To evaluate the effectiveness of videothoracoscopic surgery in the treatment of complicated parapneumonic pleural effusion and to determine whether there is a difference in the videothoracoscopic surgery outcome before or after the chest tube drainage.
MethodsThe medical records of 79 children (mean age 35 months) undergoing videothoracoscopic surgery from January 2000 to December 2011 were retrospectively reviewed. The same treatment algorithm was used in the management of all patients. Patients were divided into two groups: in group 1, videothoracoscopic surgery was performed as the initial procedure; in group 2, videothoracoscopic surgery was performed after previous chest tube drainage.
ResultsVideothoracoscopic surgery was effective in 73 children (92.4%); the other six (7.6%) needed another procedure. Sixty patients (75.9%) were submitted directly to videothoracoscopic surgery (group 1) and 19 (24%) primarily underwent chest tube drainage (group 2). Primary videothoracoscopic surgery was associated with a decrease of hospital stay (p=0.05), time to resolution (p=0.024), and time with a chest tube (p<0.001). However, there was no difference between the groups regarding the time until fever resolution, time with a chest tube, and the hospital stay after videothoracoscopic surgery. No differences were observed between groups regarding the need for further surgery and the presence of complications.
ConclusionsVideothoracoscopic surgery is a highly effective procedure for treating children with complicated parapneumonic pleural effusion. When videothoracoscopic surgery is indicated in the presence of loculations (stage II or fibrinopurulent), no difference were observed in time of clinical improvement and hospital stay among the patients with or without chest tube drainage before videothoracoscopic surgery.
Avaliar a eficácia da cirurgia torácica vídeoassistida no tratamento de derrame pleural parapneumônico complicado e determinar se há diferença no resultado da cirurgia torácica vídeoassistida realizada antes ou depois da drenagem torácica.
MétodosAnalisamos retrospectivamente prontuários médicos de 79 crianças (idade média de 35 meses) submetidas a cirurgia torácica vídeoassistida de janeiro de 2000 a dezembro de 2011. O mesmo algoritmo de tratamento foi utilizado no manejo de todos os pacientes. Os pacientes foram divididos em dois grupos: o Grupo 1 foi submetido a cirurgia torácica vídeoassistida como procedimento inicial; o Grupo 2 foi submetido a cirurgia torácica vídeoassistida após drenagem torácica prévia.
ResultadosA cirurgia torácica vídeoassistida foi eficaz em 73 crianças (92,4%); as outras seis (7,6%) necessitaram outro procedimento. Sessenta pacientes (75,9%) foram diretamente submetidos a cirurgia torácica vídeoassistida (Grupo 1) e 19 (24%) foram primeiramente submetidos a drenagem torácica (Grupo 2). A cirurgia torácica vídeoassistida primária foi associada à redução do tempo de internação (p=0,05), do tempo para resolução (p=0,024) e do tempo com o tubo torácico (p<0,001). Contudo, não houve diferença entre os grupos a respeito do tempo até que não tivessem mais febre, do tempo com o tubo torácico e do tempo de internação após a cirurgia torácica vídeoassistida. Não foram observadas diferenças entre os grupos com relação à necessidade de cirurgia adicional e à presença de complicações.
ConclusõesA cirurgia torácica vídeoassistida é um procedimento altamente eficaz para tratar crianças com derrame pleural parapneumônico complicado. Quando a cirurgia torácica vídeoassistida é indicada na presença de loculações (fase II ou fibrinopurulenta) não há diferença no tempo de melhora clínica e no tempo de internação entre os pacientes com ou sem drenagem torácica antes da cirurgia torácica vídeoassistida.
An estimate recently published by the World Health Organization (WHO)1 shows Brazil among the 15 countries with the highest absolute number of new cases of pneumonia per year (1.8 million), with an estimated incidence of 0.11 episodes per child-year. Bacterial pneumonia in children is often accompanied by pleural effusion,2 present in 40% of cases,3 of which 5%–10% may progress to complicated pleural effusion and/or empyema.4 Based on these data, it is estimated that approximately 14,000–20,000 new cases of complicated pleural effusion occur in children each year in Brazil.
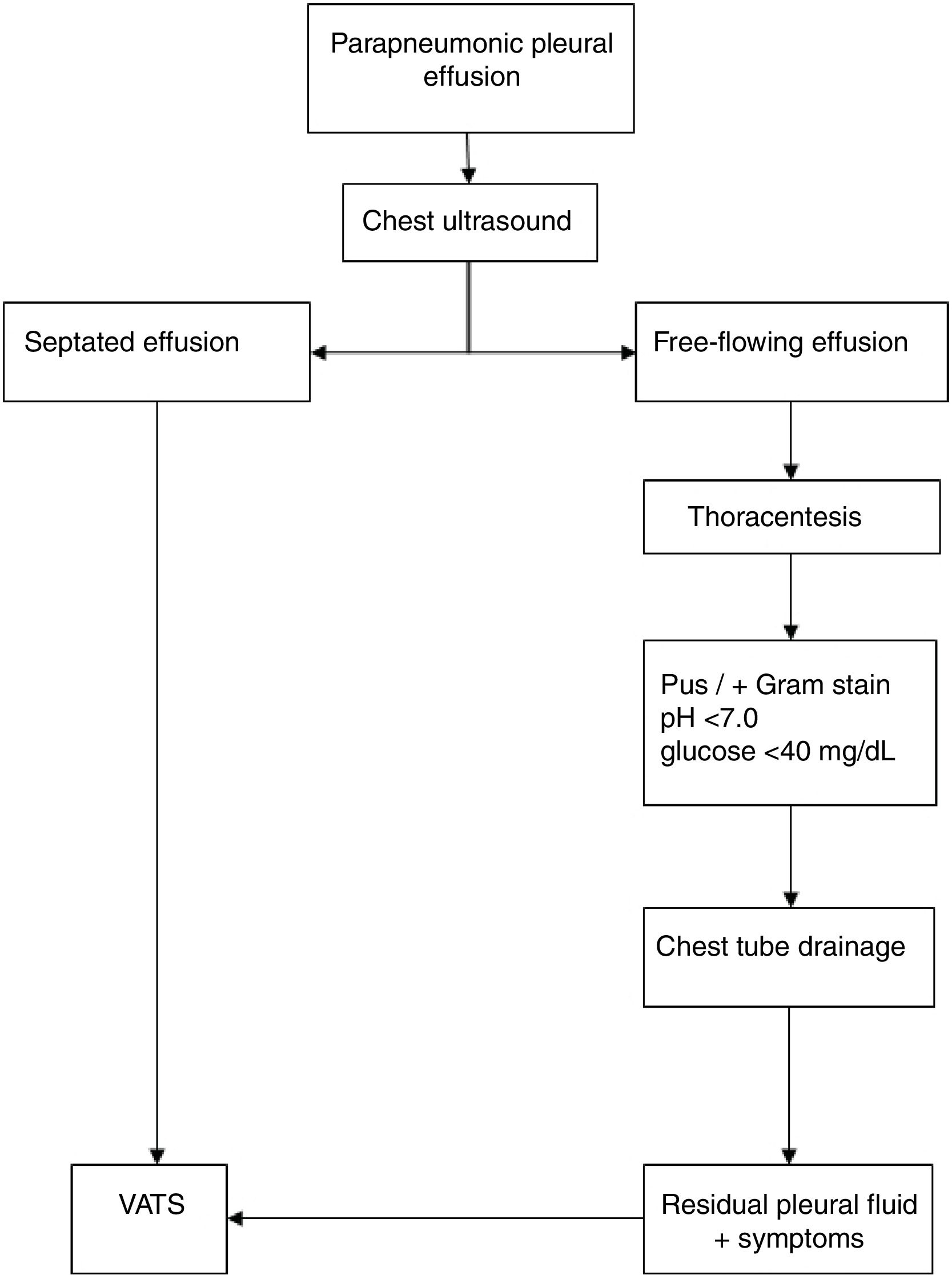
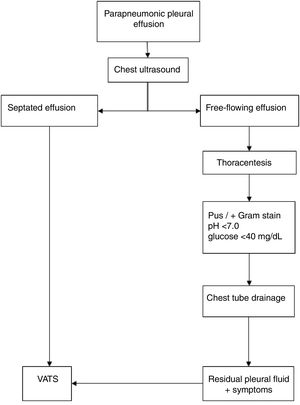
Parapneumonic pleural effusion (PPE) is regarded as complicated (CPPE) when antibiotics alone are insufficient, and a surgical procedure3 is required for an adequate drainage the infected pleural space. In this case, the pleural fluid is characterized by positive bacterial studies, a glucose level below 40mg/dL, a pH below 7.0, and progressive loculation.5
Controversy remains on the subject of the optimal treatment of pediatric CPPE.6,7 Treatment options range from simple chest tube drainage (CTD), with or without fibrinolytic instillation, and the less invasive videothoracoscopic surgery (VATS), to more invasive thoracotomy and lung decortication of the lung; the intermediate option is represented by minithoracotomy.8 The choice of surgical intervention often depends on the clinical condition of the patient, stage of CPPE at diagnosis, and expertise and experience of local staff9; the treatment option has substantial economic implications.10 It remains unclear whether the choice and timing of the different therapeutic options influence the duration of illness and the length of hospital stay.11
VATS is an effective option for pleural debridement in cases of loculated or septated effusion, allowing cleaning of the infected pleural space under direct vision and full expansion of the lung, thus preventing the morbidity associated with conventional thoracotomy. When indicated, it was proven to be effective in the treatment of empyema, with an overall success rate ranging from 83% to 97%, and associated with less postoperative pain, shorter hospital stay, and better cosmetic results.12,13 However, the question regarding the ideal time to perform the procedure persists. Some authors suggest that VATS should be performed immediately after the diagnosis of PPE,14 while others suggest waiting a few days.15 Another management option is CTD, with or without fibrinolytic instillation, as the initial procedure, reserving VATS for patients who do not present a favorable clinical evolution after this procedure.13,16–18
This study aimed to report the experience with VATS in the treatment of children with CPPE, determining its effectiveness and whether or not the use of initial CTD might influence VATS outcome.
MethodsRetrospective analysis of medical records of all children aged 1 month to 12 years, with CPPE diagnosis who underwent VATS at Hospital de Clínicas de Porto Alegre (HCPA) and Hospital Moinhos de Vento (HMV), in Porto Alegre, southern Brazil, between January 2000 and December 2011. The study was approved by the institutional review boards of HCPA (number 100166) and HMV (number 185999).
CPPE diagnosis was performed on a clinical basis (fever, dyspnea, or deterioration of general state) and confirmed through radiologic evaluation, thoracentesis, and ultrasound studies. The diagnosis of CPPE was determined by the presence of pus on the macroscopic analysis; by the presence of pleural septations or loculations in the ultrasound; or by biochemical analysis showing pH<7.0 or glucose<40mg/dL, and the presence of bacteria on Gram stain and/or culture. The same treatment algorithm was used in the management of CPPE (Fig. 1),12 and all patients received intravenous antibiotics.
Children with immunodeficiency, tuberculous effusion, and those with severe cerebral palsy associated with gastroesophageal reflux and swallowing disorders were excluded from the study.
For the study, patients were divided into two groups: primary or secondary VATS. Primary CTD was indicated for patients with CPPE with pleural fluid without septated or loculated at ultrasound; VATS was indicated as the initial (primary) procedure in the presence of persistent fever or worsening of the patient's status, accompanied by septated or loculated pleural effusion at ultrasound. The indication for secondary VATS after CTD was the presence of residual fluid after drainage, accompanied by symptoms of fever, worsening of the patient's status, or sepsis, with septations or loculations at ultrasound.
A standardized data form was completed for each patient. Time to resolution was calculated by adding the interval (in days) between diagnosis of pleural effusion and hospital admission to the length of hospital stay. Treatment failure was defined as the need for further surgical interventions after VATS.
All surgical procedures were performed or supervised by the same surgeon (JCF), in accordance with a previously described technique.12 Appropriate analgesic management was provided for all patients through intercostal nerve blocks and intravenous analgesia with morphine.
Quantitative data were expressed as mean or median, and categorical variables as percentage. Variables were compared by Student’ t, Wilcoxon–Mann–Whitney, Fisher's exact, or Pearson's chi-squared tests. Values were statistically significant when p-value<0.05. Data were analyzed using SPSS (Statistical Package for the Social Sciences, Version 17. College Station, TX, USA).
ResultsA total of 79 children were included in the analysis; 41 (51.9%) male and 38 (48.1%) female. Sixty-two (78.5%) patients were operated on at HCPA and 17 (21.5%) at HMV. Aged ranged from 2 to 137 months (mean: 35 months). Weight ranged from 5.14 to 48kg (mean: 15.0kg). Ten children (12.6%) had one or more associated diseases, and asthma was the most common. The interval between symptom onset and hospital admission ranged from 1 to 11 days. Thirty-six (45%) patients received antibiotics before admission.
Forty-seven (60%) children had right-sided effusion, and 34 (43%) patients had large effusions (over one-third of the hemithorax). Of 79 patients analyzed, two (2.5%) did not have ultrasound examination and eight (10%) required computed tomography (CT) of the chest. Bacteriological examination of pleural fluid, as well as blood and pleural fluid cultures, were performed in all children. An etiologic agent at pleural fluid was detected in 22 (27.8%) children: Streptococcus pneumoniae in 14 (17.7%); Staphylococcus aureus in seven (8.9%); and a Gram-negative rod in one (1.3%).
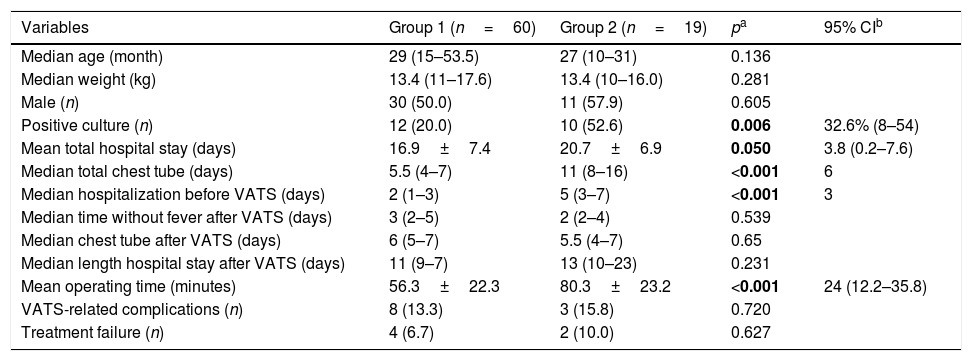
Table 1 shows the comparison between the primary VATS and secondary VATS after CTD. Sixty patients (75.9%) underwent primary VATS (Group 1). Primary VATS reduced hospitalization by 3.8 days (p=0.05), time to resolution by 4.4 days (p=0.024), and time with a chest tube by 6 days (p<0.001). CTD was performed prior to VATS (Group 2) in 19 (24%) children. CTD before VATS increased operating time by 24minutes (p<0.001) and resulted in a delay of 3 days in performing VATS (p<0.001). However, when evaluating the outcome after VATS, it was observed that time until fever resolution, time with chest tube, and length of stay were not significantly different, presenting a similar behavior on clinical outcomes in both groups. Treatment complication and failure rates were similar in both groups.
Comparison between children who underwent primary (group 1) and secondary (group 2) VATS.
| Variables | Group 1 (n=60) | Group 2 (n=19) | pa | 95% CIb |
|---|---|---|---|---|
| Median age (month) | 29 (15–53.5) | 27 (10–31) | 0.136 | |
| Median weight (kg) | 13.4 (11–17.6) | 13.4 (10–16.0) | 0.281 | |
| Male (n) | 30 (50.0) | 11 (57.9) | 0.605 | |
| Positive culture (n) | 12 (20.0) | 10 (52.6) | 0.006 | 32.6% (8–54) |
| Mean total hospital stay (days) | 16.9±7.4 | 20.7±6.9 | 0.050 | 3.8 (0.2–7.6) |
| Median total chest tube (days) | 5.5 (4–7) | 11 (8–16) | <0.001 | 6 |
| Median hospitalization before VATS (days) | 2 (1–3) | 5 (3–7) | <0.001 | 3 |
| Median time without fever after VATS (days) | 3 (2–5) | 2 (2–4) | 0.539 | |
| Median chest tube after VATS (days) | 6 (5–7) | 5.5 (4–7) | 0.65 | |
| Median length hospital stay after VATS (days) | 11 (9–7) | 13 (10–23) | 0.231 | |
| Mean operating time (minutes) | 56.3±22.3 | 80.3±23.2 | <0.001 | 24 (12.2–35.8) |
| VATS-related complications (n) | 8 (13.3) | 3 (15.8) | 0.720 | |
| Treatment failure (n) | 4 (6.7) | 2 (10.0) | 0.627 |
95% CI, 95% confidence interval; VATS, videothoracoscopic surgery.
VATS was effective in 73 (92.4%) children with CPPE. Six (7.6%) patients required other surgical procedures for the resolution of CPPE. Two needed further pleural drainage and four children required a second VATS. Three of the children undergoing a second VATS procedure showed good resolution, and one required open thoracostomy. Children requiring further surgical procedures showed a prolonged hospitalization and increased time to resolution, with a mean length of hospital stay of 30 days (p<0.001) and an additional time with a tube that was twice as long as those of with no treatment failures (p=0.011).
Eleven (13.9%) patients had VATS-related complications: subcutaneous emphysema (n=5), intraoperative decrease in oxygen saturation (n=3), small postoperative pneumothorax (n=1), intraoperative blood transfusion (n=1), and bronchopleural fistula (n=1). All of them recovery very well and no deaths were observed during the study and postoperative follow-up.
DiscussionThe present results demonstrated that VATS is a safe and effective procedure in pediatric patients with CPPE. The effectiveness of VATS was 92.4%, a rate within the range reported in the literature.12,13,19–21 Kern and Rodgers22 were the first pediatric surgeons to report the effectiveness of VATS in the treatment of children with pleural empyema. Since then, VATS has been increasingly used and currently plays an important role in the treatment of CPPE. Given the improvement of materials and minimally invasive surgical techniques, the need to perform conventional thoracotomy has decreased, being used only as rescue treatment in complex cases with lung entrapment, or for treatment of bronchopleural fistula with pyopneumothorax.23
VATS has been successfully used as the initial treatment of CPPE since 1993,22 resulting in complete empyema resolution and shorter hospitalization than CTD alone.14 However, the indication and the ideal timing of VATS remain controversial. Kalfa et al.19 demonstrated that a four-day interval between CPPE diagnosis and surgery is the main factor contributing to the effectiveness of thoracoscopic treatment of pleural empyema in children. This may be considered the ideal timing of VATS, because according to the pathophysiology of CPPE, this corresponds to the early phase of the fibrinopurulent stage, in which fibrin deposition in the pleural space is present, leading to septation and the formation of loculations.
However, some retrospective series have reported successful outcomes with primary non-operative management.7 Furthermore, prospective randomized trials comparing different treatment options have been published. Sonnappa et al.13 performed the first randomized controlled trial of pediatric CPPE at the Great Ormond Street Hospital for Children, in London. Those authors then compared VATS to chest tube and urokinase infusion. They found no significant difference in length of stay, days with a chest tube, and post-intervention fever. Similar results were obtained by St Peter et al.16 in the United States. Most recently, in Spain, Marhuenda et al.18 published a multicenter randomized trial comparing 53 patients treated with VATS and 50 patients treated with chest and urokinase infusion. Similarly to the previous studies, they found no difference in post-intervention length of stay, total length of stay, post-intervention fever days, or failure rate, but they observed fewer CTD days in the VATS group compared with the urokinase group. In 2011, in Turkey, Cobanoglu et al.,24 in a randomized study, compared 27 patients with VATS to 27 patients with tube thoracostomy and streptokinase infusion. They found that patients treated with VATS had a significantly shorter overall length of stay. Lastly, in 2016, a large prospective nationwide surveillance study, in Germany,6 did not observe a difference in the length of hospital stay and rate failure after CPPE diagnosis in children who were treated with intrapleural fibrinolytic therapy or VATS.
The current evidence suggests that management with VATS and non-operative management with CTD and fibrinolytic is equally successful; indeed, the guidelines of the British Thoracic Society state both approaches as parallel options,3 and in a very recent National Survey in Unites States, there was also disagreement as to the optimal first-line method of drainage when this is indicated, with 37.1% vs. 36.9% respondents preferring a tube thoracostomy with a fibrinolytic agent and tube thoracostomy with VATS, respectively.9 It is therefore reasonable to manage patients with either treatment strategy based upon local expertise and success rates.7
VATS is the authors’ procedure of choice in the treatment of CPPE due to several advantages. VATS allows, under direct vision, disruption of loculations, debridement, and drainage of the infected pleural space, ensuring full lung expansion.12 VATS has been compared to thoracic drainage for the treatment of CPPE, presenting a shorter hospital stay, reduced use of antibiotics and narcotics, fewer days of chest tube use, lower hospital costs, and reduction in imaging and interventional procedures.24–28 Moreover, urokinase is not commercially available in Brazil and alteplase (tissue plasminogen activator) is very expensive.
In the present study, when comparing patients undergoing primary or secondary VATS, no difference was observed between both groups, except for operating time, which was higher in the group of VATS after CTD. Thus, the authors believe that when VATS is indicated in the presence of loculations or septations, the outcome tends to be the same, regardless if it was initially performed CTD. This is due to the fact that the procedure is indicated based on the pathophysiological evolution of disease, i.e., VATS at stage II (where CTD cannot remove all fibrinopurulent material). CTD must be indicated at first stage of CPPE, with free-flowing effusion, without pleural fluid loculation or septation. The authors believe that these criteria reflect the evolution of CPPE better than isolated temporal aspects.
A limitation of this study is the fact that data were collected retrospectively from two different institutions over a long period. However, the same treatment algorithm and the same operative technique were used in the management of all patients. Because the group with treatment failure was small, it was not possible to identify, due to lack of statistical power, factors that might predict VATS failure. Although children in group 1 had significantly more positive cultures, this could indicate a more active disease at the time of CTD insertion. This may cause some bias in the results concerning time to resolution and length of hospital stay.
In conclusion, no differences in effectiveness or post-interventional CPPE course were observed when comparing VATS with or without previous CTD. The present data do not support prior reports that the four-day interval is critical for the effectiveness of VATS. The authors believe that once the indication of VATS is standardized as cases with presence of septations and/or loculations, any differences related to the clinical course of these patients will not be significant.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Knebel R, Fraga JC, Amantea SL, Isolan PB. Videothoracoscopic surgery before and after chest tube drainage for children with complicated parapneumonic effusion. J Pediatr (Rio J). 2018;94:140–145.
Study carried out at the Universidade Federal do Rio Grande do Sul (UFRGS), Faculdade de Medicina, Programa de Pós-graduação em Medicina: Ciências Cirúrgicas, Porto Alegre, RS, Brazil.