To analyze the association between the Trp64Arg polymorphism of the ADRB3 gene, maximal fat oxidation rates and the lipid profile levels in non-obese adolescents.
Methods72 schoolchildren, of both genders, aged between 11 and 17 years, participated in the study. The anthropometric and body composition variables, in addition to total cholesterol, HDL-c, LDL-c, triglycerides, insulin, and basal glycemia, were evaluated. The sample was divided into two groups according to the presence or absence of the polymorphism: non-carriers of the Arg64 allele, i.e., homozygous (Trp64Trp: n=54), and carriers of the Arg64 allele (Trp64Arg+Arg64Arg: n=18), in which the frequency of the Arg64 allele was 15.2%. The maximal oxygen uptake and peak of oxygen uptake during exercise were obtained through the symptom-limited, submaximal treadmill test. Maximal fat oxidation was determined according to the ventilatory ratio proposed in Lusk's table.
ResultsAdolescents carrying the less frequent allele (Trp64Arg and Arg64Arg) had higher LDL-c levels (p=0.031) and lower maximal fat oxidation rates (p=0.038) when compared with non-carriers (Trp64Trp).
ConclusionsAlthough the physiological processes related to lipolysis and lipid metabolism are complex, the presence of the Arg 64 allele was associated with lower rates of FATMAX during aerobic exercise, as well as with higher levels of LDL-c in adolescents.
Analisar a associação entre o polimorfismo Trp64Arg do gene ADRB3, as taxas de oxidação máxima de gorduras e as concentrações do perfil lipídico em adolescentes não obesos.
MétodosParticiparam do estudo 72 escolares, de ambos os sexos, com idade entre 11 e 17 anos. Foram avaliadas as variáveis antropométricas e de composição corporal, além do colesterol total, lipoproteina de alta densidade, lipoproteina de baixa densidade, triglicerídeos; insulina e glicemia basal. A amostra foi dividida em dois grupos, segundo a presença ou não do polimorfismo: não portadores do alelo Arg64, ou seja, homozigotos (Trp64Trp: n=54) e portadores do alelo Arg64 (Trp64Arg+Arg64Arg: n=18), em que a frequência do alelo Arg64 foi de 15,2%. O consumo máximo de oxigênio e pico de consumo máximo de oxigênio durante o exercício foram obtidos por meio do teste aeróbio submáximo de sintoma limitado em esteira. A oxidação máxima de gorduras foi determinada de acordo com a razão de trocas ventilatórias propostas na Tabela de Lusk.
ResultadosOs adolescentes portadores do alelo menos frequente (Trp64Arg e Arg64Arg) apresentaram maiores concentrações de lipoproteina de baixa densidade (p=0,031) e menores taxas de oxidação máxima de gorduras (p=0,038) quando comparados aos não portadores (Trp64Trp).
ConclusõesEmbora os processos fisiológicos relacionados à lipólise e ao metabolismo de lipídeos sejam complexos, a presença do alelo Arg64 associou-se a menores taxas de FATMAX durante exercício aeróbio, bem como maiores níveis de lipoproteina de baixa densidade em adolescentes.
Aerobic exercise is one of the main strategies in body weight regulation, prevention, and treatment of complex obesity and type 2 diabetes mellitus (DM2), because in addition to potentiating the energy expenditure, it increases glucose absorption of muscle cells and promotes a decrease in insulin resistance.1 However, the general population does not benefit from the practice of physical exercise, as, in addition to the exercise itself, these effects depend on other environmental factors, such as diet and individual genetic background.2 The significant role that specific genes play in weight regulation through the action of their products on energy expenditure, substrate oxidation, appetite modulation, lipid metabolism, thermogenesis, and cell differentiation has been well described.2,3
In this context, the adrenergic is one of the most important system, acting on the energy balance regulation through brown adipose tissue thermogenesis and white adipose tissue lipolysis, both in humans and in other species.4,5 Part of this system is found in the β3 receptor, located on chromosome 8p 11.23, expressed mainly in visceral and subcutaneous adipose tissue, acting as lipolysis mediator and, in brown adipose tissue, as thermogenesis regulator, with both functions performed in response to catecholamine stimulation.6,7
Changes in the functionality and amount of the expressed β3 receptors may permeate individual differences in energy expenditure in response to physical activities. Some of its variants associated with metabolic dysfunctions include the single nucleotide polymorphism (SNP) Trp64Arg (rs4994), which consists in the substitution of a thymine by a cytosine (T>C), resulting in the exchange of a tryptophan for an arginine at position 64 of the mature protein and corresponding to the first intracellular loop of the β38 receptor. Arg64 allele carriers have shown to be more resistant to weight loss and decreased visceral fat,9 in addition to being more likely to have lipid alterations, obesity, DM2,7 and reduced rates of fat oxidation.10
Both the normal metabolic processes that culminate in fat oxidation and the metabolic changes related to the lipid profile are complex systems involving multiple pathways and interactions of exogenous (environmental) and endogenous factors.11 Despite the underlying complexity of these processes, studies have sought to identify the small portion that can be attributed to allelic variants of genes whose products may influence the abovementioned physiological processes. Therefore, the present study aimed to verify the association between the Trp64Arg polymorphism, the maximal fat oxidation (FATMAX) rates, and the lipid profile levels in non-obese adolescents.
MethodsStudy designThis was a cross-sectional and observational study, characterized by initial anthropometric screening of all adolescents from public and urban schools in the South of Brazil. A total of 1077 schoolchildren of both genders and aged between 11 and 17 years participated in the study. The prevalence of non-obese adolescents classified between the 5th and 97th percentiles, as proposed by the World Health Organization,12 was 93%. Subsequently, all students were invited to participate in a physical activity program, in which 165 subjects were volunteers. Participants were evaluated by a single pediatrician through complete physical examination, anthropometric tests, and sexual maturation analysis as proposed by Tanner.13
The inclusion criteria were: absence of chronic diseases such as DM2, uncontrolled hypothyroidism, endocrine diseases, infections, and/or use of medications that promote changes in adiposity and metabolic and inflammatory parameters. The exclusion criterion used was the obese nutritional status (n=85), considered above the 97th percentile by the World Health Organization.12 All volunteers and parents signed the informed consent form, according to the research project approved by the Institutional Ethics Committee (protocol number 2460.067/2011-03-UFPR).
After applying the inclusion and exclusion criteria, the selection resulted in 80 non-obese adolescents (38 boys and 42 girls), who did not perform more than 120min of physical activity a week and did not have any contraindications to the examinations (including absence of heart, pulmonary, and osteo-articular diseases) and were submitted to submaximal treadmill test and blood collection. Of these, five adolescents did not perform the cardiorespiratory evaluation tests and in three it was not possible to perform the genotyping of the Trp64Arg polymorphism of the ADRB3 gene, resulting in 72 non-obese adolescents (33 boys and 39 girls).
Evaluation of anthropometric and physiological variablesBody mass (BM) (kg) was obtained using a platform scale, with an accuracy of 0.1 kilograms (kg) and a maximum capacity of 150kg. Height was measured in a stadiometer fixed to the wall, with an accuracy of 0.1cm and an amplitude of 220cm. Abdominal circumference (AC) measurement was evaluated following the recommendations of the Centers for Disease Control and Prevention.14
Body fat composition assessment was performed with dual energy X-ray absorptiometry (DXA), using a Lunar Prodigy Primo device (General Electric, Healthcare, MA, USA). Lean mass (LM) and fat mass (FM) were determined with an accuracy of 1g and described in kilograms (kg). To reconstruct the image of the LM and FM, the enCore 2008 software version 12.30 (General Electric, Healthcare, MA, USA) was used.
The maximal oxygen consumption (VO2max) and peak oxygen consumption (VO2peak) were measured on a treadmill using a K4b2® Portable Gas Analyzer (Cosmed®, SP, Brazil) with the following protocol: initial velocity of 4km/h, progressive increase of 0.3km/h at every 30seconds and fixed slope of 1% kept constant during the test. Heart rate (HR) was monitored during the test, using a cardiofrequencimeter (Polar®, USA), a portable wireless transmission–reception system. The adolescents were advised by a nutritionist to eat a light meal with a predominance of carbohydrates before the test, according to the daily food recommendations.15 The test was considered maximal when two of the following criteria were observed: A) exhaustion or inability to maintain the required speed; B) R≥1.09; C) reaching the maximal HR predicted by the formula 208−(0.7×age), proposed by Tanaka.16
To obtain the FATMAX values, the ventilatory exchange ratio (R) observed during the symptom-limited submaximal treadmill test were used, according to Lusk's table.17 This variable was obtained through the product between the caloric equivalent of R at the FATMAX point and the oxygen consumption observed at the same point.
Evaluation of biochemical variablesBlood samples were collected in the morning, after a 12-h fast, and stored in appropriate tubes. Plasma levels of total cholesterol, HDL-cholesterol, and triglycerides were determined in mg/dL by enzymatic-colorimetric assay. LDL-cholesterol was calculated by the Friedewald equation in mg/dL.18 Glucose values were determined by the enzymatic method (Glucose Oxidase – Labtest, SP, Brazil) and insulin was measured by the chemiluminescence technique using an immunometric immunoassay in uU/mL, in an automated equipment.
Genotyping of the Trp64Arg polymorphism of the ADRB3 geneDNA extraction from the blood samples was performed using the QIAamp DNA Mini Kit (QIAGEN, MD, USA), according to the Lahiri and Nurnberger19 method. Genotyping of the Trp64Arg polymorphism of the ADRB3 gene was performed using the TaqMan allelic discrimination assay using the 7500™ real-time PCR system (Applied Biosystems®, CA, USA), the reactions were performed on an Eppendorf Matercycler Realplex 2 (Biocompare®, CA, USA) apparatus, and analyses were performed with the software Eppendorf Realplex v. 1.5 (Biocompare®, CA, USA).
DNA extraction was carried out by obtaining the leukocyte layer by centrifugation of the collected whole blood, lysis of erythrocytes, precipitation of proteins, and precipitation of genomic DNA. The PCR technique allowed the detection, cycle by cycle, with high sensitivity and specificity, of the intensity of fluorescence emitted as a result of the amplification of the target DNA sequence. At the end of the reaction, according to the genotype, one or another fluorescence was present in the case of homozygotes and in the case of heterozygotes, both types of fluorescence were present. The genotype distribution among the adolescents enrolled in the study was: Trp/Trp (n=54; 75%), Trp/Arg (n=14; 19.5%), and Arg/Arg (n=4; 5.5%). The frequency of the Arg64 allele was 15.2%, indicating that the data are in Hardy–Weinberg equilibrium, that is, the observed values are similar to those expected (p=0.165). The models of recessive, dominant, and dominance-free allele interaction were tested by adopting the dominant allele interaction model, due to the small number of Arg64 allele carriers in homozygosis and also due to the observed effect. Thus, individuals were grouped into carriers (Arg/Arg+Trp/Arg) and non-carriers (Trp/Trp) of the Arg64 allele.
Statistical analysesThe data were tested for normality and variance homogeneity by the Kolmogorov–Smirnov test and Lilliefors’ correction of significance, respectively. The results are shown as means and standard deviation for the variables with normal distribution, and medians and interquartile ranges for those with non-normal distribution. Student's t-test was used for variables with normal distribution and Mann–Whitney's U test, for variables that did not meet the normality assumption. Fisher's exact test was used for comparisons of proportions between groups regarding the maturation stage. Spearman's correlation was used to analyze the correlation between the biochemical variables and FATMAX. Genotype frequencies were obtained by direct counting, and the comparisons of proportions between the groups regarding gender and Hardy–Weinberg equilibrium groups were verified using the chi-squared test (χ2). Multiple linear regression was used to verify the degree of association between sexual maturation, gender, fat mass, and the Trp64Arg polymorphism of the ADRB3 gene (independent variables) and the lipid profile and FATMAX (outcome). The power of the study was calculated using the G*power3 software (G Power, V.3.1.7, Dusseldorf, Germany) with the present sample, and a power (1−β) of 0.66 was identified for the comparisons between groups, of 0.84 for the correlations and of 0.82 for regressions. Data were analyzed with SPSS for Windows (IBM SPSS Statistics for Windows, version 22.0. NY, USA), and the significance level was set at p<0.05.
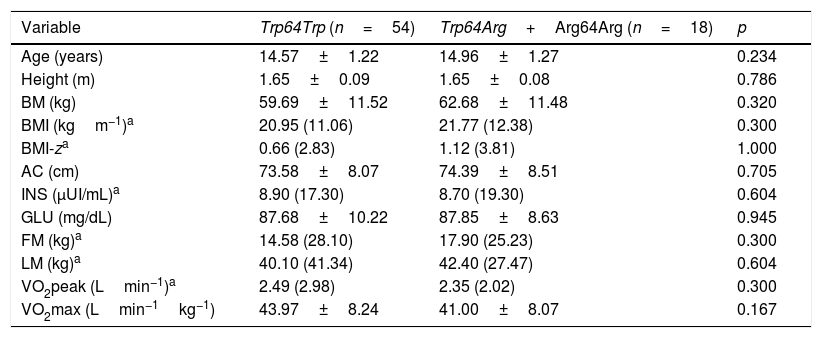
ResultsTable 1 shows the anthropometric, body composition, biochemical, and cardiorespiratory fitness variables of non-carrier adolescents (Trp64Trp) and the less frequent allele carriers (Trp64Arg+Arg64Arg), which were similar. No statistically significant differences were observed between the groups regarding Tanner stage.
Comparison of means±standard deviation or medians (confidence intervals) of anthropometric, body composition, biochemical and cardiorespiratory fitness variables among non-carrier adolescents (Trp64Trp) and carriers of the less frequent allele (Trp64Arg+Arg64Arg) of the ADRB3 gene.
| Variable | Trp64Trp (n=54) | Trp64Arg+Arg64Arg (n=18) | p |
|---|---|---|---|
| Age (years) | 14.57±1.22 | 14.96±1.27 | 0.234 |
| Height (m) | 1.65±0.09 | 1.65±0.08 | 0.786 |
| BM (kg) | 59.69±11.52 | 62.68±11.48 | 0.320 |
| BMI (kgm−1)a | 20.95 (11.06) | 21.77 (12.38) | 0.300 |
| BMI-za | 0.66 (2.83) | 1.12 (3.81) | 1.000 |
| AC (cm) | 73.58±8.07 | 74.39±8.51 | 0.705 |
| INS (μUI/mL)a | 8.90 (17.30) | 8.70 (19.30) | 0.604 |
| GLU (mg/dL) | 87.68±10.22 | 87.85±8.63 | 0.945 |
| FM (kg)a | 14.58 (28.10) | 17.90 (25.23) | 0.300 |
| LM (kg)a | 40.10 (41.34) | 42.40 (27.47) | 0.604 |
| VO2peak (Lmin−1)a | 2.49 (2.98) | 2.35 (2.02) | 0.300 |
| VO2max (Lmin−1kg−1) | 43.97±8.24 | 41.00±8.07 | 0.167 |
BM, body mass; BMI, body mass index; BMI-z, body mass index Z-score; AC, abdominal circumference; INS, basal insulin; GLU, basal glucose; FM, fat mass; LM, lean mass.
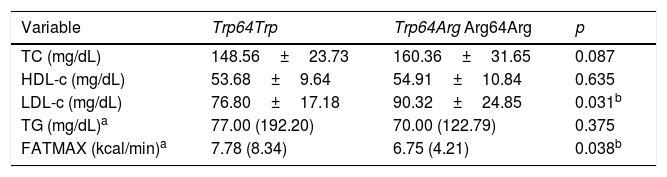
Table 2 shows that the group carrying the Arg64 allele had higher levels of LDL-c when compared with the non-carrier group (p=0.031). Levels of TC, HDL-c, and TG were similar between groups. The group carrying the Arg64 allele had lower FATMAX rates (p=0.038) when compared with the non-carrier group (Trp64Trp).
Comparison of means±standard deviation or medians (confidence intervals) of the lipid profile and maximal fat oxidation variables in non-carrier adolescents (Trp64Trp) and carriers of the less frequent allele (Trp64Arg+Arg64Arg) of the ADRB3 gene.
| Variable | Trp64Trp | Trp64Arg Arg64Arg | p |
|---|---|---|---|
| TC (mg/dL) | 148.56±23.73 | 160.36±31.65 | 0.087 |
| HDL-c (mg/dL) | 53.68±9.64 | 54.91±10.84 | 0.635 |
| LDL-c (mg/dL) | 76.80±17.18 | 90.32±24.85 | 0.031b |
| TG (mg/dL)a | 77.00 (192.20) | 70.00 (122.79) | 0.375 |
| FATMAX (kcal/min)a | 7.78 (8.34) | 6.75 (4.21) | 0.038b |
TC, total cholesterol; HDL-c, high-density lipoprotein; LDL-c, low-density lipoprotein; TG, triglycerides; FATMAX, maximal fat oxidation.
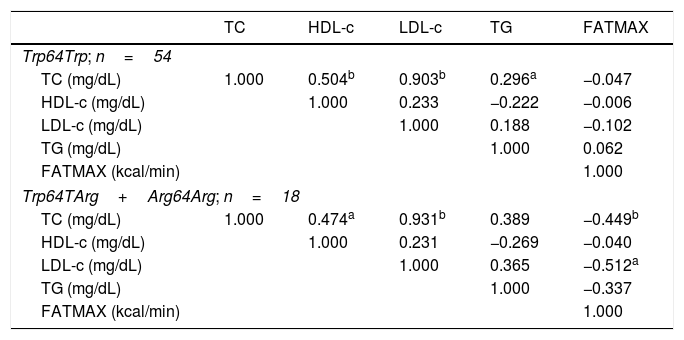
Table 3 shows Spearman's correlation values, in which levels of HDL-c (p=0.000, r=0.504), LDL-c (p=0.000, r=0.903), and TG (p=0.035) of non-carriers of the Arg64 allele were directly correlated with total cholesterol (TC). Among the carriers of the Arg64 alleles, FATMAX showed an indirect correlation with LDL-c (p=0.018, r=0.512) and TC levels (p=0.041, r=0.449). Additionally, serum levels of HDL-c (p=0.030, r=0.474) and LDL-c (p=0.000; r=0.931) were directly correlated with total cholesterol. No significant correlations were observed with the other variables.
Matrix of Spearman's correlation coefficients between the lipid profile and maximal fat oxidation variables in homozygous adolescents for the most frequent allele (Trp64Trp; n=54) and for carriers of the less frequent allele (Trp64TArg+Arg64Arg; n=18).
| TC | HDL-c | LDL-c | TG | FATMAX | |
|---|---|---|---|---|---|
| Trp64Trp; n=54 | |||||
| TC (mg/dL) | 1.000 | 0.504b | 0.903b | 0.296a | −0.047 |
| HDL-c (mg/dL) | 1.000 | 0.233 | −0.222 | −0.006 | |
| LDL-c (mg/dL) | 1.000 | 0.188 | −0.102 | ||
| TG (mg/dL) | 1.000 | 0.062 | |||
| FATMAX (kcal/min) | 1.000 | ||||
| Trp64TArg+Arg64Arg; n=18 | |||||
| TC (mg/dL) | 1.000 | 0.474a | 0.931b | 0.389 | −0.449b |
| HDL-c (mg/dL) | 1.000 | 0.231 | −0.269 | −0.040 | |
| LDL-c (mg/dL) | 1.000 | 0.365 | −0.512a | ||
| TG (mg/dL) | 1.000 | −0.337 | |||
| FATMAX (kcal/min) | 1.000 | ||||
TC, total cholesterol; HDL-c, high-density lipoprotein; LDL-c, low-density lipoprotein; TG, triglycerides; FATMAX, maximal fat oxidation.
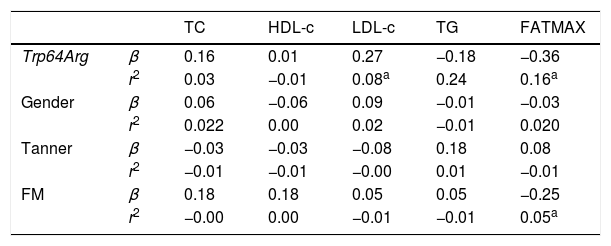
The multiple linear regression test (Table 4) showed that the Trp64Arg polymorphism of the ADRB3 gene explained 8% (adjusted r2=0.08) of the LDL-c levels (β=0.27, p<0.01) and 16% (adjusted r2=0.16) of FATMAX (β=−0.36, p<0.01), whereas FM explained 5% (adjusted r2=0.05) of FATMAX (β=−0.25, p<0.01).
Linear regression coefficient (β) and r2 for gender, sexual maturation, Trp64Arg polymorphism of ADRB3 gene, lipid profile, and maximal fat oxidation variables in adolescents.
| TC | HDL-c | LDL-c | TG | FATMAX | ||
|---|---|---|---|---|---|---|
| Trp64Arg | β | 0.16 | 0.01 | 0.27 | −0.18 | −0.36 |
| r2 | 0.03 | −0.01 | 0.08a | 0.24 | 0.16a | |
| Gender | β | 0.06 | −0.06 | 0.09 | −0.01 | −0.03 |
| r2 | 0.022 | 0.00 | 0.02 | −0.01 | 0.020 | |
| Tanner | β | −0.03 | −0.03 | −0.08 | 0.18 | 0.08 |
| r2 | −0.01 | −0.01 | −0.00 | 0.01 | −0.01 | |
| FM | β | 0.18 | 0.18 | 0.05 | 0.05 | −0.25 |
| r2 | −0.00 | 0.00 | −0.01 | −0.01 | 0.05a |
TC, total cholesterol; HDL-c, high-density lipoprotein; LDL-c, low-density lipoprotein; TG, triglycerides; FATMAX, maximal fat oxidation, FM, fat mass.
The present study aimed to investigate the association of the Trp64Arg polymorphism on FATMAX and biochemical variables in non-obese adolescents. These physiological parameters show great variation in the general population, including among adolescents, due to the interaction of multiple determinant genetic factors, and of these with environmental components, such as eating habits and lifestyle.
The results suggest that FATMAX rates were significantly lower and LDL-c levels were higher among adolescents with the Arg64 allele when compared with those without the allele. Similar results were observed in adults with normal weight, both at rest and during aerobic exercise, in whom the Trp64Arg polymorphism was associated with the reduction of FATMAX.10 Moreover, in this sense, in vitro research with human adipocytes suggested reduced lipolytic activity in homozygous individuals that were carriers of the Arg64 allele.20 Although it is a complex physiological process, the genetic variant of the ADRB3 gene appears to contribute to the variation in FATMAX values in response to physical activity, as well as to a lower response capacity to weight loss.8
The prescription of physical activities to the point of FATMAX is an important factor that can contribute to lipid metabolism improvement and weight maintenance,21 as well as the increase in insulin sensitivity.22 Training in the FATMAX zone suggests significant increases in this parameter, as observed in obese female adolescents who also showed favorable changes in adipocytokine levels.23 Thus, understanding the factors that contribute to the individual variation of FATMAX rates may allow the prescription of more individualized training, which will result in more positive outcomes.
Although the present study did not consider functional assays to demonstrate the effect of changes in the functioning of pathways triggered by the β3 receptor activation, previous studies have demonstrated its functionality. A study carried out in rats demonstrated that the Arg64 allele carrier showed a reduction in the amount of cAMP in response to catecholamines, when compared to the presence of the 64Trp allele.24 The same effect was also observed in other cells (ovary cells in hamsters and human embryonic renal cells),4,5 suggesting that lipolysis may be decreased in the presence of the Arg64 variant in β3 receptors, when compared with a receptor without the alteration. Still in this sense, such genetic alteration may reflect a less favorable metabolic profile when associated with brown adipose tissue thermogenesis, body weight and early onset of T2DM in some populations.6,7
This study indicates that the Arg64 allele carriers had higher levels of LDL-c, which may represent greater risks for cardiovascular disease development in the future. Likewise, a study carried out with adult Japanese individuals with normal weight showed a significant correlation between LDL-c levels, the Arg64Arg genotype, and age, with an annual increase in BMI.25 However, the literature shows heterogeneity of results. In Italian non-obese adults, no influence of the Arg64 allele was observed on lipid levels; however, this allele appears to influence abdominal adiposity.26 A study in Hungarian children with different nutritional states found no influence of the presence of the Arg64 allele on TG, TC, HDL-c, and glucose levels; however, the authors observed higher values of body mass, fat mass, and fasting insulin, when compared with non-carriers of the allele.27 In another study, Arg64Arg homozygous individuals had significantly lower insulin secretion and higher fasting glucose levels when compared with overweight Trp64Trp homozygous individuals.7
Furthermore, the results suggest that the higher rates of FATMAX in the non-carrier group of the Arg64 allele of the ADRB3 gene showed a negative correlation with TC and HDL-c levels. This relationship is known, since the increase in energy expenditure leads to changes in the lipid profile secondary to physical exercise, through the stimulation of metabolic reactions and the potentiation of energy substrate use by active muscles, which occurs both acutely and by physiological adaptations that stimulate metabolism throughout the day.21 Additionally, although it has been observed in the regressions that the Trp64Arg polymorphism of the ADRB3 gene was associated with LDL-c and FATMAX levels, it must considered the effect of genetic variants on the complex traits in such physiological processes, which can be attributed to the age range and the subjects’ nutritional status, as well as the ethnic heterogeneity of the genetic background.
The Arg64 allele is found at different frequencies in the assessed ethnicities; in the present study the observed frequency was 15.3%. Studies in other populations reported 5.77% in Hungarian obese children,7 8% in Euro-North Americans,25 and 11% in Brazilian adults with different nutritional status.28
The present study has some limitations that should be mentioned. First, it had a cross-sectional design; therefore, it was not possible to establish a cause-and-effect association between the presence of polymorphism, FATMAX, and lipid profile, considering that this condition is multifactorial. Second, the small sample size (n=72) made it impossible to perform an individualized analysis by gender and, therefore, caution should be adopted when generalizing the results obtained in this study, considering the influence that gender may have in this context. Third, the use of a convenience sample hindered the homogeneity between the selected groups, because differences in gender and Tanner stage may alter FATMAX rates and lipid profile levels. However, no difference was found in the comparisons between genders and Tanner stage in the groups divided by the presence or absence of the Arg64 allele. Nevertheless, other aspects support the present findings, such as the method used for the analysis of body fat composition (DXA), considered the gold standard; the strict inclusion and exclusion criteria; and the use of the Lusk protocol for assessing FATMAX, which shows good agreement with invasive techniques such as arterial cannulation and muscle biopsies, as well as the easy access and applicability by health professionals.29
Identifying the influence of polymorphisms in candidate genes on the maximal rates of fat oxidation and lipid metabolism may contribute to the implementation of more individualized and effective exercise protocols, able to more effectively contribute to health care provision. However, the association between the Arg64 allele of the Trp64Arg polymorphism of ADRB3 gene, FATMAX, and LDL-cholesterol levels should be interpreted with caution, as it may represent a small portion of complex physiological processes related to lipolysis and metabolism lipids.
FundingCAPES and CNPq.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Jesus ÍC, Alle LF, Munhoz EC, Silva LR, Lopes WA, Tureck LV, et al. Trp64Arg polymorphism of the ADRB3 gene associated with maximal fat oxidation and LDL-C levels in non-obese adolescents. J Pediatr (Rio J). 2018;94:425–31.
Study carried out at Universidade Federal do Paraná (UFPR), Curitiba, PR, Brazil.