiratory failure, and those with hyperkalemia or metabolic acidosis refractory to clinical treatment.
Children were divided into two groups: those who were in use of HAART prior to the admission and those who were not. Demographical, clinical, and laboratory data of the two groups were compared.
TreatmentThe HAART drugs used in the treatment were: zidovudine (AZT), didanosine (ddI), lamivudine (3TC), stavudine (D4T), abacavir (ABC), tenofovir disoproxil fumarate (TDF), lopinavir (LPV), nelfinavir (NFV), saquinavir (SQV), ritonavir (RTV), amprenavir (APV), efavirenz (EFZ), and nevirapine (NPV), according to the protocols of the Brazilian Ministry of Health.
Statistical analysisThe SPSS software for Windows, release 20.0 (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, version 20.0, USA) was used for statistical analysis. Chi-squared test was used to analyze frequencies in the patients’ groups. All independent variables were tested for normal distribution using the Kolmogorov–Smirnov test. Differences between two independent variables were evaluated using Student's t-test or Mann–Whitney test as appropriate. Data were expressed as means±SD, and a p≤0.05 was considered statistically significant.
A multivariate logistic regression was performed to analyze the possible risk factors associated with AKI. Initially, a univariate analysis was done with all dichotomous variables available, including gender, presence of each symptom and comorbidity, HAART use, viral load >100,000copies, and CD4 count <200/mm3. These parameters were evaluated for significant difference between AKI and non-AKI groups, using the chi-squared test and crosstabs. Secondly, parameters included in the multivariate model were those that presented a significance level (p≤0.05) in the univariate analysis. Only one of them (HAART use) was significantly different, so it was evaluated as a risk factor for AKI, by calculating the adjusted odds ratio (OR) and 95% confidence interval (95% CI).
EthicsThe study protocol was reviewed and approved by Ethics Committees from Walter Cantídio University Hospital and São José Infectious Diseases Hospital.
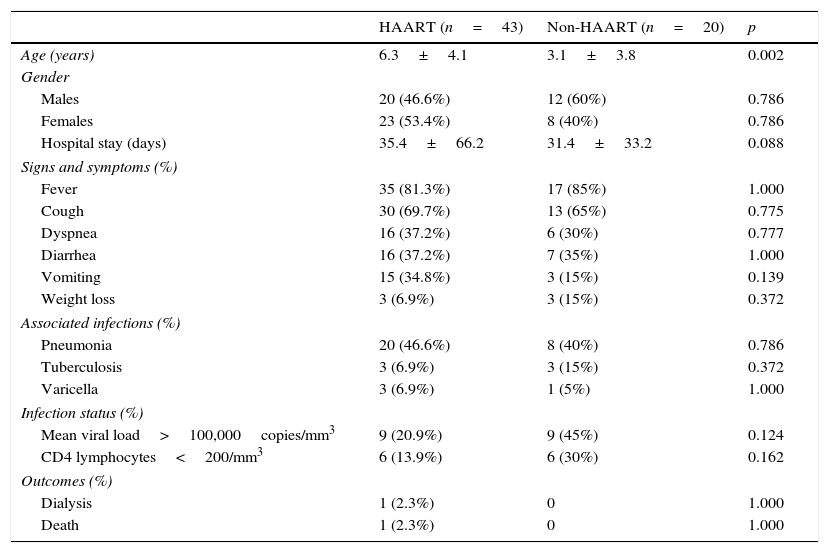
ResultsSixty-three children were included, with mean age 5.3±4.27 years (range 1–14 years); 44 (69.8%) were younger than 7 years; 35 five (55.6%) were females. Forty-three (68.3%) patients were using HAART prior to admission, while 20 (31.7%) were not. Among all patients using HAART, 37 (58.7%) were using 3TC; 31 (49.2%), AZT. 16 (35.4%), LPV; and five (7.9%) TDF. Duration of hospital stay ranged from 1 to 352 days (mean 34.2±57.8 days). One patient needed hemodialysis (3.2%) and one patient died (3.2%). None of the patients needed intensive care. Only 16 children had urine samples. One of them presented microscopic hematuria and three presented proteinuria, none in the nephrotic range of proteinuria. Comparison of demographic data, main signs and symptoms, associated infections, immunologic status and outcomes between the two groups is summarized in Table 1. Among all patients, the most frequent opportunistic infections (OIs) were pneumonia (44.4%), pulmonary tuberculosis (9.5%), and varicella zoster/chickenpox (6.3%).
Demographic data, clinical manifestations, infection status, and outcomes of children with human immunodeficiency virus (HIV) according to the use of highly active antiretroviral therapy (HAART).
| HAART (n=43) | Non-HAART (n=20) | p | |
|---|---|---|---|
| Age (years) | 6.3±4.1 | 3.1±3.8 | 0.002 |
| Gender | |||
| Males | 20 (46.6%) | 12 (60%) | 0.786 |
| Females | 23 (53.4%) | 8 (40%) | 0.786 |
| Hospital stay (days) | 35.4±66.2 | 31.4±33.2 | 0.088 |
| Signs and symptoms (%) | |||
| Fever | 35 (81.3%) | 17 (85%) | 1.000 |
| Cough | 30 (69.7%) | 13 (65%) | 0.775 |
| Dyspnea | 16 (37.2%) | 6 (30%) | 0.777 |
| Diarrhea | 16 (37.2%) | 7 (35%) | 1.000 |
| Vomiting | 15 (34.8%) | 3 (15%) | 0.139 |
| Weight loss | 3 (6.9%) | 3 (15%) | 0.372 |
| Associated infections (%) | |||
| Pneumonia | 20 (46.6%) | 8 (40%) | 0.786 |
| Tuberculosis | 3 (6.9%) | 3 (15%) | 0.372 |
| Varicella | 3 (6.9%) | 1 (5%) | 1.000 |
| Infection status (%) | |||
| Mean viral load>100,000copies/mm3 | 9 (20.9%) | 9 (45%) | 0.124 |
| CD4 lymphocytes<200/mm3 | 6 (13.9%) | 6 (30%) | 0.162 |
| Outcomes (%) | |||
| Dialysis | 1 (2.3%) | 0 | 1.000 |
| Death | 1 (2.3%) | 0 | 1.000 |
Age and hospital stay are presented as mean±SD. The rest of the data are presented as number (percentage). Student's t-test and the chi-squared test were used. p-values≤0.05 were considered statistically significant.
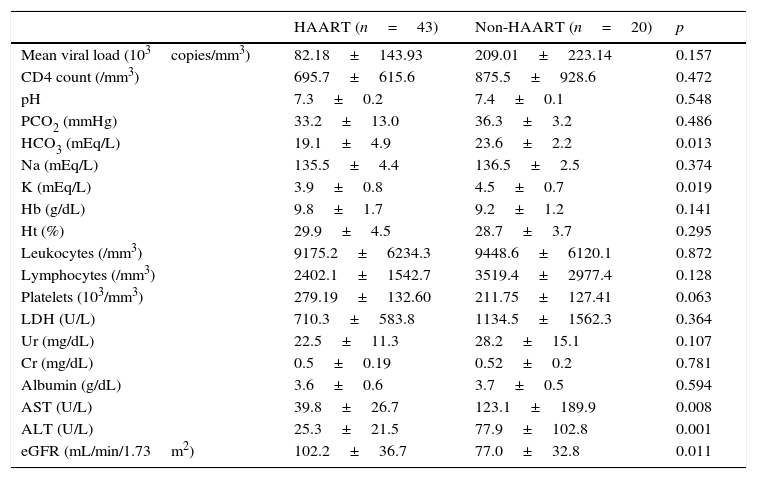
Comparing groups, it was noticed that those patients on HAART presented significantly lower levels of serum bicarbonate (19.1±4.9 vs. 23.6±2.2mEq/L, p=0.013) and serum potassium (3.9±0.8 vs. 4.5±0.7mEq/L, p=0.019) than those not on HAART, respectively. Also, those patients not on HAART presented significantly higher levels of AST (123.1±189.9U/L vs. 39.8±26.7, p=0.008) and ALT (77.9±102.8U/L vs. 25.3±21.5, p=0.001) than those on HAART, respectively. Moreover, eGFR was remarkably higher in patients on HAART than in those not on HAART (102.2±36.7 vs. 77.0±32.8mL/min/1.73m2, p=0.011). There was no significant difference between the two groups when comparing percentage of patients who presented CD4<200/mm3 and VL>100,000copies/mm3. A comparison of laboratory data between groups is presented in Table 2.
Comparison of laboratory data between children with human immunodeficiency virus (HIV) according to use of highly active antiretroviral therapy (HAART).
| HAART (n=43) | Non-HAART (n=20) | p | |
|---|---|---|---|
| Mean viral load (103copies/mm3) | 82.18±143.93 | 209.01±223.14 | 0.157 |
| CD4 count (/mm3) | 695.7±615.6 | 875.5±928.6 | 0.472 |
| pH | 7.3±0.2 | 7.4±0.1 | 0.548 |
| PCO2 (mmHg) | 33.2±13.0 | 36.3±3.2 | 0.486 |
| HCO3 (mEq/L) | 19.1±4.9 | 23.6±2.2 | 0.013 |
| Na (mEq/L) | 135.5±4.4 | 136.5±2.5 | 0.374 |
| K (mEq/L) | 3.9±0.8 | 4.5±0.7 | 0.019 |
| Hb (g/dL) | 9.8±1.7 | 9.2±1.2 | 0.141 |
| Ht (%) | 29.9±4.5 | 28.7±3.7 | 0.295 |
| Leukocytes (/mm3) | 9175.2±6234.3 | 9448.6±6120.1 | 0.872 |
| Lymphocytes (/mm3) | 2402.1±1542.7 | 3519.4±2977.4 | 0.128 |
| Platelets (103/mm3) | 279.19±132.60 | 211.75±127.41 | 0.063 |
| LDH (U/L) | 710.3±583.8 | 1134.5±1562.3 | 0.364 |
| Ur (mg/dL) | 22.5±11.3 | 28.2±15.1 | 0.107 |
| Cr (mg/dL) | 0.5±0.19 | 0.52±0.2 | 0.781 |
| Albumin (g/dL) | 3.6±0.6 | 3.7±0.5 | 0.594 |
| AST (U/L) | 39.8±26.7 | 123.1±189.9 | 0.008 |
| ALT (U/L) | 25.3±21.5 | 77.9±102.8 | 0.001 |
| eGFR (mL/min/1.73m2) | 102.2±36.7 | 77.0±32.8 | 0.011 |
Data are presented as mean±SD.
pH, serum hydrogenionic potential; PCO2, carbon dioxide partial pressure; HCO3, serum bicarbonate; Na, serum sodium; K, serum potassium; Hb, serum hemoglobin; Ht, hematocrit; LDH, lactate dehydrogenase; Ur, serum urea; Cr, serum creatinine; Albumin, serum albumin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; eGFR, estimated glomerular filtration rate. Student's t-test and Mann–Whitney tests were used. p-values≤0.05 were considered statistically significant.
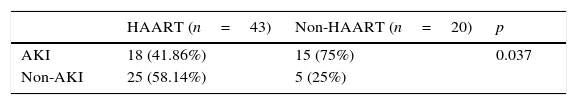
AKI was observed in 33 (52.3%) children. Nineteen were classified as Risk (57.5%), 13 as injury (39.4%), and one as failure (3.1%). Prevalence of AKI was lower in those on HAART than those not on HAART (41.86% vs. 75%, p=0.037). In the multivariate analysis, use of HAART prior to admission was a protective factor for AKI (p=0.036; OR=0.30; 95% CI=0.097–0.926). Comparison of AKI prevalence between groups is shown in Table 3.
Comparison of acute kidney injury (AKI) prevalence according to use of highly active antiretroviral therapy (HAART).
| HAART (n=43) | Non-HAART (n=20) | p | |
|---|---|---|---|
| AKI | 18 (41.86%) | 15 (75%) | 0.037 |
| Non-AKI | 25 (58.14%) | 5 (25%) |
Data were presented as number (percentage). The chi-squared test was used.
p-values≤0.05 were considered statistically significant.
This was the first study to evaluate demographical, clinical, and laboratory data of HIV-infected children admitted to an infectious diseases hospital in Fortaleza, state of Ceará, Brazil, focusing on renal function and the development of AKI. It was observed that HAART appears to be a protective factor against AKI in children with HIV.
In the present study, mean age of the children was 5.3 years, with a predominance of females, which is similar to past studies that evaluated renal disease in HIV-infected children.12–14 Furthermore, the most prevalent symptoms in the present study were fever and cough, which reflect main associated infections, the leading cause of hospitalization. In two recent pediatric studies, the main symptoms were identical to those of the present cohort.15,16 Regarding viral and immunologic status, most patients presented high levels of mean viral load and low CD4 count, but there was not significant difference between groups.
Among all children in the present study, hypokalemia was observed in 22.2% of them. Mean serum potassium was significantly lower in patients on HAART than in those not on HAART. This electrolyte disorder has been associated with antiretroviral use, especially TDF, most likely due to its well-known nephrotoxicity.17,18 In a study performed by Kohler et al., it was observed that TDF's renal toxicity and the hypokalemia that follows happen because of the renal proximal tubular mitochondrial ultrastructural abnormalities induced by this drug.19 In addition, lower body weight and high-dose of the drug were significantly associated with TDF-induced nephrotoxicity in HIV-infected children from the United Kingdom.20
In addition to hypokalemia, lactic acidosis has also been reported as a consequence of HAART toxicity, specifically by the nucleoside reverse transcriptase inhibitors (NRTIs). This is an important cause of acidosis in HIV-infected patients, both children and adults.21,22 In the present study, lower mean levels of serum bicarbonate were observed in patients on HAART than in those not on HAART, which indicates higher incidence of metabolic acidosis in the first group. Furthermore, mitochondrial toxicity in proximal tubule cells is the responsible for another well-described cause of acidosis in HIV patients using HAART: Fanconi syndrome.23,24 Hence, HAART toxicity is most likely the major cause of metabolic acidosis in the present cohort instead of eGFR decrease, since serum bicarbonate was lower in patients on HAART, who presented higher levels of eGFR.
Other interesting findings in the present study were the higher mean levels of AST and ALT in patients not on HAART when compared to those on HAART, which may indicate liver injury. According to a recent review of liver disease in HIV-infected adult patients, there are several factors related to liver injury and HIV, including hepatitis B and C viruses co-infection; steatosis and non-alcoholic steatohepatitis (NASH); metabolic changes such as insulin resistance; liver toxicity of the medications, especially HAART; and also a direct effect of the HIV on the liver.25 Some of these factors probably contributed to AST and ALT elevation in the present cohort. Since higher levels of liver enzymes were presented by the patients who were not on HAART, liver impairment is most likely due to causes other than drug toxicity.
Since the introduction of HAART, chronic conditions such as renal disease have been rising as important causes of morbidity and mortality of patients with HIV. In the present study, it was observed that those not on HAART presented an important renal function impairment and higher incidence of AKI when compared to patients on HAART. In addition, AKI is a common complication in ambulatory HIV-infected adult patients treated with HAART, having been associated with previous renal impairment, lower CD4 levels, AIDS, hepatitis C virus co-infection and liver disease.26,27 Usually, the development of AKI in these patients is multifactorial, including sepsis, nephrotoxic drugs, volume depletion, and use of radiocontrast.28
In the multivariate analysis, use of HAART prior to the admission was a protective factor for AKI. This might be explained by the benefits of HAART in reducing viral load and then decreasing renal effects of HIV. There is evidence to prove the efficacy of HAART in preventing HIV-associated nephropathy and delaying progression to end-stage renal disease in HIV-infected adult patients.29,30
In conclusion, renal disease is rising as an important long-term complication of HIV infection in children worldwide. The comparison between the two groups showed that children using HAART prior to the admission had higher eGFR, but lower levels of potassium and bicarbonate. HAART use was shown to be a protective factor for AKI. These findings may indicate that the use of HAART prior to the admission preserved glomerular filtration and reduced incidence of AKI, but increased medication side effects, such as hypokalemia and renal metabolic acidosis, which are mainly associated with some antiretorvirals, such as TDF.
Study limitationsStudy limitations include some data missing due to the retrospective nature of the research, and the sample selection, which was based only on hospitalized patients. The authors did not have access to data on children's urine output, as this was a retrospective study. In general, non-critical care units do not accurately record urine output, especially in the pediatric population. Some anthropometric data, such as height and weight, were also not available in children's charts, so it was necessary to obtain some of them from WHO Growth Data in order to estimate GFR by using Schwartz formula. Duration of HAART was also not available in patient's records. Serum creatinine within three months before admission was also not available, thus a baseline eGFR of 120mL/min/1.73m2 was assumed for all children, since they did not present any identified factors for previous chronic kidney disease and it was not possible to estimate the real GFR. Using AKI as the outcome of interest may have reduced the accuracy of logistic regression results, since the number of AKI patients was limited (only 33), but did not undermine the importance of the multivariate analysis findings.
FundingConselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq (Brazilian Research Council). Edson Queiroz Foundation/University of Fortaleza.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank the team of physicians, residents, medical students, and nurses from the São José Infection Disease Hospital for providing technical support to develop this research and for the exceptional assistance provided to the patients. This research was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; Brazilian Research Council).
Please cite this article as: Soares DS, Cavalcante MG, Ribeiro SM, Leitão RC, Vieira AP, Pires Neto RJ, et al. Acute kidney injury in HIV-infected children: comparison of patients according to the use of highly active antiretroviral therapy. J Pediatr (Rio J). 2016;92:631–7.