To gather current evidence on the use of fiber for constipation treatment in pediatric patients.
Source of dataSystematic review with meta-analysis of studies identified through Pubmed, Embase, LILACS and Cochrane databases published up to 2016.
Inclusion criteriaRandomized controlled trials; patients aged between 1 and 18 years and diagnosed with functional constipation receiving or not drug treatment for constipation; articles published in Portuguese, English, Spanish, French, and German in journals accessible to the researchers.
Synthesis of dataA total of 2963 articles were retrieved during the search and, after adequate evaluation, nine articles were considered relevant to the study objective. A total of 680 children were included, of whom 45% were boys. No statistical significance was observed for bowel movement frequency, stool consistency, therapeutic success, fecal incontinence, and abdominal pain with fiber intake in patients with childhood constipation. These results should be interpreted with care due to the high clinical heterogeneity between the studies and the methodological limitation of the articles selected for analysis.
ConclusionsThere is a scarcity of qualified studies to evaluate fiber supplementation in the treatment of childhood constipation, generating a low degree of confidence in estimating the real effect of this intervention on this population. Today, according to the current literature, adequate fiber intake should only be recommended for functional constipation, and fiber supplementation should not be prescribed in the diet of constipated children and adolescents.
Reunir evidências atuais sobre o uso de fibras no tratamento da constipação funcional em pacientes pediátricos.
Fontes dos dadosRevisão sistemática com metanálise de estudos identificados por pesquisa nas bases de dados Pubmed, Embase, LILACS e Cochrane publicados até o ano de 2016. Critérios de inclusão: estudos controlados randomizados; pacientes com idade entre 1 a 18 anos com diagnóstico de constipação funcional em uso ou não de tratamento medicamentoso para constipação; artigos publicados em língua portuguesa, inglesa, espanhola, francesa e alemã em revistas acessíveis aos pesquisadores.
Síntese dos dadosForam encontrados 2.963 artigos na busca e, após avaliação adequada, nove artigos mostraram-se relevantes frente aos objetivos do estudo. Um total de 680 crianças foram incluídas, sendo 45% meninos. Não foi demonstrado significância estatística da frequência evacuatória, da consistência evacuatória, do sucesso terapêutico, da incontinência fecal e da dor abdominal com o uso de fibras nos pacientes com constipação infanto-juvenil. Esses resultados devem ser interpretados com atenção devido à alta heterogeneidade clínica entre os estudos e à limitação metodológica dos artigos analisados.
ConclusõesExiste uma grande falta de estudos qualificados para avaliar a suplementação de fibras no tratamento da constipação infanto-juvenil, gerando um baixo grau de confiança para se estimar o efeito real dessa intervenção na população em questão. Até esse momento, conforme a literatura atual, deve-se apenas recomendar a ingestão adequada de fibras na constipação funcional, não se podendo prescrever a suplementação de fibras na dieta das crianças e adolescentes constipados.
In pediatrics, constipation is defined as a delay in or resistance to evacuate, with a history of two or fewer bowel movements per week, associated with fecal incontinence, fecal retention, and/or pain during bowel movement.1,2 It is classified as functional when, after clinical evaluation and physical examination of the pediatric patient, it cannot be attributed to any intestinal or extra-intestinal disorder, according to the ROME IV consensus.2
Functional constipation is the result of voluntary fecal retention by the child or adolescent related to the fear of evacuating. After frequent unsuccessful attempts to evacuate, a vicious cycle is created: the greater the refusal to evacuate, the greater the stool retention, which will dry out and increase in volume, thus causing more discomfort.1,3
Constipation is frequently observed in the pediatric age group, being the main complaint in 3–5% of consultations with pediatricians and in 25% of consultations with pediatric gastroenterologists.4,5 Worldwide, the prevalence ranges from 3% to 29.6%6,7; in Brazil, it ranges between 17.5 and 38.4%,4,8 due to the different diagnostic criteria used for the definition of functional constipation. Its peak incidence occurs during the sphincter training phase, affecting both genders and with no differences between social classes,1,2 homogeneously affecting all age ranges.7 When chronic, functional constipation has a negative impact on the quality of life of pediatric patients and their families.5,6
A low dietary fiber intake has been considered a risk factor for the development of functional constipation,9 and the increase in fiber consumption is an important factor in its prevention and treatment.4,10 Dietary fibers are divided into insoluble and soluble. Insoluble fibers increase the fecal volume because they resist the action of digestive enzymes and the colonic microflora, absorbing water from the intestinal lumen. Soluble fibers, fermented by the intestinal flora, release adsorbed water and produce fatty acids that result in the co-absorption of electrolytes and fecal water.11
Usually, the initial treatment of constipation in children and adolescents consists in the prescription of fibers by most healthcare professionals.12,13 However, there is still no clear evidence to corroborate the routine use of fiber supplementation in this population's diet as part of functional constipation treatment.10,14
One of the most recent recommendations in the literature on the management of functional constipation in children and adolescents, the consensus of the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition – North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN – NASPGHAN) of 2014, emphasized for the first time that there was no evidence to justify the prescription of fiber.14 This consensus was based on scientific articles published until the year 2011. Aiming to gather more current evidence on the use of fibers in the treatment of functional constipation in pediatric patients, a systematic review with meta-analysis was proposed.
MethodsThis was a systematic review with meta-analysis of randomized controlled trials, with a convenience sample including all articles identified in the search. The studies were identified through a search carried out in the Pubmed, Embase, Lilacs and Cochrane databases. For the search, structured Medical Subject Headings (MeSH) terms were used for PubMed, Emtree for Embase, and Health Sciences Descriptors (DeCS) for Lilacs. The authors also searched for relevant bibliographical references in the gray literature.
The search strategy in the Pubmed database included: “Child”[Mesh] OR “Child” OR “Children” OR “Child, Preschool”[Mesh] OR “Child, Preschool” OR “Preschool Child” OR “Children, Preschool” OR “Preschool Children” OR “Adolescent”[Mesh] OR “Adolescent” OR “Adolescents” OR “Adolescence” OR “Teens” OR “Teen” OR “Teenagers” OR “Teenager” OR “Youth” OR “Youths” OR “Adolescents, Female” OR “Adolescent, Female” OR “Female Adolescent” OR “Female Adolescents” OR “Adolescents, Male” OR “Adolescent, Male” OR “Male Adolescent” OR “Male Adolescents” AND “Constipation”[Mesh] OR “Constipation” OR Dyschezia OR “Colonic Inertia” AND (randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized controlled trials[mh] OR random allocation[mh] OR double-blind method[mh] OR single-blind method[mh] OR clinical trial[pt] OR clinical trials[mh] OR (“clinical trial”[tw]) OR ((singl*[tw] OR doubl*[tw] OR trebl*[tw] OR tripl*[tw]) AND (mask*[tw] OR blind*[tw])) OR (“latin square”[tw]) OR placebos[mh] OR placebo*[tw] OR random*[tw] OR research design[mh:noexp] OR follow-up studies[mh] OR prospective studies[mh] OR cross-over studies[mh] OR control*[tw] OR prospectiv*[tw] OR volunteer*[tw]) NOT (animal[mh] NOT human[mh]).15
Randomized controlled trials (written in Portuguese, English, Spanish, French, and German, published in journals accessible to the researchers) with patients aged between 1 and 18 years, without breast milk consumption and with a diagnosis of functional constipation receiving or not medical treatment for constipation were considered eligible. Studies in which fiber use was not associated with the treatment of functional constipation and studies with incomplete data were excluded.
Two reviewers independently assessed the titles and abstracts of the studies identified during the electronic search, in accordance with the previously established eligibility criteria. In the absence of adequate information in the abstract, the full-text articles were assessed. The reviewers’ assessment was not masked regarding the authors and the results of the studies. A third reviewer was invited to participate in case of divergence regarding the articles selected by the first reviewers. After a consensus was achieved, all studies retrieved were stored in the EndNoteWeb program (EndNoteWeb, Microsoft®, WA, USA).
The Cochrane tool was used to assess the risk of bias in the studies,16 as well as the Jadad scale,17 which allows rating the quality of the studies through five simple questions, with a value of 0–5 points being assigned to each study; a score equal to or lower than 3 reflects a lower quality study.
The studies were grouped for the meta-analysis. Dichotomous variables were expressed as proportions (percentage) and continuous variables as mean and standard deviation (SD). The summary measure based on the standardized mean difference (SMD) was used for continuous variables and odds ratio (OR) was used for binary variables. These summary measures and their respective 95% confidence intervals (95% CI) were obtained from a random effect model. The inconsistency test (I2) was used to assess the heterogeneity between the studies. Only one analysis per subgroup was performed, due to the small number of available articles. A p-value<0.05 was considered as statistically significant.
Mozaffarpur et al. categorized fecal consistency data based on a visual scale ranging from 0 to 100, with 0 defining soft and comfortable consistency and 100, hardened.18 As this scale uses a reverse value direction when compared with the Bristol Scale (1–7, with 1 being very hard and 7 liquid stool),19 the means were subtracted from the scale's maximum value to reflect the results obtained in the other scales, as suggested by the Cochrane Handbook for Systematic Reviews of Interventions.16
Nimrouzi et al. showed stool frequency and fecal consistency as median and IQ (interquartile range),20 as Chmielewska et al. presented the evacuation frequency results.21 The conversion calculation into means described by the Cochrane Handbook for Systematic Reviews of Interventions was used by subtracting the IQ values and subsequent division by 1.35.16 A sensitivity analysis was performed excluding the two articles of each outcome in which they were included.
Kokke et al. presented the results of fecal consistency as mean and statistical significance (p-value) for the Student's t-test, not presenting the SD,22 which was calculated based on the calculations available in the Cochrane Handbook for Systematic Reviews of Interventions.16
Weber et al. used therapeutic failure as the primary outcome, considering the therapeutic success in the final analysis as the total number of participants in the study minus the percentage of failure.23 Moreover, the patients’ individual data of this study were obtained directly from the researchers and were calculated as mean and SD.
The sensitivity analysis was performed through the sequential omission of each study, using one-by-one exclusion for each mentioned outcome. Forest plot charts were reported for each outcome. A funnel plot of each analyzed outcome was assessed for publication bias. The statistical analysis was performed using the program Review Manager (Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
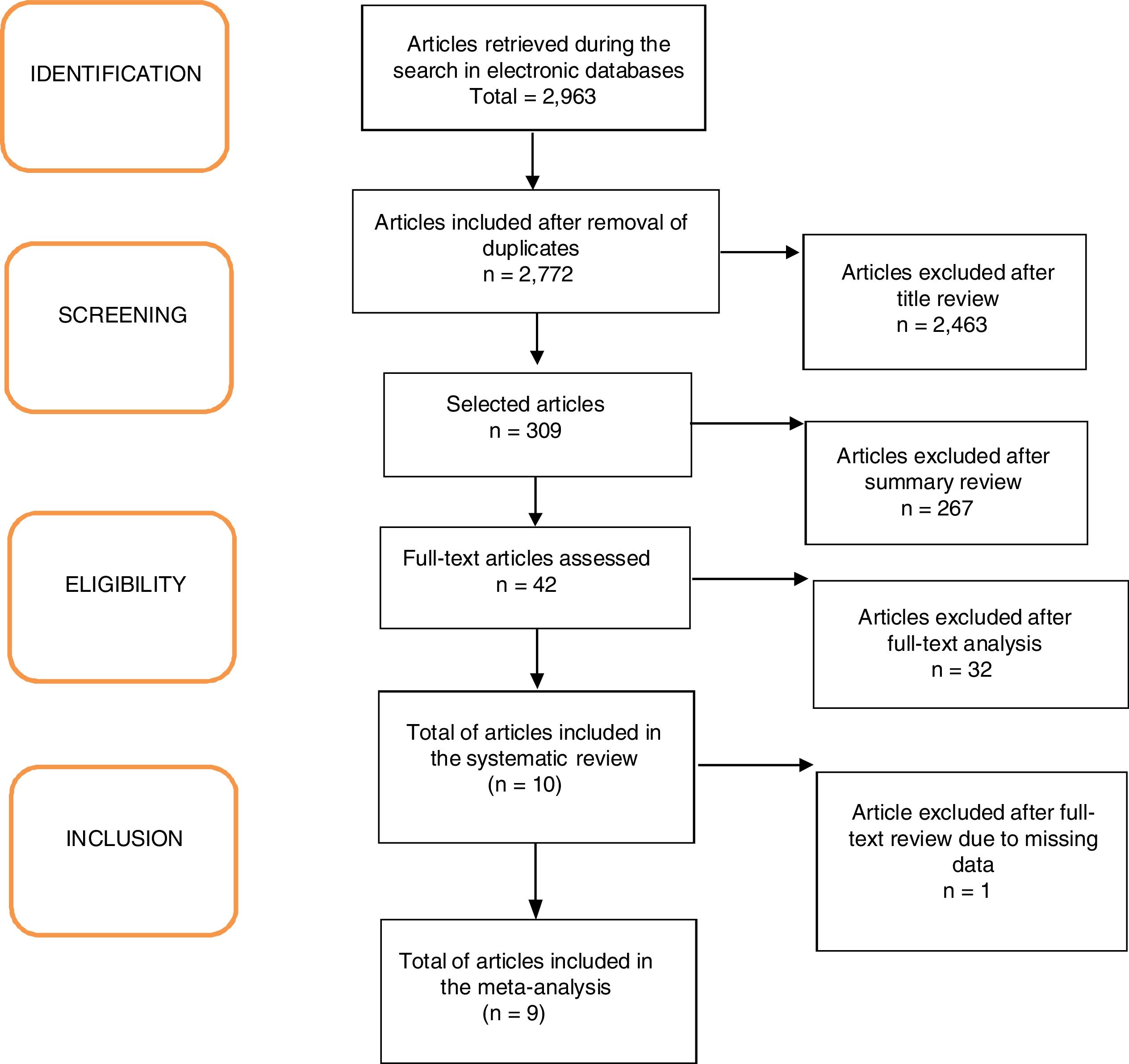
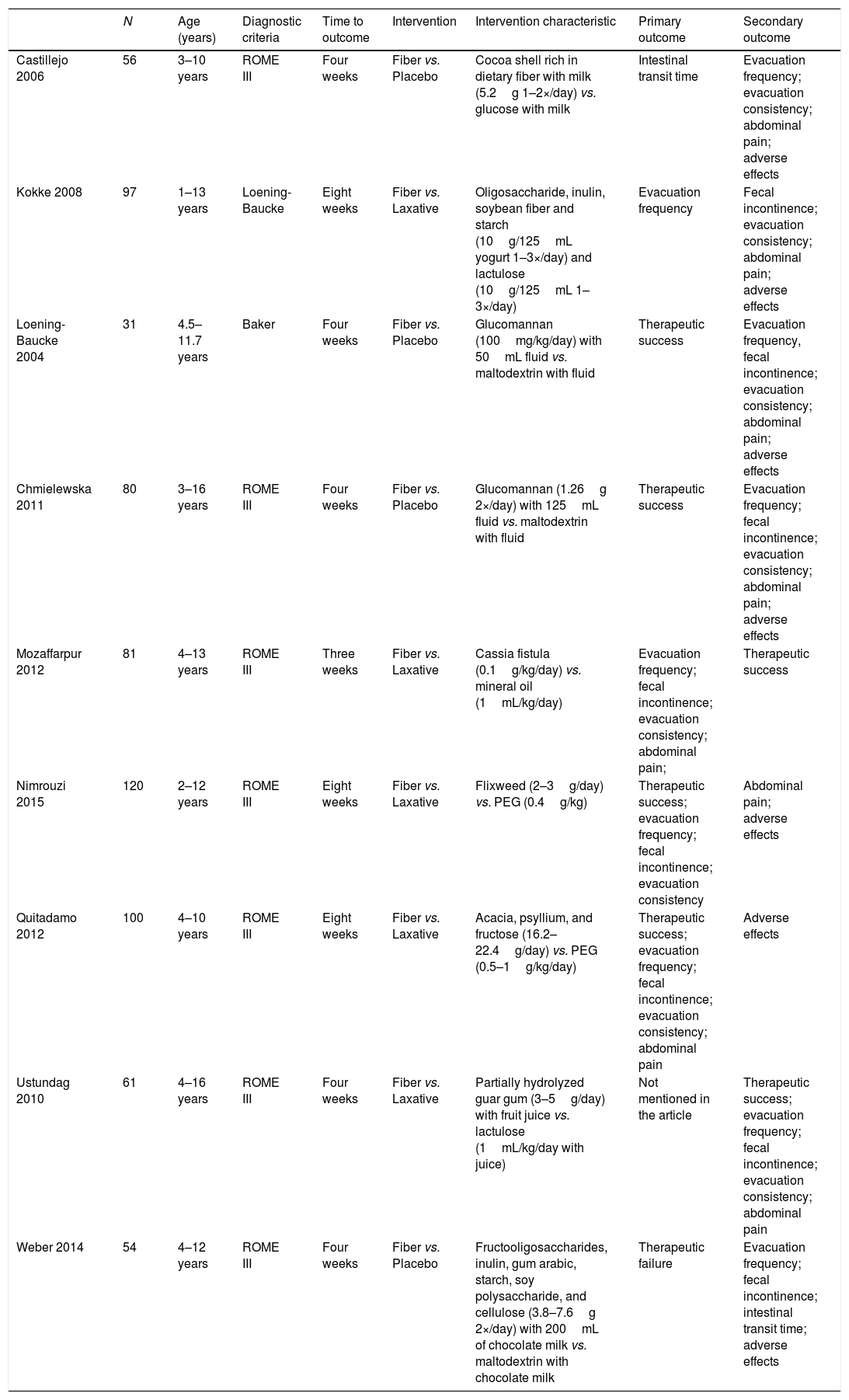
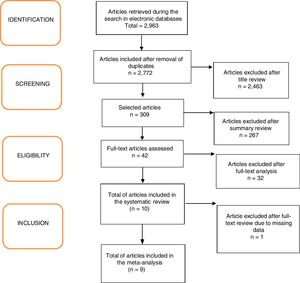
ResultsA total of 2963 articles were retrieved. Of these, 191 were removed because they were duplicated, 2463 were excluded after title analysis and 256 after reading the abstract. After the complete assessment of the articles, 32 did not meet the inclusion criteria of the study and one article did not depict its data correctly. In the end, nine articles were considered relevant,18,20–27 as shown in the flowchart (Fig. 1). A total of 680 children were included, 45% of whom were boys (only the study by Ustundag et al. did not provide this information).24 The characteristics of the included studies are depicted in Table 1.
Characteristics of the studies included in the final analysis.
| N | Age (years) | Diagnostic criteria | Time to outcome | Intervention | Intervention characteristic | Primary outcome | Secondary outcome | |
|---|---|---|---|---|---|---|---|---|
| Castillejo 2006 | 56 | 3–10 years | ROME III | Four weeks | Fiber vs. Placebo | Cocoa shell rich in dietary fiber with milk (5.2g 1–2×/day) vs. glucose with milk | Intestinal transit time | Evacuation frequency; evacuation consistency; abdominal pain; adverse effects |
| Kokke 2008 | 97 | 1–13 years | Loening-Baucke | Eight weeks | Fiber vs. Laxative | Oligosaccharide, inulin, soybean fiber and starch (10g/125mL yogurt 1–3×/day) and lactulose (10g/125mL 1–3×/day) | Evacuation frequency | Fecal incontinence; evacuation consistency; abdominal pain; adverse effects |
| Loening-Baucke 2004 | 31 | 4.5–11.7 years | Baker | Four weeks | Fiber vs. Placebo | Glucomannan (100mg/kg/day) with 50mL fluid vs. maltodextrin with fluid | Therapeutic success | Evacuation frequency, fecal incontinence; evacuation consistency; abdominal pain; adverse effects |
| Chmielewska 2011 | 80 | 3–16 years | ROME III | Four weeks | Fiber vs. Placebo | Glucomannan (1.26g 2×/day) with 125mL fluid vs. maltodextrin with fluid | Therapeutic success | Evacuation frequency; fecal incontinence; evacuation consistency; abdominal pain; adverse effects |
| Mozaffarpur 2012 | 81 | 4–13 years | ROME III | Three weeks | Fiber vs. Laxative | Cassia fistula (0.1g/kg/day) vs. mineral oil (1mL/kg/day) | Evacuation frequency; fecal incontinence; evacuation consistency; abdominal pain; | Therapeutic success |
| Nimrouzi 2015 | 120 | 2–12 years | ROME III | Eight weeks | Fiber vs. Laxative | Flixweed (2–3g/day) vs. PEG (0.4g/kg) | Therapeutic success; evacuation frequency; fecal incontinence; evacuation consistency | Abdominal pain; adverse effects |
| Quitadamo 2012 | 100 | 4–10 years | ROME III | Eight weeks | Fiber vs. Laxative | Acacia, psyllium, and fructose (16.2–22.4g/day) vs. PEG (0.5–1g/kg/day) | Therapeutic success; evacuation frequency; fecal incontinence; evacuation consistency; abdominal pain | Adverse effects |
| Ustundag 2010 | 61 | 4–16 years | ROME III | Four weeks | Fiber vs. Laxative | Partially hydrolyzed guar gum (3–5g/day) with fruit juice vs. lactulose (1mL/kg/day with juice) | Not mentioned in the article | Therapeutic success; evacuation frequency; fecal incontinence; evacuation consistency; abdominal pain |
| Weber 2014 | 54 | 4–12 years | ROME III | Four weeks | Fiber vs. Placebo | Fructooligosaccharides, inulin, gum arabic, starch, soy polysaccharide, and cellulose (3.8–7.6g 2×/day) with 200mL of chocolate milk vs. maltodextrin with chocolate milk | Therapeutic failure | Evacuation frequency; fecal incontinence; intestinal transit time; adverse effects |
PEG, polyethylene glycol.
Most studies chose to use evacuation frequency, stool consistency, and/or treatment success as primary outcomes, despite the diversity when defining the criteria for treatment success. The study by Ustundag et al. did not mention primary outcomes, only secondary.24
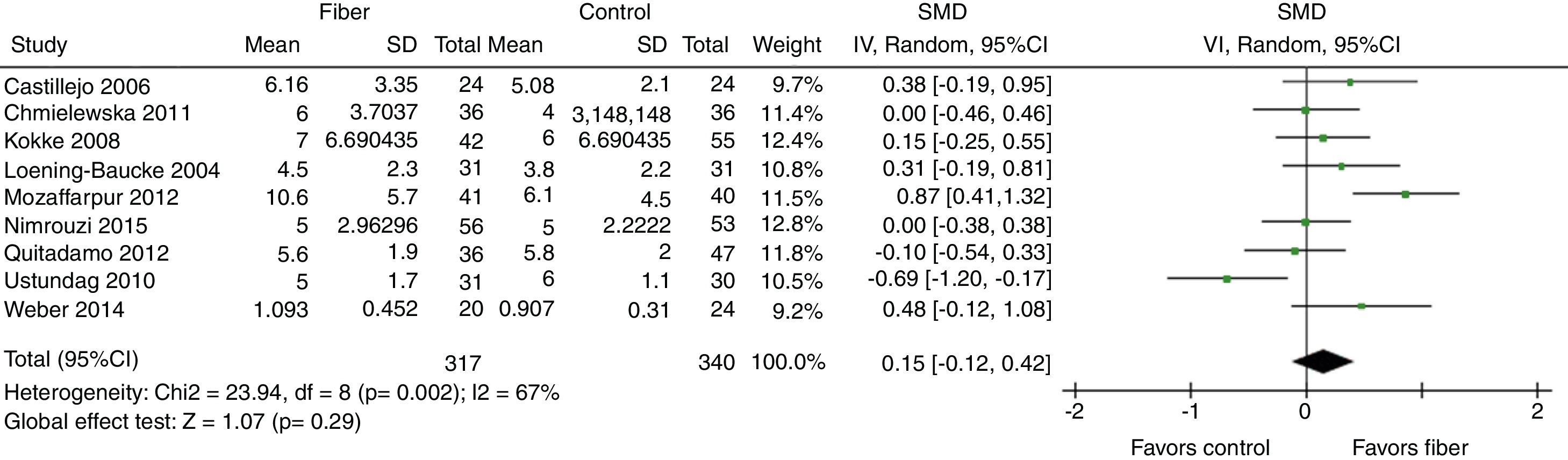
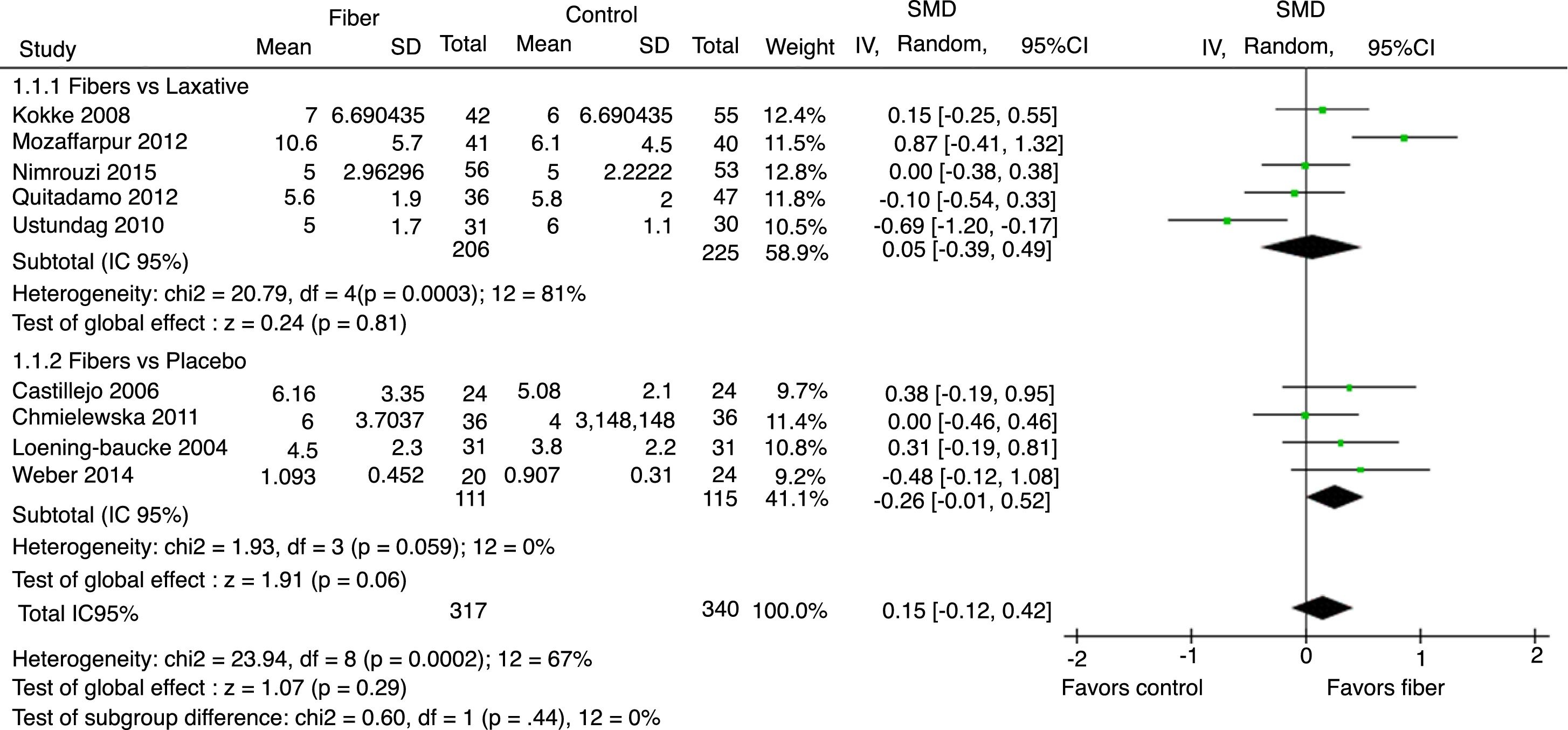
Evacuation frequency was reported in nine studies. The results indicated that there was no significant increase in the number of bowel movements per week in the fiber group when compared with the control group, with SMD=0.15 (95% CI=−0.12 to 0.42, p=0.29; I2=67%, p=0.002; Fig. 2).
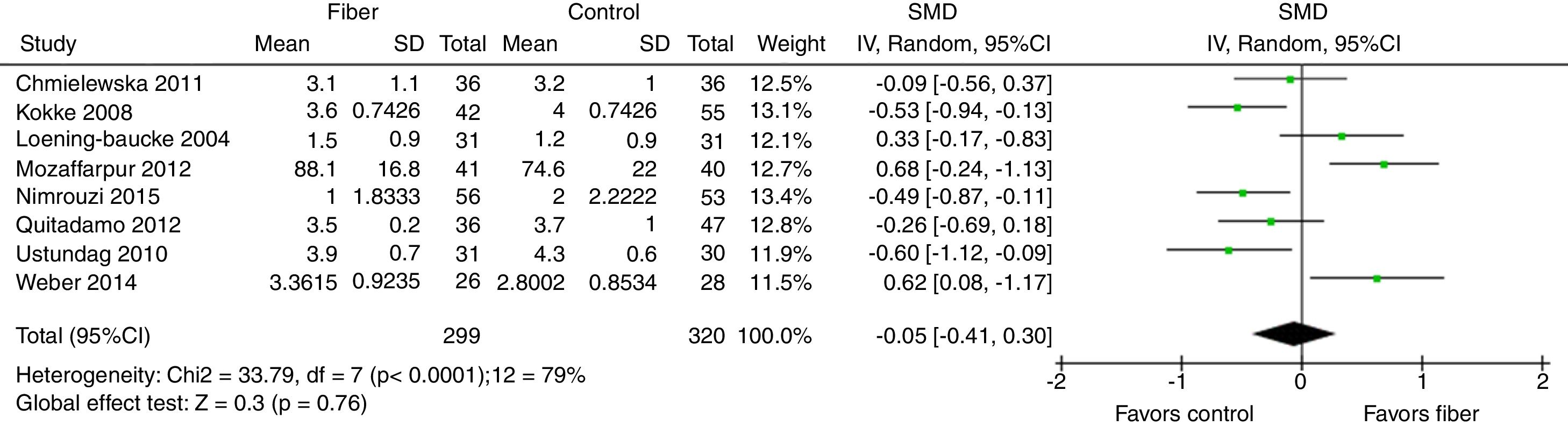
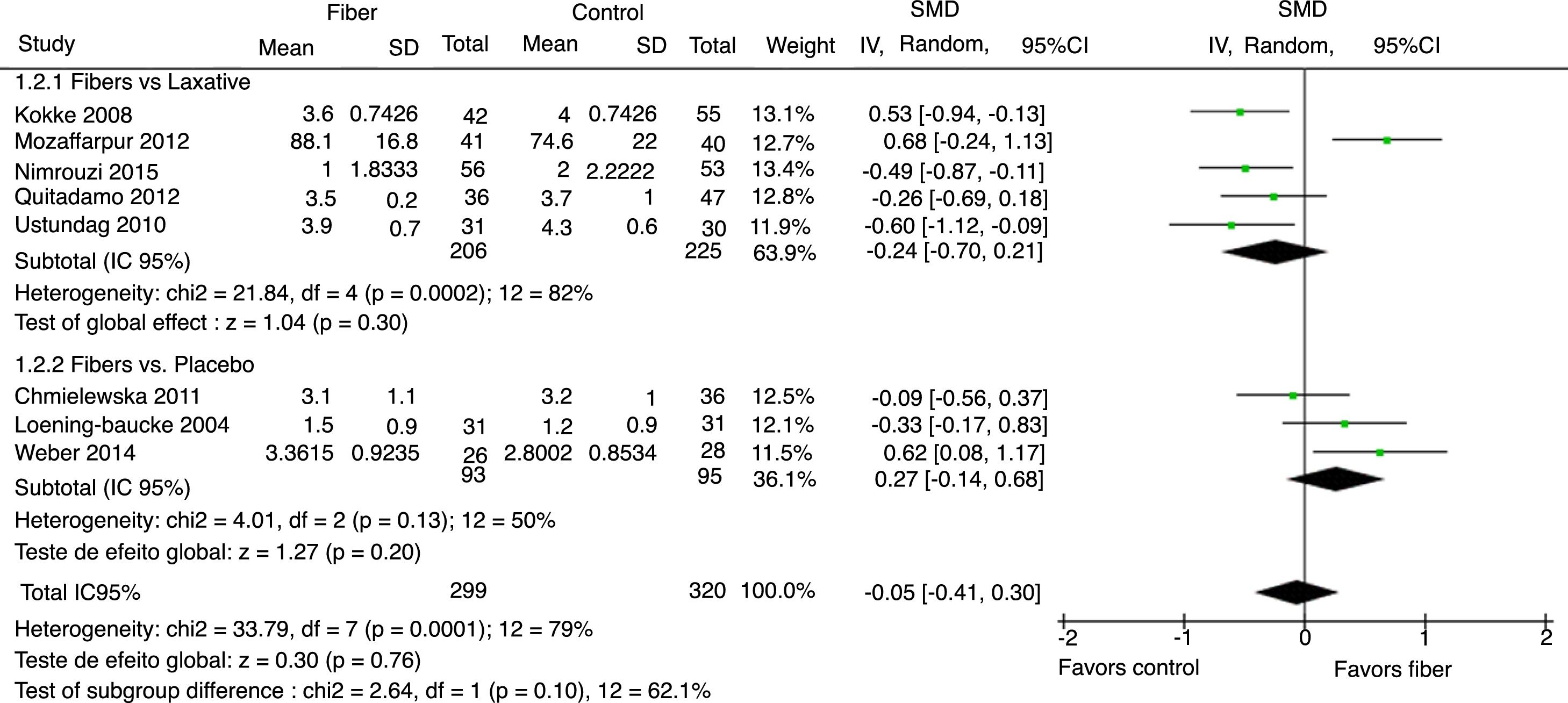
Stool consistency was assessed by eight studies and in six of them it was categorized as the mean bowel movements according to the Bristol Scale. The study by Mozzafarpur et al. used a visual scale ranging from 0 to 100.18 The study by Castillejo et al. provided dichotomous data and was not included in the final analysis.25 The results showed that there was no statistically significant difference between the fiber group and the control group, with SMD=−0.05 (95% CI=−0.41 to 0.30, p=0.76; I2=79%, p<0.0001; Fig. 3).
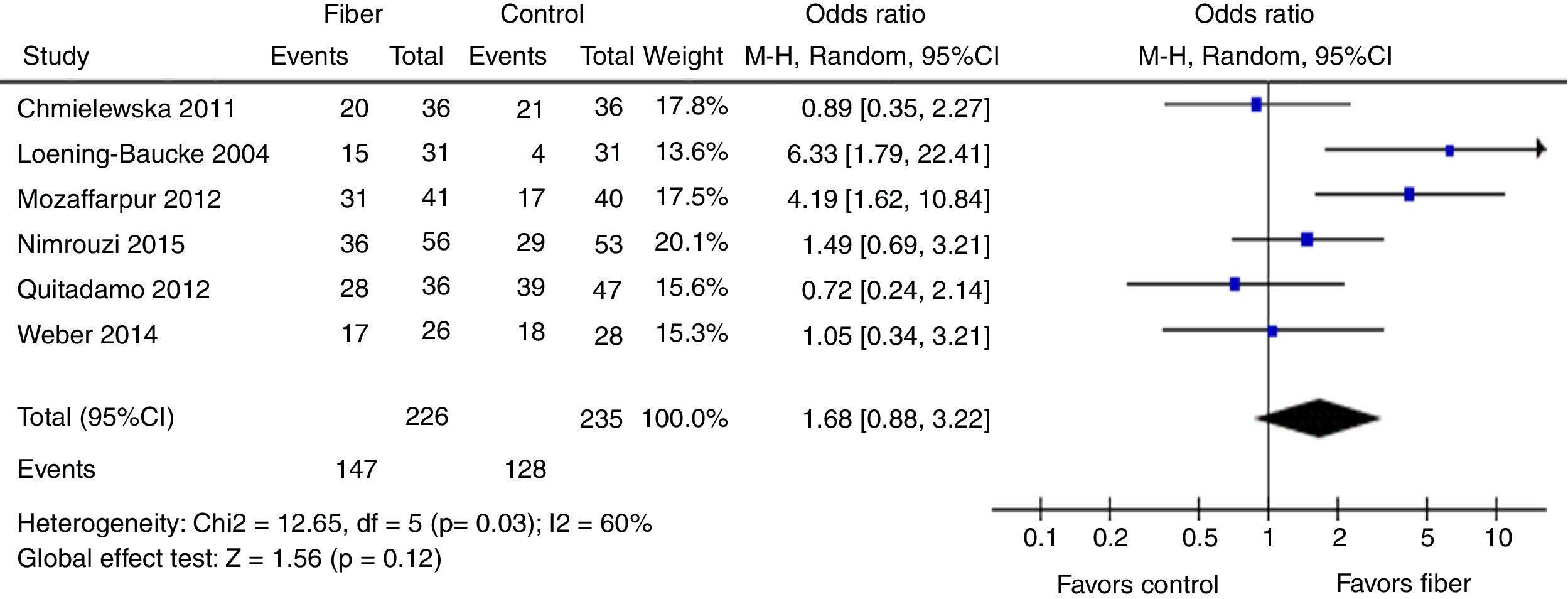
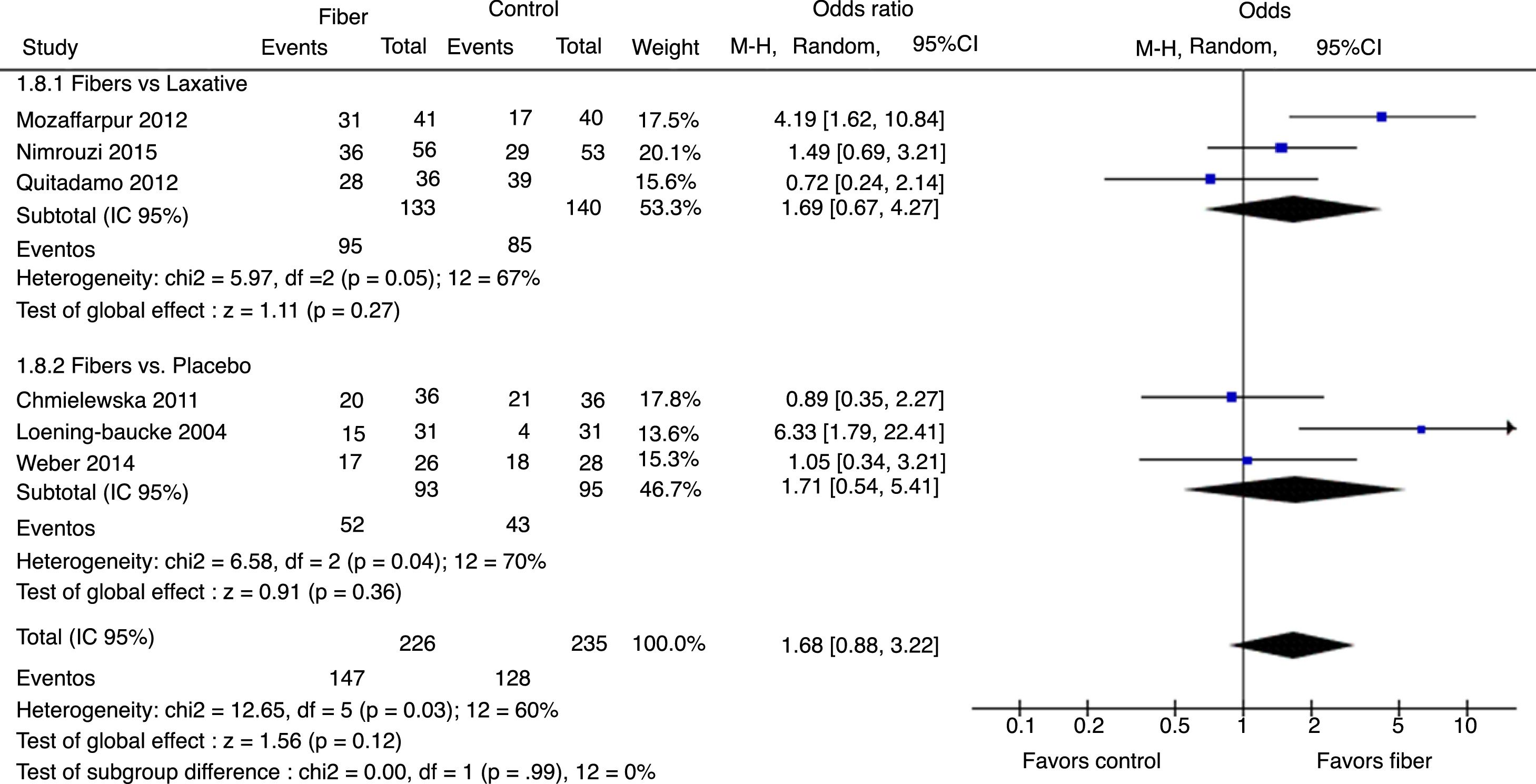
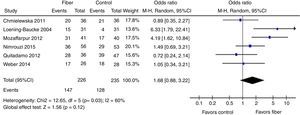
Therapeutic success was evaluated by six studies. The final results showed that there was no statistically significant difference between the fiber and control groups with OR=1.68 (95% CI=0.88–3.22, p=0.12; I2=60%, p=0.03; Fig. 4). There was great discrepancy in the definition of therapeutic success used by each author (Table 1).
The analysis per subgroup was performed, comprising the separate evaluation of the outcomes: evacuation frequency, fecal consistency, and therapeutic success, between studies using placebo or laxative drugs as control vs. fibers as intervention. No outcome showed a statistically significant change in subgroup results (Figs. 5–7). Nonetheless, a trend favoring fibers was observed, when compared with placebo, for the evacuation frequency (p=0.06), which was not observed in the fiber vs. laxative drug analysis (p=0.81).
In the sensitivity analysis through one-by-one exclusion of the studies, the consistency of the main results was observed. No significant differences were observed after the simultaneous exclusion of Nimrouzi et al. and Chmielewska et al.20,21 results. The only exception was the exclusion of the article by Ustundag et al. regarding evacuation frequency,24 which showed a significant increase of evacuations in the fiber group, with SMD=0.24 (95% CI=0.01–0.46; p=0.04; I2=47%).
There is evidence of publication bias in the funnel plots of the outcomes evacuation frequency, stool consistency and therapeutic success. The bias risk assessment of the included articles showed a moderate degree of inconsistency due to the great heterogeneity of the studies and a high risk of selection, allocation, and blinding bias due to methodological deficiency of the article designs (Annex 1). Moreover, an analysis of the methodological quality of the articles was performed using the Jadad Scale,17 which showed that most of the selected articles had adequate quality (Annex 2).
DiscussionAlthough functional constipation is one of the most prevalent diagnoses in the pediatric age group and one of the most frequent gastrointestinal manifestations in childhood, this area still shows a scarcity of studies, as only nine articles were included in this systematic review. The meta-analysis showed no statistical significance in any of the assessed outcomes. Moreover, it was not possible to study other outcomes, such as intestinal transit velocity, use of laxatives, presence of pain during bowel movement, and adverse effects after fiber intake, due to the absence of these data in the selected studies.
No statistical significant difference was observed in the final analysis regarding the evacuation frequency, one of the main diagnostic criteria for constipation and its management, and an important well-being parameter in the pediatric age group. However, in the sensitivity analysis after the exclusion of the article by Ustundag et al.,24 who used laxatives as control group and fibers as the intervention group, a significant increase in the evacuation frequency was observed with the use of fibers. Previous systematic reviews have also demonstrated an increased evacuation frequency with fiber supplementation.28,29 However, the systematic review by Gordon et al.30 showed no difference in evacuation frequency in this population.
Fecal consistency is a diagnostic criterion for constipation, and is a factor that generates pain and worsens the quality of life of constipated children and adolescents. In the present study, however, no statistically significant difference was observed in the final analysis. The same result was found in another systematic review.28
The therapeutic success outcome showed the highest degree of heterogeneity, due to the varied definitions used by the authors. No statistically significant difference was observed in the final analysis, a similar result to that found in previous systematic reviews.28,29,31 However, two other systematic reviews, one using glucomannan vs. placebo32 and another psyllium vs. placebo,33 showed that these fibers could be beneficial for functional constipation treatment.
Although the subgroup analysis showed no statistically significant differences between the analyzed outcomes, a trend was observed favoring fiber use when compared with placebo in increasing the evacuation frequency, a fact not observed in the comparison with laxatives. This fact makes sense from some biological standpoints, because the treatments were short lived and the use of laxatives in this situation should demonstrate better results than placebo.
The ESPGHAN-NASPGHAN and the National Institute for Health and Care Excellence (NICE) consensuses recommend a normal fiber intake for children and adolescents with constipation, and do not recommend the use of dietary fiber supplements alone to treat functional constipation in the pediatric population, mainly due to the lack of scientific evidence to prove its efficacy and effectiveness.14,34 The ROME IV consensus only mentions the recommendation of adequate fiber intake for each age, emphasizing that there are no strong and well-designed studies that support the use of any dietary supplementation for the treatment of functional constipation in children and adolescents.1,2 The present study reached the same conclusion, while demonstrating and creating new evidence to prove these claims.
New, well-designed, double-blind randomized clinical trials, using globally recommended and updated diagnostic criteria and treatment protocols for functional constipation are necessary in order to homogenize future publications on the use of fiber for constipation treatment in children and adolescents and, perhaps, to reproduce the efficacy of fiber supplementation use in the treatment of functional constipation.
LimitationsThe low number of randomized clinical trials evaluating the use of fiber in the treatment of constipation in children and adolescents was a strong limitation of the study. For this reason, the authors chose to include articles with low methodological quality and those with in parallel and crossover groups in the final analysis.
High heterogeneity was found in all outcomes of the included studies. Most of the studies used different definitions of functional constipation, did not quantify fiber intake before and during the intervention period, and chose to use distinct interventions with different fiber types and individualized doses. Moreover, the studies differed regarding the statistical analysis and the use of different control groups, comparing the use of fibers with the use of placebo or laxatives. Still, there was a high rate of loss in some studies, which had a small number of participants.
The sensitivity analysis helped to explain the great heterogeneity among all the outcomes, but the subgroup analysis was limited by the low number of studies in the meta-analysis. The authors chose to use the random effects model due to the important heterogeneity of the study outcomes.
The standardized mean difference was used as an effect measure for the continuous outcomes, since the selected studies did not use the same scores to classify the outcomes. Additionally, it was necessary to standardize the provided results to combine them in the meta-analysis. This measure has methodological validity, but the final results are difficult to apply in daily clinical practice, since the clinical interpretation of the scores used is lost. The median transformation (IQ) was performed on a mean (SD), as recommended by the Cochrane Collaboration.16 This transformation was performed due to the small number of articles in the literature, which can result in a greater risk of not including a study, since the selection bias or non-publication bias of studies can result in a more important influence.
The authors included studies whose quality of evidence was reduced by selection, allocation, and blinding biases and heterogeneity. The inclusion of these studies was considered appropriate due to the absence of others with better methodological criteria.
ConclusionsBased on the results of this systematic review with meta-analysis, there is no scientific evidence to corroborate the prescription of fiber supplementation in the diet of constipated children as part of the treatment of this condition. This meta-analysis may help in the current scenario, since there is a great scarcity of qualified studies to evaluate fiber supplementation in the treatment of functional constipation in children and adolescents, generating a low degree of confidence to estimate the real effect of this intervention in this population. Therefore, more studies with high methodological quality to evaluate the effects of fiber supplementation in the treatment of functional constipation are needed.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank Hospital de Clínicas de Porto Alegre and Universidade Federal do Rio Grande do Sul.
Please cite this article as: Picoli de Mello P, Eifer DA, Daniel de Mello E. Use of fibers in childhood constipation treatment: systematic review with meta-analysis. J Pediatr (Rio J). 2018;94:460–70.
Study carried out at Universidade Federal do Rio Grande do Sul (UFRGS) and Hospital de Clínicas de Porto Alegre (HCPA), Porto Alegre, RS, Brazil.