To evaluate the impact of invasive mechanical ventilation associated with two serum inflammatory cytokines and clinical indicators, on the second day of life, as predictors of bronchopulmonary dysplasia in very low birth weight preterm infants. It was hypothesized that the use of invasive mechanical ventilation in the first hours of life is associated with biomarkers that may predict the chances of preterm infants to develop bronchopulmonary dysplasia.
MethodsProspective cohort of 40 preterm infants with gestational age <34 weeks and birth weight <1500 g. The following were analyzed: clinical variables; types of ventilator support used (there is a higher occurrence of bronchopulmonary dysplasia when oxygen supplementation is performed by long periods of invasive mechanical ventilation); hospitalization time; quantification of two cytokines (granulocyte and macrophage colony stimulating factor [GM-CSF] and eotaxin) in blood between 36 and 48 h of life. The preterm infants were divided in two groups: with and without bronchopulmonary dysplasia.
ResultsThe GM-CSF levels presented a significantly higher value in the bronchopulmonary dysplasia group (p = 0.002), while eotaxin presented higher levels in the group without bronchopulmonary dysplasia (p = 0.02). The use of continuous invasive mechanical ventilation was associated with increased ratios between GM-CSF and eotaxin (100% sensitivity and 80% specificity; receiver operating characteristic area = 0.9013, CI = 0.7791–1.024, p < 0.0001).
ConclusionsThe duration of invasive mechanical ventilation performed in the first 48 h of life in the very low birth weight infants is a significant clinical predictor of bronchopulmonary dysplasia. The use of continuous invasive mechanical ventilation was associated with increased ratios between GM-CSF and eotaxin, suggesting increased lung injury and consequent progression of the disease.
The implementation of new therapies, such as antenatal corticosteroids, surfactants, and non-invasive ventilation in the care of very low birth weight (VLBW) preterm infant has increased overall survival. However, the inadequate management of these resources increases morbidity due to hospitalization, especially respiratory diseases such as bronchopulmonary dysplasia (BPD), and child mortality up to 5 years of age.1,2 BPD is a multifactorial disease that has specific clinical, radiological, and histological characteristics, and is defined as oxygen dependence at concentrations above 21% for a period ≥28 days and/or at 36 weeks post-menstrual age.3 There is a higher occurrence of BPD when oxygen supplementation is performed by long periods of invasive mechanical ventilation (IMV), since this causes lesions in pulmonary structure.4,5
The clinical characteristics of BPD have begun to change, which has led researchers to recognize the need to redefine the disease in the current context.6,7 However, even with changes in disease characteristics and technological advances, the incidence of BDP remains high, which has considerable economic impact on health services.1,8 The discovery of biomarkers associated with BPD could delimit new immunomodulators associated with this disease, facilitating its definition, pathophysiology, and, consequently, diagnosis.6 Therefore, it is still necessary to investigate new biomarkers that will be useful in the prediction, diagnosis, and prognosis of BPD. The objective of this study was to evaluate the impact of IMV, associated with two serum inflammatory cytokines and clinical indicators, on the second day of life, as predictors of BPD in VLBW preterm infants. The hypothesis was that the use of IMV in the first hours of life is associated with biomarkers that may predict the chances of VLBW preterm infants in developing BPD.
Materials and methodsThis study is a part of the prospective cohort entitled Development and Evaluation of Biomarker Associated with Pathologies in the Neonatal Period and Diagnostic and Prognostic Implications. The prospective cohort study was carried out from September 2015 to April 2016 in a neonatology department of a reference university hospital in Brazil. The study was approved by the Institution's Ethics and Research Committee (No. 2360814) and the parents who agreed to the participation of their infants signed the informed consent.
Considering the total number of eligible infants in the analyzed period (n = 60), the unidirectional odds ratio of the standard normal distribution (α = 5%), p = 0.12 (based on the incidence used in the ongoing cohort), and sample error = 0.05, the sample for this study was defined with 40 preterm infants.
Premature infants admitted to the neonatal intensive care unit of both sexes, of all racial/ethnic groups, with gestational age less than 34 weeks, birth weight less than 1500 g and who did not have congenital malformations were selected. Those who died at less than 28 days or without authorized participation in the survey were excluded.
Maternal clinical data (chronic and/or gestational hypertension, diabetes, chorioamnionitis, antennal steroid, peripartum hemorrhage, premature rupture of membranes >18 h, and pre-eclampsia) and neonatal gestational age, sex, birth weight, Apgar score at 5th minute, birth diagnosis, intrauterine growth restriction, infectious risk, days of hospitalization, use of mechanical ventilation on day 2, use of the nasal continuous positive airway pressure (nCPAP), use of antibiotics on day 2, surfactant performance, presence of pulmonary hypertension, early sepsis, persistent ductus arteriosus, and the Score for Neonatal Acute Physiology with Perinatal Extension-II (SNAPPE II) were obtained.
Blood collection was performed between 36 and 48 h of life of the infants in a tube with clot activator plus gel, which is a routine procedure. The blood was centrifuged and the serum stored in a 0.5 mL Safe-Lock Tube; 25 μL of serum was used to quantify levels of the cytokines eotaxin and granulocyte and macrophage colony stimulating factor (GM-CSF) by a multiplex system in a BioPlex Magpix analyzer (Bio-Rad). The assay was performed in 86-well plates according to the manufacturer’s instructions, similar to the sandwich enzyme-linked immunosorbent assay. During the procedure, capture antibodies against the cytokines were coupled to magnetic microspheres, which were reacted with the sample containing the cytokines of interest. After several washes to remove proteins that did not bind the magnetic microspheres, a biotinylated detection antibody was added to create the sandwich complex. To obtain the result, the streptavidin-phycoerythrin conjugate was added to the reaction. After several washes, the median fluorescence intensity of each sample was recorded. Data analysis was performed using Bio-Plex Magpix equipment software (Bio-Rad) and the concentration of the cytokines in the samples was determined by comparison with standard curves equivalent to each cytokine. Values were expressed in pg/mL.
Preterm infants have a predisposition toward eosinophilia after birth, especially those with BPD, which is why eotaxin was chosen.9–11 To identify the impact of IMV on pulmonary structure, GM-CSF was evaluated because it is important in the maturation of alveolar macrophages12 and is present in bronchial aspirates of infants under invasive ventilatory support. BPD was defined as the need for supplemental oxygen at 28 days and severity classification at 36 weeks post-menstrual age.1,2
Statistical analysisThe Shapiro–Wilk test was used to evaluate the normality of the data; Mann–Whitney, Student’s t, or chi-squared tests were used to compare study groups, cytokines, and clinical data, respectively. Odds ratios were used to determine the odds of developing the disease, considering the type and time of ventilatory support used in the first 48 h. Binary logistic regression with stepwise forward was used to identify clinical variables strongly related to BPD. Statistical significance was set at p < 0.05.
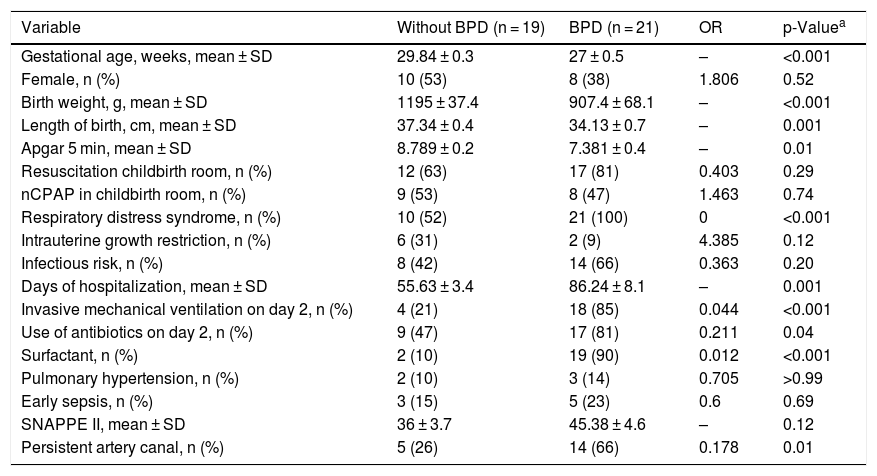
ResultsCharacterization of the sampleOf the 60 preterm infants born in the study’s reference hospital, 17 parents for the preterm infants did not authorize participation, only 43 were considered eligible and three were excluded for having died before 28 days of life. In total, 40 preterm infants with MBPP were divided into two groups: preterm infants who did not develop BPD (n = 19); and premature infants who developed BPD (n = 21). The groups were similar in terms of maternal characteristics regarding diabetes, chronic and/or gestational hypertension, antenatal steroidS, peripartum hemorrhage, premature rupture of membranes at more than 18 h, and pre-eclampsia. The infants’ characteristics with the greatest clinical relevance for the BPD development were selected (Table 1).
Clinical characteristics of the neonates included in the study.
| Variable | Without BPD (n = 19) | BPD (n = 21) | OR | p-Valuea |
|---|---|---|---|---|
| Gestational age, weeks, mean ± SD | 29.84 ± 0.3 | 27 ± 0.5 | – | <0.001 |
| Female, n (%) | 10 (53) | 8 (38) | 1.806 | 0.52 |
| Birth weight, g, mean ± SD | 1195 ± 37.4 | 907.4 ± 68.1 | – | <0.001 |
| Length of birth, cm, mean ± SD | 37.34 ± 0.4 | 34.13 ± 0.7 | – | 0.001 |
| Apgar 5 min, mean ± SD | 8.789 ± 0.2 | 7.381 ± 0.4 | – | 0.01 |
| Resuscitation childbirth room, n (%) | 12 (63) | 17 (81) | 0.403 | 0.29 |
| nCPAP in childbirth room, n (%) | 9 (53) | 8 (47) | 1.463 | 0.74 |
| Respiratory distress syndrome, n (%) | 10 (52) | 21 (100) | 0 | <0.001 |
| Intrauterine growth restriction, n (%) | 6 (31) | 2 (9) | 4.385 | 0.12 |
| Infectious risk, n (%) | 8 (42) | 14 (66) | 0.363 | 0.20 |
| Days of hospitalization, mean ± SD | 55.63 ± 3.4 | 86.24 ± 8.1 | – | 0.001 |
| Invasive mechanical ventilation on day 2, n (%) | 4 (21) | 18 (85) | 0.044 | <0.001 |
| Use of antibiotics on day 2, n (%) | 9 (47) | 17 (81) | 0.211 | 0.04 |
| Surfactant, n (%) | 2 (10) | 19 (90) | 0.012 | <0.001 |
| Pulmonary hypertension, n (%) | 2 (10) | 3 (14) | 0.705 | >0.99 |
| Early sepsis, n (%) | 3 (15) | 5 (23) | 0.6 | 0.69 |
| SNAPPE II, mean ± SD | 36 ± 3.7 | 45.38 ± 4.6 | – | 0.12 |
| Persistent artery canal, n (%) | 5 (26) | 14 (66) | 0.178 | 0.01 |
nCPAP, nasal continuous positive airway pressure; SNAPPE, Score for Neonatal Acute Physiology with Perinatal Extension-II; BPD, bronchopulmonary dysplasia.
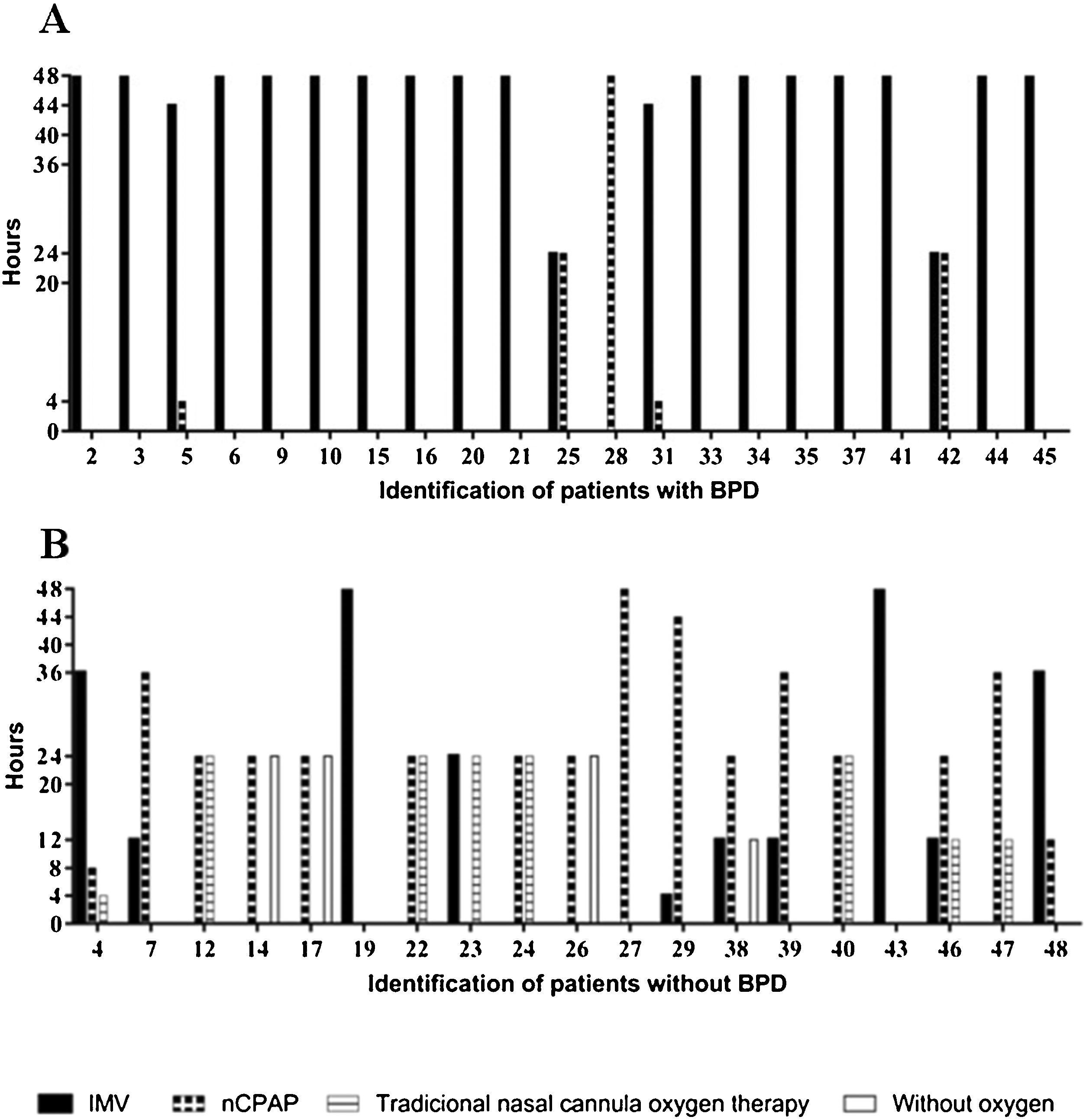
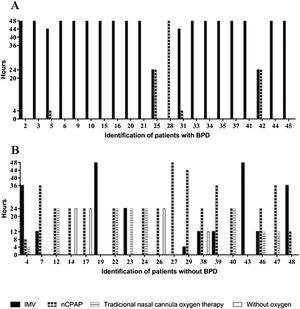
All of the preterm infants included in this study used some ventilatory support or oxygen supplementation in the first 48 h, with a predominance of IMV in the group with BPD and nasal continuous positive airway pressure (nCPAP) or traditional nasal cannula oxygen therapy in the group without BPD (Fig. 1).
Characterization of the types of ventilatory support used in the first 48 h of life by very low birth weight preterm infants and duration of treatments.
nCPAP, nasal continuous positive airway pressure; IMV, invasive mechanical ventilation; traditional nasal cannula oxygen therapy and without oxygen of preterm infants with and without bronchopulmonary dysplasia (BPD). Types of ventilatory support used of preterm infants with BPD in the first 48 h of life (A), types of ventilatory support used of preterm infants without BPD in the first 48 h of live (B).
Preterm infants who used more than 40 continuous hours (OR: 51; CI: 6.786–262.2; p ≤ 0.0001) of IMV and less than six continuous hours of nCPAP (OR: 32; CI: 5.255–137.4; p ≤ 0.0001) had an increased chance of developing BPD.
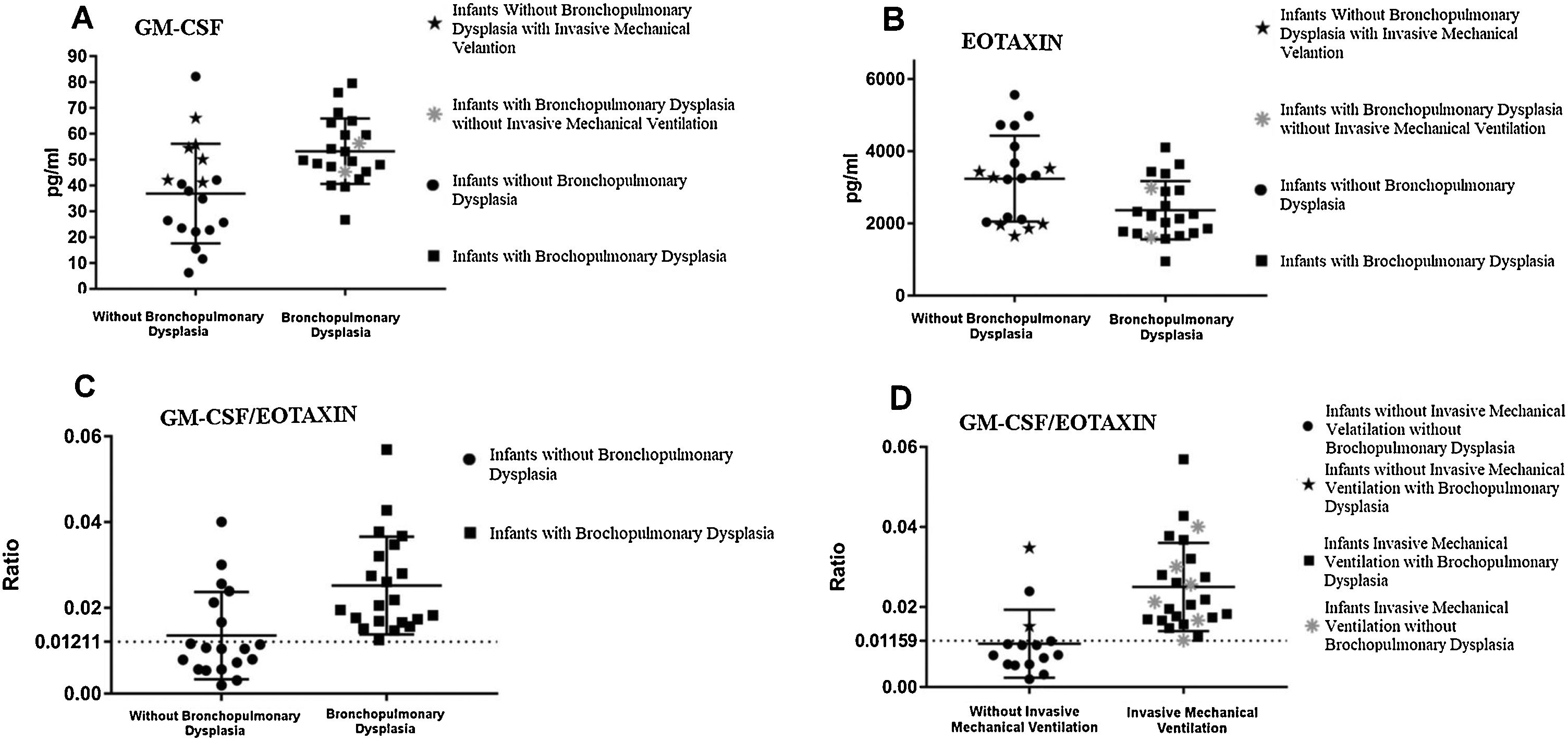
Cytokines analyzed in peripheral blood and ventilatory supportThere was a statistically significant difference between the groups GM-CSF (p = 0.0024; CI = 6.24–27.14) and eotaxin (p = 0.0205; CI = −1588 to −124.6; Fig. 2A and B). Serum level of GM-CSF was higher in the BPD group and eotaxin level was higher in the non-BPD group.
Analysis of the serum levels of granulocyte and macrophage colony stimulating factor (GM-CSF) and eotaxin in the first 48 h of life in groups with and without bronchopulmonary dysplasia.
A, Serum levels of GM-CSF in the first 48 h of life in the groups with and without bronchopulmonary dysplasia (BPD); B, Serum levels of eotaxin in the first 48 h of life in the groups with and without bronchopulmonary dysplasia (BPD); C, GM-CSF and eotaxin ratio analysis of preterm infants of very low birth weight in the first 48 h of life, with and without bronchopulmonary dysplasia (BPD); D, GM-CSF/eotaxin ratio related to the use of mechanical ventilation during the first 36–48 h of life, and stratification of patients with and without bronchopulmonary dysplasia (BPD).
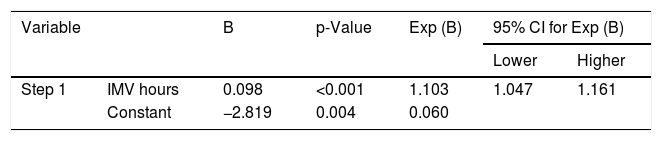
It was possible to observe that the time in IMV hours was determinant for BPD development (Table 2).
Stepwise binary logistic regression with the clinical and laboratory variables of the studied neonates.
| Variable | B | p-Value | Exp (B) | 95% CI for Exp (B) | ||
|---|---|---|---|---|---|---|
| Lower | Higher | |||||
| Step 1 | IMV hours | 0.098 | <0.001 | 1.103 | 1.047 | 1.161 |
| Constant | −2.819 | 0.004 | 0.060 | |||
IMV, invasive mechanical ventilation; CI, confidence interval; B, stepwise binary logistic regression.
The ratio between GM-CSF and eotaxin was used in an attempt to understand the underlying immune mechanism related to BPD, which allowed for significant differentiation between groups (p = 0.0004; CI = 0.006197 to 0.01653). When the cutoff value was set to 0.01211 through the receiver operating characteristic (ROC) curve (100% sensitivity and 68.42% specificity; area = 0.8145, CI 0.6711 to 0.958, p = 0.0007), it was possible to identify cases of BPD (Fig. 2C).
When analyzing the groups according to the use of IMV during the first 36 and 48 h of life, regardless of whether or not they developed BPD, it was possible to visualize how many infants were using IMV at the time of collection using the ROC curve (100% sensitivity and 80% specificity; area = 0.9013, CI = 0.7791–1.024, p < 0.0001) when the cut off value was defined as 0.01159 (Fig. 2D).
DiscussionThis investigation showed that the type and duration of mechanical ventilatory support used in the first 48 h of life by preterm infants is critical for BPD development. It was revealed that during the first 48 h of life the time under continuous IMV was the only predictive variable for BPD. It was also shown that the GM-CSF and eotaxin levels were associated with BPD due to the duration of IMV in the first 48 h of life. Interestingly, the two markers presented opposite levels, and higher ratios between GM-CSF and eotaxin were able to predict aggression of the pulmonary structure generated by IMV with 100% sensitivity.
It is known that IMV induces pulmonary lesions as a result of necrosis, apoptosis, and inflammation that compromise type I and II alveolar epithelial cells, fibroblasts, and endothelial cells, and alter the alveolar-capillary barrier. These changes lead to the release of pro-inflammatory cytokines, increased permeability, and influx of neutrophils and macrophages into the lung.13,14
It was also observed that during the first two days of life, infants who did not develop BPD used predominantly nCPAP and traditional nasal cannula oxygen therapy. This result reinforces indication of the use of non-invasive ventilation in the conduction of respiratory distress syndrome in VLBW preterm infants in an attempt to avoid the harmful effects of IMV.15–17 Previous studies have shown that increased IMV time causes pulmonary structural deformity and, consequently, ventilation/perfusion impairment and increased oxygen dependence.4,5
In recent years, there has been a significant increase in the use of non-invasive ventilatory support. However, this did not reduce oxygen dependence at 36 weeks and no improvement in lung function was observed in childhood.18 Therefore, an alternative would be to associate the use of biomarkers in clinical management to identify early neonates at risk for developing BPD. Many studies have evaluated biomarkers in bronchial aspirate fluids, which cannot be performed in preterm infants who are not using IMV.19 Thus, the present study analyzed blood samples for the levels of GM-CSF and eotaxin. Few studies have reported similar methodologies with blood samples, but have not analyzed eotaxin levels.20
The level of GM-CSF in the BPD group presented higher values compared to the control group, which may suggest macrophage stimulation and increase by GM-CSF, in an attempt to control the aggression suffered by the lung. GM-CSF is important in the maturation of alveolar macrophages,12 and thus exerts a beneficial effect in the infected lung, promoting the differentiation, accumulation, activation, and localization of alveoli infected by dendritic cells and alveolar macrophages.21
Eotaxin presented high levels in the group without BPD, which suggests that these VLBW preterm infants were more sensitive to eosinophilic changes. There is a tendency for preterm infants to develop eosinophilia after birth due to procedures and interventions during hospitalization.9 However, high levels are common in infants with BPD and in extremely low birth weight preterm infants,9,11 which differs from the data found in this study. This result can be explained by the attempt to create a compensatory mechanism, since the concomitant overexpression of macrophages and eosinophils generates irreversible fibrosis.22
Another relevant result observed in this study was the analysis of the GM-CSF and eotaxin ratio, in an attempt to evaluate the behavior of the underlying immunity, as well as to verify the balance of T helper (Th) cells during the first days of life.23 Th1 cells form a heterogeneous set of protective cells, which are responsible for the activation of phagocytes and protection against intracellular microorganisms. Th2 cells protect the body mainly from parasites, and Th17 cells protect against fungi and extracellular bacteria.24,25
Zielinski, in a 2014 study with mice, observed that GM-CSF had a Th17 function related to autoimmune or chronic diseases, but when evaluated in human cells, expressed Th1 characteristics.26 In neuroinflammatory autoimmune diseases affecting children, GM-CSF has a different signaling pathway than Th1 and Th17, which raises the possibility of an alternative pathway for GM-CSF stimulation.27,28
Eotaxin is a member of the Th2 cytokine family11 and binds CCR3 in the lungs, which, in turn, has a profibrinogenic capacity and may lead to pulmonary remodeling.11,29 On the second day investigated, there was a balance between the Th cells triggered by GM-CSF and Th2 cells, which suggests that when there is an increase in GM-CSF levels there is a decrease in eotaxin, and vice versa. This may be a result of the body’s attempt to avoid definitive fibrosis.22
When the use of IMV in both groups between 36 and 48 h was associated with the GM-CSF/eotaxin ratio, it was possible to see how the cytokines reacted to the use of IMV, since the use of IMV resulted in higher values. With this result, it is possible to affirm that short periods of IMV activate a pro-inflammatory cascade.30
Despite the differences between groups, cytokines alone did not show a prediction for the diagnosis of BPD; they only tended to indicate the influence of IMV on the body of VLBW preterm infants. Further studies are needed to expand the investigation of the role of serum inflammatory cytokines, clinical variables, and IMV associated with the prediction and diagnosis of BPD.
In conclusion, after monitoring the second day of life, the duration of IMV used was considered a predictive factor for BPD, since the longer its continuous use, the greater the chances of preterm infants developing the disease. The clinical variables and cytokines evaluated were not related to prediction of the disease, but only indicated the aggression generated by IMV in the pulmonary structure of the preterm infant. Future research is needed not only to validate the use of both markers as predictors, but also to determine whether they are potential therapeutic targets for BPD.
FundingBrazilian funding agencies, CNPq, CAPES, and FAPEMIG, for providing financial support to the National Institute of Science and Technology in Theranostics and Nanobiotechnology – INCT-TeraNano (CNPq/CAPES/FAPEMIG, grant numbers CNPq-465669/2014-0 and FAPEMIG-CBB-APQ-03613-17).
Conflicts of interestThe authors declare no conflicts of interest.
The authors are grateful to Coordination for the Higher Education Personnel – Brazil (CAPES).