To evaluate the prevalence of vitamin D deficiency in obese children and adolescents when compared to eutrophic controls.
MethodsSystematic review with meta-analysis covering studies with patients aged 0–18 years old diagnosed with obesity and vitamin D deficiency and control group of eutrophic patients. The studies were retrieved in the PubMed, Embase, and LILACS databases in December 2019. The search used the terms “obesity” in combination with “pediatric population” and “vitamin D”.
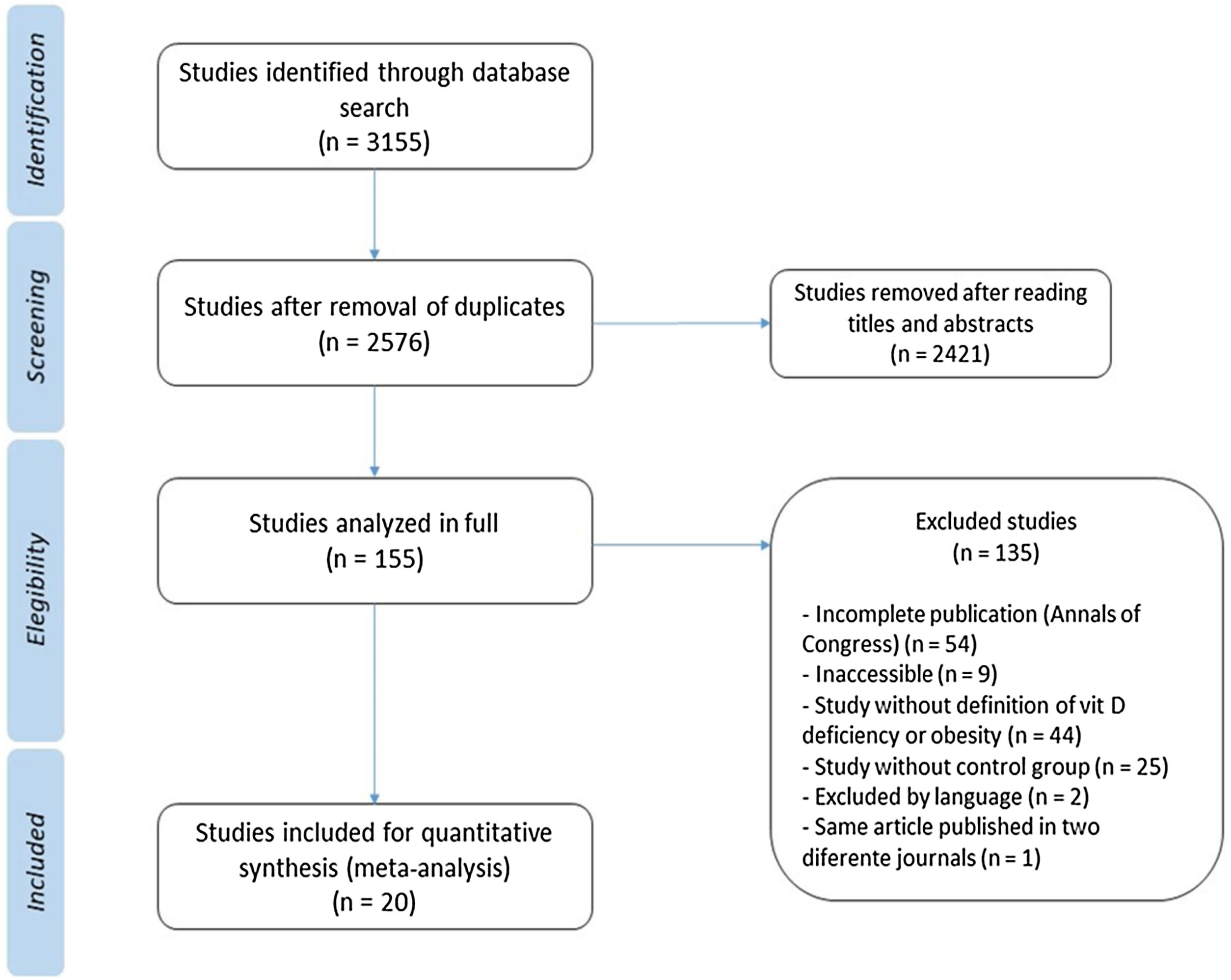
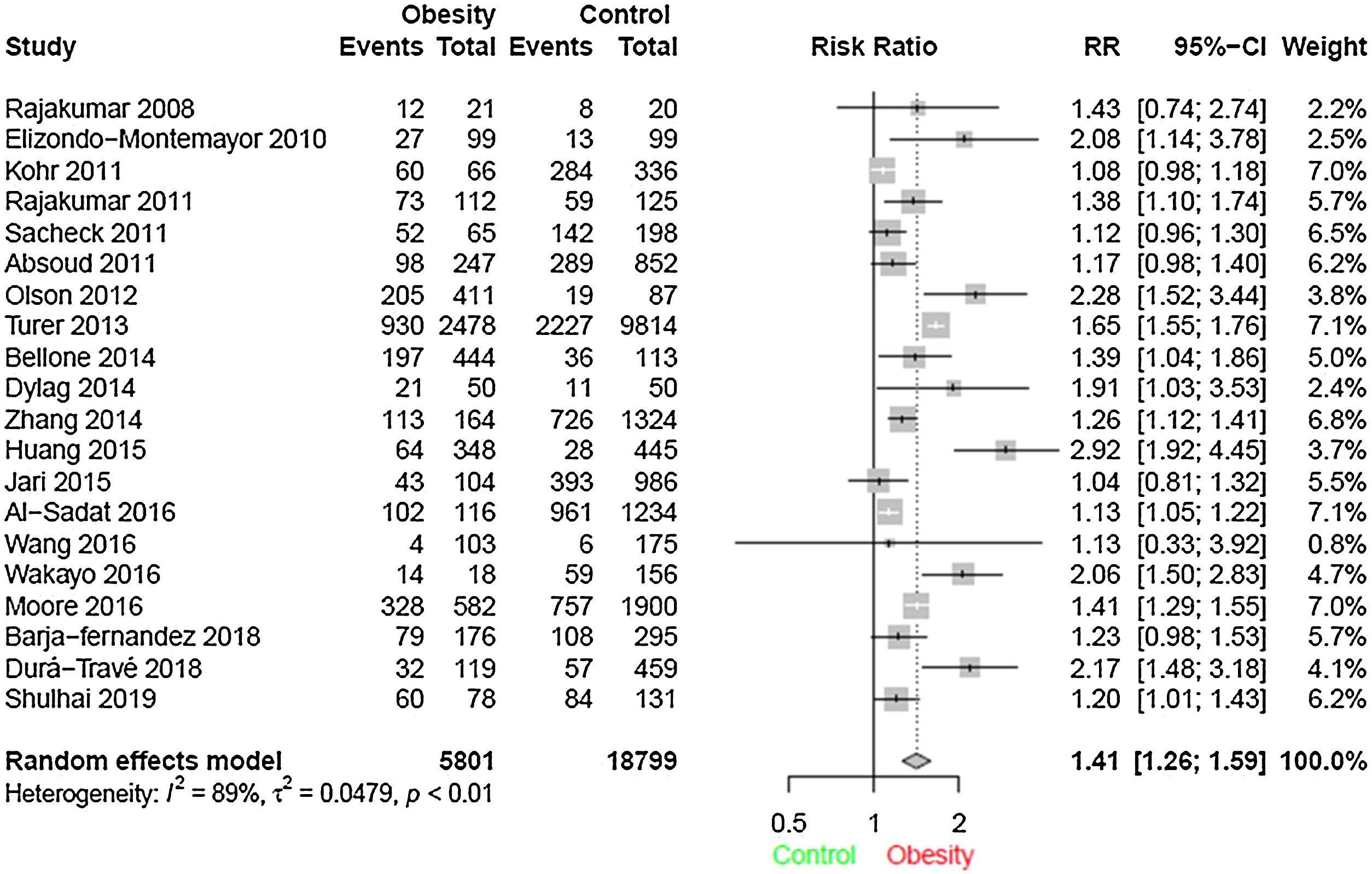
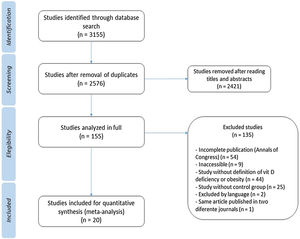
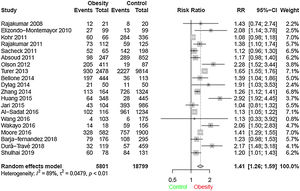
ResultsThrough the search 3155 articles were retrieved; and after analysis, 20 studies were selected according to the study objectives. A total of 24,600 children and adolescents were included. Through meta-analysis, the relative risk for the association between obesity and vitamin D deficiency in the pediatric population was 1.41 (95% CI: 1.26–1.59) (I² = 89%, p < 0.01).
ConclusionChildren and adolescents with obesity have higher risk of vitamin D deficiency.
Childhood obesity is a worldwide problem.1 It is known that overweight in childhood and adolescence is an important risk factor for obesity in adulthood, as well as for the development of comorbidities.1
Regarding vitamin D (vit D), in addition to its role in bone health and calcium and phosphorus metabolism, its role in immune functions and in decreasing the risk of chronic illnesses has been considered.2,3 Vit D deficiency varies by geographic region, with an estimated prevalence of 15% in a study with the general pediatric population in the United States of America from 1 to 11 years of age.4 A similar prevalence of 14% was found in a cross-sectional survey in a representative sample of adolescents from that country aged 12–19.5 The best indicator to assess vit D status is the metabolite 25-hydroxy vitamin D (25[OH]D).6
The relationship between hypovitaminosis D and obesity has been widely studied in the general population. A meta-analysis published in 2015 showed an association between vit D deficiency and obesity, with OR 3.43 (95% CI: 2.33–5.06).7
Vit D deficiency and excess body fat have mutual negative effects, resulting from metabolic processes that generate accumulation of inactive forms and decreased vit D bioavailability, in addition to decreased tissue secretion and sensitivity to insulin.8 There is no consensus as to why vit D levels are lower in obese individuals. The main hypothesis would be the absorption of vit D, which is fat soluble, by the adipose tissue.9
Thus, it is known that obesity is related with hypovitaminosis D, with consequent greater chance of changes in glycemic control and metabolic syndrome in general population.10,11 Although this relationship is clear in the adult population, there is no consensus on the literature regarding a higher frequency of vit D deficiency in children and adolescents with obesity.
MethodsThe literature search, study selection based on title and abstract, and data extraction were carried out independently by two trained reviewers. In cases of disagreement, it was resolved by the reviewers. In case of duplicate articles, only one was considered. The meta-analysis is registered on the PROSPERO Platform (PROSPERO, University of York, England) under number CRD42019137788.
Search strategyThe studies were identified through research in the PubMed, Embase, and LILACS databases, performed in December 2019. For the search, the term “obesity” was used in combination with “pediatric population” and “vitamin D”, through structured Medical Subject Heading (MeSH) keywords for PubMed, Emtree for Embase, and Health Science Descriptors (DeCS) for LILACS. The terms used in the search and the number of articles retrieved per database are depicted in Table S1 of the Supplementary material.
Eligibility criteriaThe inclusion criteria were as follows: studies published in Portuguese, English, Spanish, or French, published on any date, evaluating patients aged 0–18 years old with diagnosis of obesity and vit D deficiency, with control group of eutrophic patients. Data related to patients with comorbidities that alter vit D metabolism (e.g. kidney, liver, gastrointestinal, or endocrine disease) or using medications that act in the metabolism of this vitamin (e.g., glucocorticoids), and studies with incomplete or data not published in full were excluded.
Data collectionAfter assessing the title, abstract, and full text of the studies according to the eligibility criteria, the data of interest were collected using a standard form. The following information was collected: authors, study place, design, year of publication, age of participants, 25(OH)D levels characterized as deficiency, method for assessing 25(OH)D levels, obesity definition by body mass index (BMI), number of patients with obesity and the respective number of patients with vit D deficiency, and number of eutrophic patients and the respective number of patients with vit D deficiency in this group.
Statistical analysisThe studies were grouped in a meta-analysis. The summary measure used was relative risk (RR) with 95% confidence interval, weighted by the study power, using the random effect model. The inconsistency test (I²) was used to assess heterogeneity between studies. A p-value <0.05 was considered to be statistically significant. The statistical analysis was performed using the “meta” package of the R program (R Core Team [2017]. R: A language and environment for statistical computing. R Foundation for Statistical Computing, v. 3.5.1 — Vienna, Austria).
ResultsDuring the search 3155 articles were retrieved, of which 20 studies were relevant to the objectives of this study (Table 112–31). The flowchart showing the search results and selection details is depicted in Fig. 1. A total of 24,600 children were included.
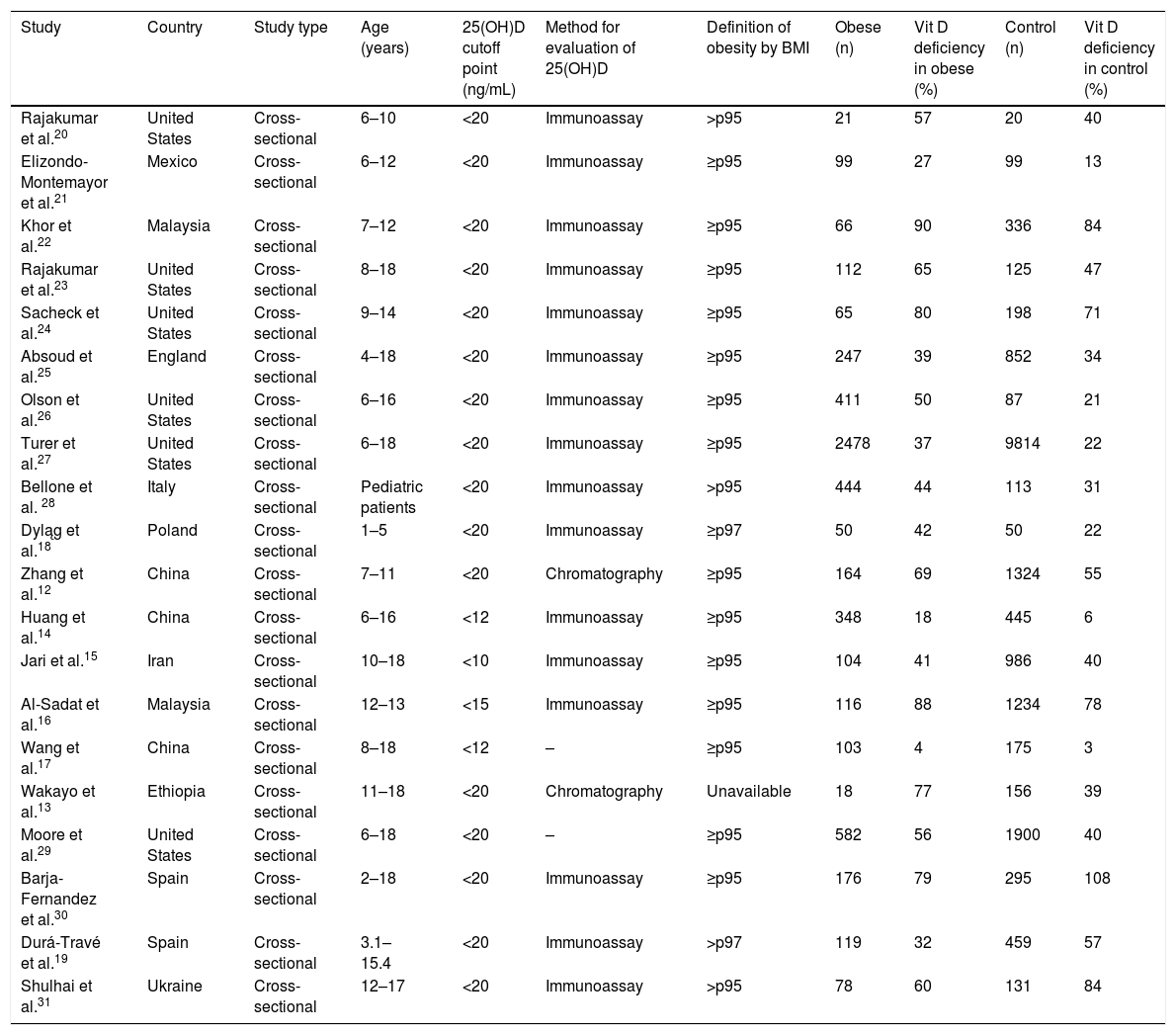
Characteristics of the studies included in the final analysis.
| Study | Country | Study type | Age (years) | 25(OH)D cutoff point (ng/mL) | Method for evaluation of 25(OH)D | Definition of obesity by BMI | Obese (n) | Vit D deficiency in obese (%) | Control (n) | Vit D deficiency in control (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Rajakumar et al.20 | United States | Cross-sectional | 6–10 | <20 | Immunoassay | >p95 | 21 | 57 | 20 | 40 |
| Elizondo-Montemayor et al.21 | Mexico | Cross-sectional | 6–12 | <20 | Immunoassay | ≥p95 | 99 | 27 | 99 | 13 |
| Khor et al.22 | Malaysia | Cross-sectional | 7–12 | <20 | Immunoassay | ≥p95 | 66 | 90 | 336 | 84 |
| Rajakumar et al.23 | United States | Cross-sectional | 8–18 | <20 | Immunoassay | ≥p95 | 112 | 65 | 125 | 47 |
| Sacheck et al.24 | United States | Cross-sectional | 9–14 | <20 | Immunoassay | ≥p95 | 65 | 80 | 198 | 71 |
| Absoud et al.25 | England | Cross-sectional | 4–18 | <20 | Immunoassay | ≥p95 | 247 | 39 | 852 | 34 |
| Olson et al.26 | United States | Cross-sectional | 6–16 | <20 | Immunoassay | ≥p95 | 411 | 50 | 87 | 21 |
| Turer et al.27 | United States | Cross-sectional | 6–18 | <20 | Immunoassay | ≥p95 | 2478 | 37 | 9814 | 22 |
| Bellone et al. 28 | Italy | Cross-sectional | Pediatric patients | <20 | Immunoassay | >p95 | 444 | 44 | 113 | 31 |
| Dyląg et al.18 | Poland | Cross-sectional | 1–5 | <20 | Immunoassay | ≥p97 | 50 | 42 | 50 | 22 |
| Zhang et al.12 | China | Cross-sectional | 7–11 | <20 | Chromatography | ≥p95 | 164 | 69 | 1324 | 55 |
| Huang et al.14 | China | Cross-sectional | 6–16 | <12 | Immunoassay | ≥p95 | 348 | 18 | 445 | 6 |
| Jari et al.15 | Iran | Cross-sectional | 10–18 | <10 | Immunoassay | ≥p95 | 104 | 41 | 986 | 40 |
| Al-Sadat et al.16 | Malaysia | Cross-sectional | 12–13 | <15 | Immunoassay | ≥p95 | 116 | 88 | 1234 | 78 |
| Wang et al.17 | China | Cross-sectional | 8–18 | <12 | – | ≥p95 | 103 | 4 | 175 | 3 |
| Wakayo et al.13 | Ethiopia | Cross-sectional | 11–18 | <20 | Chromatography | Unavailable | 18 | 77 | 156 | 39 |
| Moore et al.29 | United States | Cross-sectional | 6–18 | <20 | – | ≥p95 | 582 | 56 | 1900 | 40 |
| Barja-Fernandez et al.30 | Spain | Cross-sectional | 2–18 | <20 | Immunoassay | ≥p95 | 176 | 79 | 295 | 108 |
| Durá-Travé et al.19 | Spain | Cross-sectional | 3.1–15.4 | <20 | Immunoassay | >p97 | 119 | 32 | 459 | 57 |
| Shulhai et al.31 | Ukraine | Cross-sectional | 12–17 | <20 | Immunoassay | >p95 | 78 | 60 | 131 | 84 |
BMI, body mass index.
The studies were all carried out in countries located in the Northern Hemisphere. None of the studies performed in tropical countries fulfilled the inclusion criteria. The United States is the country with the most publications (n = 6), followed by three studies carried out in China.
Zhang et al.12 and Wakayo et al.13 evaluated 25(OH)D levels using chromatography, all others studies used immunoassay techniques. Regarding the cutoff point used to define 25(OH)D deficiency, the value of 20 ng/mL was the most frequently used, with only four studies using other cutoff points — Huang et al.,14 Jari et al.,15 Al-sadat et al.,16 and Wang et al.17 There were also different definitions of obesity among the studies. Although most of them have used the definition of BMI equal or higher 95th percentile for age, Dyląg et al.18 and Durá-Travé et al.19 used the 97th percentile for age the as cutoff point.
In meta-analysis, the relative risk for the association between vit D deficiency and obesity was 1.41 (95% CI = 1.26–1.59), (I² = 89%, p < 0,01), as shown in the forest plot (Fig. 2).
Heterogeneity was found among the studies, although the random effect model was used for meta-analysis. The study that contributed the most for heterogeneity was Turer et al.27 A sensitivity analysis was performed by excluding this study from the present analysis, and the relative risk did not change drastically.
Some studies reported other factors associated with vit D deficiency. Khor et al.22 demonstrated higher prevalence of vit D deficiency in girls (77.5%) than boys (66.1%), with statistical significance (p = 0.01). Rajakumar et al.23 and Turer et al.27 evidenced lower prevalence of hypovitaminosis D in individuals with white skin color, and in the summer and autumn seasons. The study by Shulhai et al.31 with Ukrainian children and adolescents reported a significant effect in the development of vit D deficiency in individuals who spend more than four hours a day in front of computer or television (p = 0.027).
Two studies also evaluated insulin resistance and its relationship with hypovitaminosis D. Wang et al.17 used fasting glucose and serum insulin measurements to estimate insulin resistance through homeostatic model assessment (HOMA-IR) in 278 children and adolescents. In multiple linear regression, there was association among HOMA-IR, BMI, and vit D deficiency (p < 0.001). Huang et al.14 also reported negative association between serum vit D and HOMA-IR in obese children and adolescents.
The severity of obesity also seems to be related with 25(OH)D levels. Turer et al.27 demonstrated that half of the children with severe obesity presented with hypovitaminosis D and even after statistical adjustment for confounding variables, these children have more than double the risk to present with vit D deficiency compared to eutrophic controls.
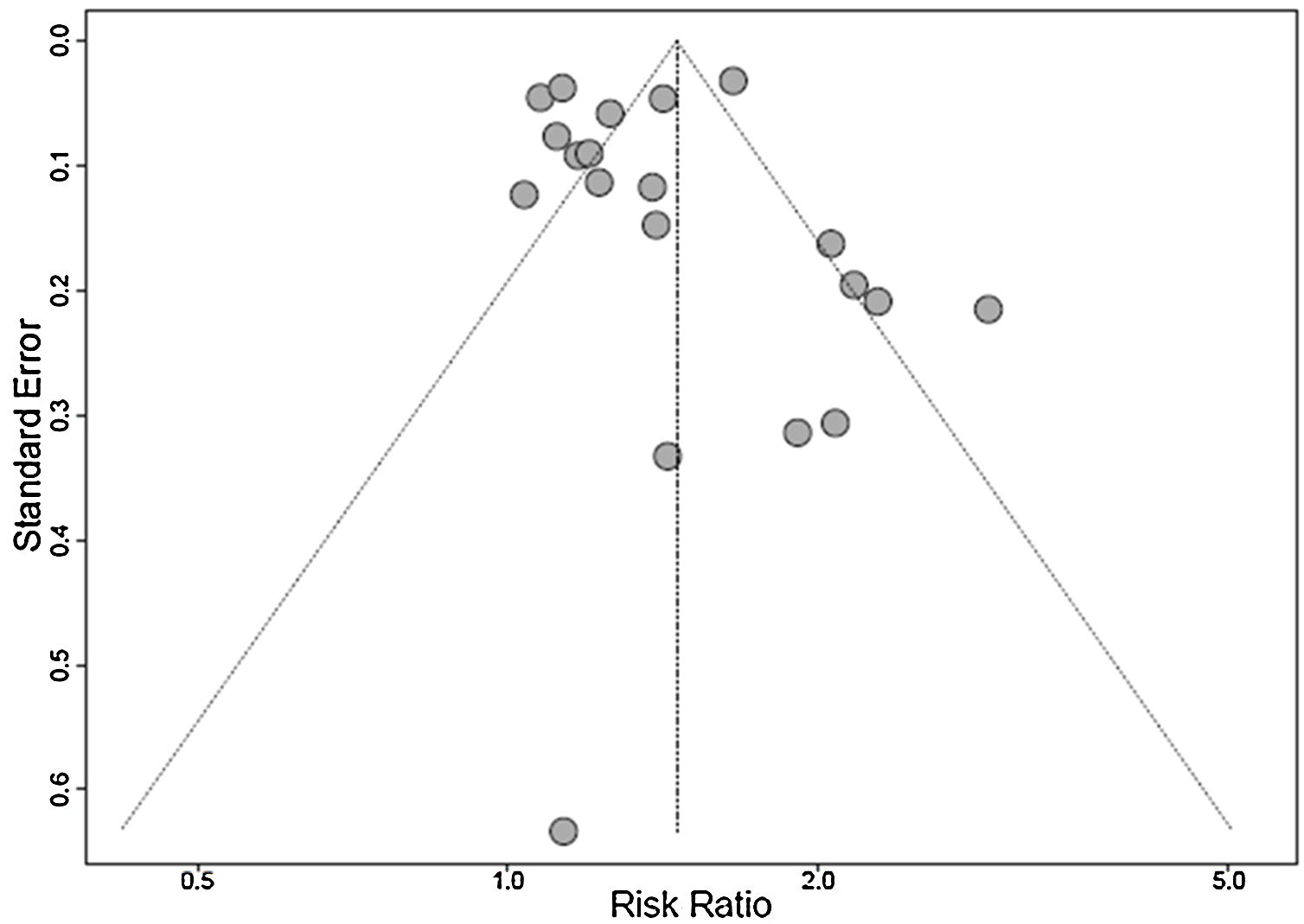
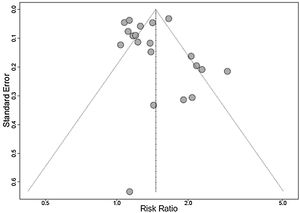
A funnel plot analysis showed the presence of publication bias (Fig. 3).
DiscussionThis meta-analysis summarized the data from 20 cross-sectional studies that assessed vit D deficiency in obese and also in eutrophic children and adolescents, comprising a total of 24,600 patients.
The hypothesis that children and adolescents with excess weight present higher prevalence of vit D deficiency when compared to control group of eutrophic patients was confirmed. The most accepted physiopathologic basis is that vit D, being fat-soluble, is over-absorbed by adipose tissue.9 The data that excessive screen time (television, computers, and tablets) is also related to lower vit D levels reinforces the idea that the association between childhood obesity and hypovitaminosis D appears to be multifactorial, also influenced by diminished exposure to outdoor activities and sunlight.27,31
It should be noted that all studies included in the review were carried out in the Northern Hemisphere. Although in Brazil the majority of the population resides in regions with adequate sun exposure, hypovitaminosis D is also a common problem. A study with 135 healthy Brazilian adolescents showed a prevalence of vit D deficiency of 60%.32 In another Brazilian study performed with healthy infants aged between six and 24 months, 6% presented deficient serum vit D concentration, which suggests that vit D deficiency may also vary with age.33 The Brazilian Society of Pediatrics recommends the prevention of deficiency through the supplementation of vit D in children on exclusive breastfeeding, children using fortified milk formula that ingest volumes less than 1000 mL/day, children and adolescents who do not ingest at least 600 IU/day of vit D in the diet or that are not exposed to the sun regularly, regardless of the region of the country.34
Epidemiological data on the prevalence of childhood obesity and hypovitaminosis D in pediatrics are worrying. Knowing the immunological functions of vit D and its influence on insulin resistance mechanisms, it is necessary to discuss therapeutic measures that can minimize the consequences on the current and future health of children and adolescents with excess weight and disability from vit D deficiency.35
Studies show that the obese pediatric population does not show the same response to vit D supplementation in the doses routinely used when compared with eutrophic controls.36,37 There is no consensus on the universally recommended dose for the treatment of hypovitaminosis D in this population; however, authors suggest an increase in the dose used regularly.36,38
It is also essential to reinforce the importance of lifestyle changes in the pediatric population. It is necessary to encourage the practice of physical exercise, the reduction of screen time, and the adoption of healthy eating habits in order to reduce the prevalence of overweight and obesity in children and adolescents and the impact of associated comorbidities, including vit D deficiency.39
LimitationsSome limitations of this meta-analysis must be recognized. The systematic review was limited to the published literature and the publication bias should be considered. There was high heterogeneity between the studies evaluated, which was minimized by the use of the random effect model. The studies used different cutoff points for 25(OH)D deficiency, a factor involved in heterogeneity. Also, hypovitaminosis D has a known multifactorial etiology, which is a possible confounding factor in the analysis. As it is a meta-analysis of cross-sectional studies, it is not possible to infer a cause-effect relationship between the variables studied. Despite the language limitation in inclusion criteria for the convenience of the researchers, the impact was minimal, since only two studies were excluded due to this criterion.
ConclusionThis meta-analysis demonstrated an association between obesity and vit D deficiency in the pediatric population. This finding reinforces the importance of stimulating healthy lifestyle habits and highlights the need to assess levels of 25(OH)D in obese children and adolescents.
Conflicts of interestThe authors declare no conflicts of interest.