Acute lymphoblastic leukemia is the most common childhood cancer, yet surprisingly, very few studies have reported the treatment outcomes and the relapse rate of patients from low/middle-income countries.
MethodThis study was a 5-year retrospective cohort study. It was conducted at Oncology Center of Mansoura University in Egypt and aimed to estimate the treatment outcomes and the relapse rates of newly diagnosed acute lymphoblastic leukemia in children.
ResultsTwo hundred children suffering from acute lymphoblastic leukemia were studied; forty-six patients (23%) died during induction and most of those deaths were related to infection. Forty-one patients (27%) relapsed out of the 152 patients who achieved complete remission. The most common site of relapse was the bone marrow, followed by the isolated central nervous system, 53.7% and 31.7%, respectively. Seventy-eight percent of relapses occurred very early/early rather than later. The majority of relapse patients’ deaths were related to infection and disease progression. The 5-year overall survival rate for patients was 63.1% (82.1% for non-relapsed compared to 36.6% for relapsed patients).
ConclusionThere was a high incidence of induction deaths related to infection and high percentages of very early/early relapses, with high mortalities and low 5-year overall survival rates. These findings suggest the urgent need for modification of chemotherapy regimens to be suitable for the local conditions, including implementation of supportive care and infection control policies. There is also a requirement for antimicrobial prophylaxis during induction period combined with the necessary increase in government healthcare spending to improve the survival of acute lymphoblastic leukemia in Egyptian children.
Estimar os desfechos do tratamento e as taxas de recidiva de crianças recém-diagnosticadas com leucemia linfoblástica aguda. É o câncer infantil mais comum, mas surpreendentemente poucos estudos relataram os desfechos do tratamento e a taxa de recidiva em pacientes de países de renda baixa/média.
MétodoEstudo de coorte retrospectivo de cinco anos. Foi feito no Centro de Oncologia da Universidade de Mansoura, no Egito.
ResultadosForam estudadas 200 crianças com leucemia linfoblástica aguda, das quais 46 (23%) morreram durante a indução e a maioria dessas mortes estava relacionada à infecção. Dos 152 pacientes que alcançaram a remissão completa, 41 (27%) apresentaram recidiva. O local mais comum de recidiva foi a medula óssea, seguido pelo sistema nervoso central isolado, com 53,7% e 31,7% dos casos, respectivamente. Das recidivas, 78% ocorreram muito precocemente ou precocemente, em vez de tardiamente. A maioria das mortes de pacientes com recidiva estava relacionada à infecção e progressão da doença. A taxa de sobrevida global em cinco anos para os pacientes foi de 63,1% (82,1% para não recidivados em comparação com 36,6% para os recidivados).
ConclusãoHouve uma alta incidência de mortes na indução relacionadas à infecção e altos percentuais de recidivas muito precoces ou precoces, com altas taxas de mortalidade e baixas taxas de sobrevida global em cinco anos. Nossos achados sugerem a necessidade urgente de modificação dos esquemas quimioterápicos para adequação às nossas condições locais, implantação de políticas de cuidados de suporte e controle de infecções. Há também a necessidade de profilaxia antimicrobiana durante o período de indução, junto com um aumento necessário nos gastos governamentais com a saúde, para melhorar a capacidade de sobrevivência das crianças egípcias com leucemia linfoblástica aguda.
Acute lymphoblastic leukemia (ALL), the most common childhood cancer worldwide, is characterized by high cure rates and good treatment outcomes of >80% in high-income countries (HIC),1 yet little is known of the treatment outcomes and the relapse rates of ALL children in low/middle-income countries (LIC/MIC), with very few studies being conducted in LIC/MIC.2–6 Egypt, as a developing MIC, suffers from economic difficulties and limited resources that prevent achieving and providing better cure rates compared to developed HIC. This study reports the treatment outcomes and relapse rates of ALL children treated at the Pediatric Oncology Unit of the Oncology Center of Mansoura University, which is located in the North-Eastern Nile Delta of Egypt.
Patients and methodsThe Institutional Research Board and Ethics Committee of Mansoura University approved this retrospective cohort chart-review study of a 5-year period (June 2011–June 2016). This research endeavor analyzed data of newly diagnosed ALL children who were treated, free of charge, at the Pediatric Oncology unit, which serves five governorates with a combined population of >10 million people. The unit has 32 inpatient beds (three beds per room), with an additional four intermediate care beds (two beds per room, with patient monitors and oxygen supply). There is access to two pediatric intensive care beds, with mechanical ventilators that are shared with the adult intensive care unit (ICU). There is no nearby housing facility for patients traveling from distant areas.
This study consecutively included all newly diagnosed children with ALL (age 1–18 years). Patients with missing or incomplete data and those who abandoned treatment were excluded. The following information was collected from the patient's medical records: sex, age at diagnosis, residence (whether from urban areas of the city of Mansoura and nearby towns/cities, or from rural areas in nearby villages or distant agricultural areas), white blood cell (WBC) count, immunophenotype profiles of leukemia, central nervous system (CNS) involvement, response to chemotherapy based on day 29 bone marrow (BM) examination, clinical status (whether complete remission, relapse, last follow-up, death or lost follow-up, cause of death).
ALL diagnosisBM examination was the basis of ALL diagnosis, whenever the BM exhibited blast cells ≥25% with additional positive ALL diagnosis confirmations conducted through flow cytometric analysis. Patients with <5% lymphoblasts in their BM aspirate (29 days after induction treatment) while showing normal hematopoiesis were defined as being in complete remission (CR). CNS involvement was evaluated through cerebrospinal fluid (CSF) analysis cytocentrifuge slide examination and classified as follows: CNS1: <5 WBCs/mm3, no blasts; CNS2: <5 WBCs/mm3 with blasts; CNS3: >5 WBCs/mm3 with blasts.7 Financial and resource restrictions deterred cytogenetic studies and minimal residual disease (MRD) in most of the patients.
Risk stratification and treatment protocolsPatients aged between <1 year and >10 years, with initial WBC count >50×109L−1, exhibiting blast cell infiltration of the CNS during presentation, displaying CD10-negative or precursor T-cell immunophenotype, and showing no signs of remission confirmed through BM examination, on 29th day of induction therapy, were then classified as being high-risk ALL subjects; otherwise, they were considered to be standard-risk ALL patients. ALL treatment protocols were based on the modified Berlin-Frankfurt-Münster 90 (ALL-BFM 90)8 (see supplementary material for further details). Prophylactic and therapeutic radiotherapy (RT) information is detailed in the treatment tables (Supplementary material Tables S1 and S2).
Definition and classification of relapseIdentification of relapse was based on BM re-infiltration with ≥25% blasts or blasts in extra-medullary sites. Relapse classification was based on the time of relapse occurrence after initial diagnosis: ‘very early’ relapse was defined as occurring <18 months from diagnosis, ‘early’ relapse occurring between 18 and 36 months from diagnosis, and ‘late relapse’ was considered to occur after >36 months from diagnosis.7
Abandonment of treatment was defined as care termination by the parent/caregiver and/or if more than 4 weeks was exceeded with a no-show/non-attendance for a scheduled treatment by the patient.9
Assessment of non-compliance was established during the maintenance phase, more aptly described as patients stopping medication intake without physician indication, through either chart review, pharmacy medication dispensing records review, or during clinical visits and discussions with families and/or with the patients themselves. It was not possible to measure the metabolite of 6-mercaptopurine (6-MP) due to financial restrictions and non-availability of testing facilities.
Statistical analysisSPSS (Statistical Package for the Social Sciences for Windows, version 18.0. Chicago, USA) was used for data analysis. Student's t-test was used for comparing numerical data, the chi-squared test was used for comparing non-numerical data, while the overall survival (OS) was measured based on the time of diagnosis until the patients’ death or their last documented follow-up visit. Event-free survival (EFS) calculations were based on the time of diagnosis to the date of the first event, be that induction failure, relapse at any site, or death. Relapse-free survival (RFS) calculations were based on end of induction time until patients relapse for those who achieved CR. Kaplan–Meier survival curves estimated RFS and OS and were compared using the log-rank test. The Cox proportional-hazard regression model was utilized for univariate and multivariate analysis of prognostic factors with 95% confidence intervals (95% CIs). p-values of <0.05 were considered significant.
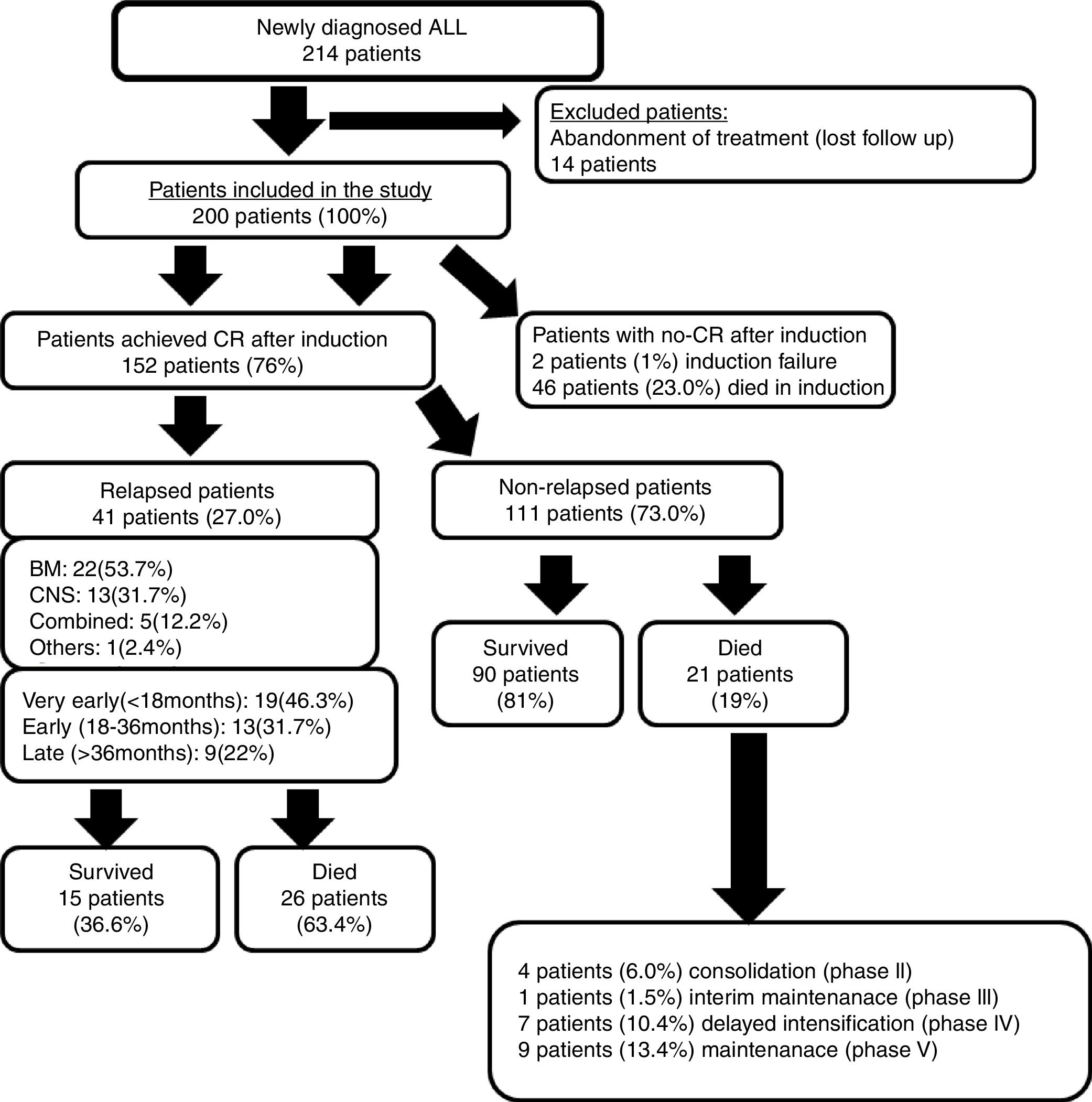
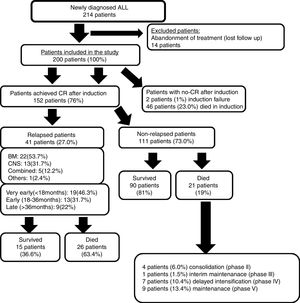
ResultsTwo-hundred newly diagnosed ALL patients were studied out of the 214 patients who were initially reviewed, since 14 patients (6.5%) abandoned treatment when follow-up contact was lost. One hundred fifty-two (76%) of the 200 studied patients achieved CR after the induction phase. Induction failure only occurred in two patients (1%), while induction deaths occurred in 46 patients (23%).
Forty-one patients (27%) experienced relapse out of the 152 patients who achieved CR. Fig. 1 illustrates the study's clinical flow chart. The median follow-up time of this study was 2.4 years (range: 0.25–5.9 years).
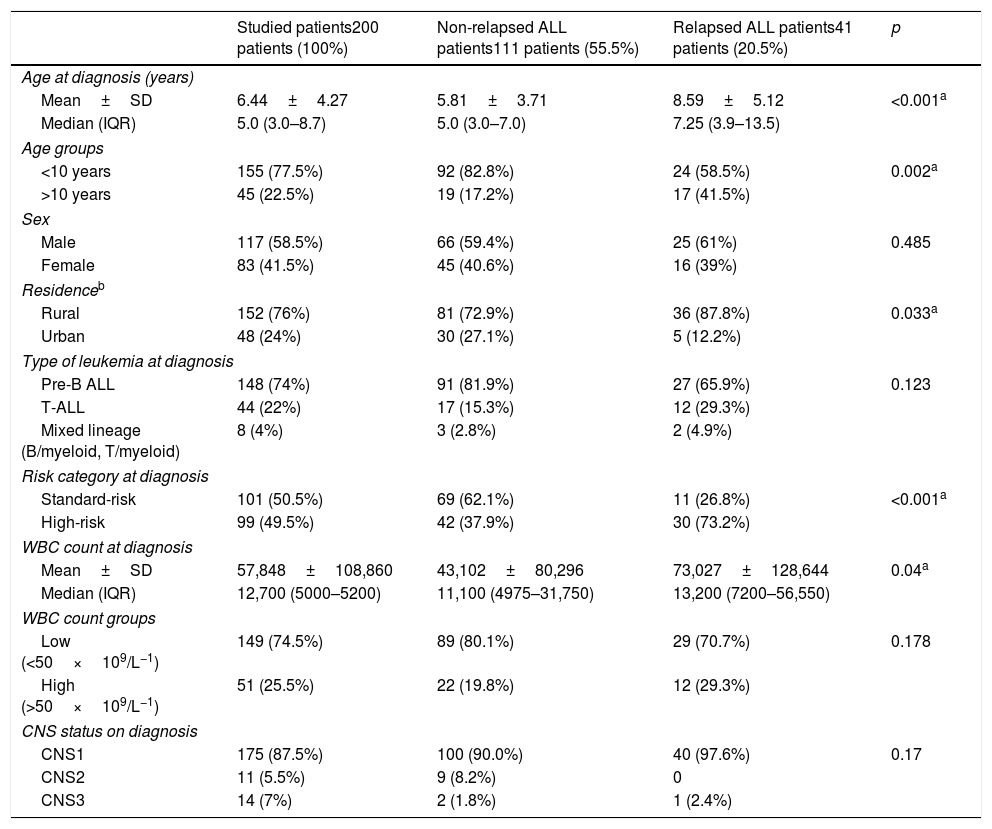
Different clinical characteristics of the studied patients and a comparison between relapsed and non-relapsed patients are shown in Table 1. Twenty-three percent (46/200) of the studied patients died during the induction phase.
Different characteristics of subjects, and the comparison between relapsed vs. non-relapsed ALL patients (Student's t-test and chi-squared test).
| Studied patients200 patients (100%) | Non-relapsed ALL patients111 patients (55.5%) | Relapsed ALL patients41 patients (20.5%) | p | |
|---|---|---|---|---|
| Age at diagnosis (years) | ||||
| Mean±SD | 6.44±4.27 | 5.81±3.71 | 8.59±5.12 | <0.001a |
| Median (IQR) | 5.0 (3.0–8.7) | 5.0 (3.0–7.0) | 7.25 (3.9–13.5) | |
| Age groups | ||||
| <10 years | 155 (77.5%) | 92 (82.8%) | 24 (58.5%) | 0.002a |
| >10 years | 45 (22.5%) | 19 (17.2%) | 17 (41.5%) | |
| Sex | ||||
| Male | 117 (58.5%) | 66 (59.4%) | 25 (61%) | 0.485 |
| Female | 83 (41.5%) | 45 (40.6%) | 16 (39%) | |
| Residenceb | ||||
| Rural | 152 (76%) | 81 (72.9%) | 36 (87.8%) | 0.033a |
| Urban | 48 (24%) | 30 (27.1%) | 5 (12.2%) | |
| Type of leukemia at diagnosis | ||||
| Pre-B ALL | 148 (74%) | 91 (81.9%) | 27 (65.9%) | 0.123 |
| T-ALL | 44 (22%) | 17 (15.3%) | 12 (29.3%) | |
| Mixed lineage (B/myeloid, T/myeloid) | 8 (4%) | 3 (2.8%) | 2 (4.9%) | |
| Risk category at diagnosis | ||||
| Standard-risk | 101 (50.5%) | 69 (62.1%) | 11 (26.8%) | <0.001a |
| High-risk | 99 (49.5%) | 42 (37.9%) | 30 (73.2%) | |
| WBC count at diagnosis | ||||
| Mean±SD | 57,848±108,860 | 43,102±80,296 | 73,027±128,644 | 0.04a |
| Median (IQR) | 12,700 (5000–5200) | 11,100 (4975–31,750) | 13,200 (7200–56,550) | |
| WBC count groups | ||||
| Low (<50×109/L−1) | 149 (74.5%) | 89 (80.1%) | 29 (70.7%) | 0.178 |
| High (>50×109/L−1) | 51 (25.5%) | 22 (19.8%) | 12 (29.3%) | |
| CNS status on diagnosis | ||||
| CNS1 | 175 (87.5%) | 100 (90.0%) | 40 (97.6%) | 0.17 |
| CNS2 | 11 (5.5%) | 9 (8.2%) | 0 | |
| CNS3 | 14 (7%) | 2 (1.8%) | 1 (2.4%) | |
CNS, central nervous system; IQR, inter-quartile range 25–75%; SD, standard deviation; WBC, white blood cell.
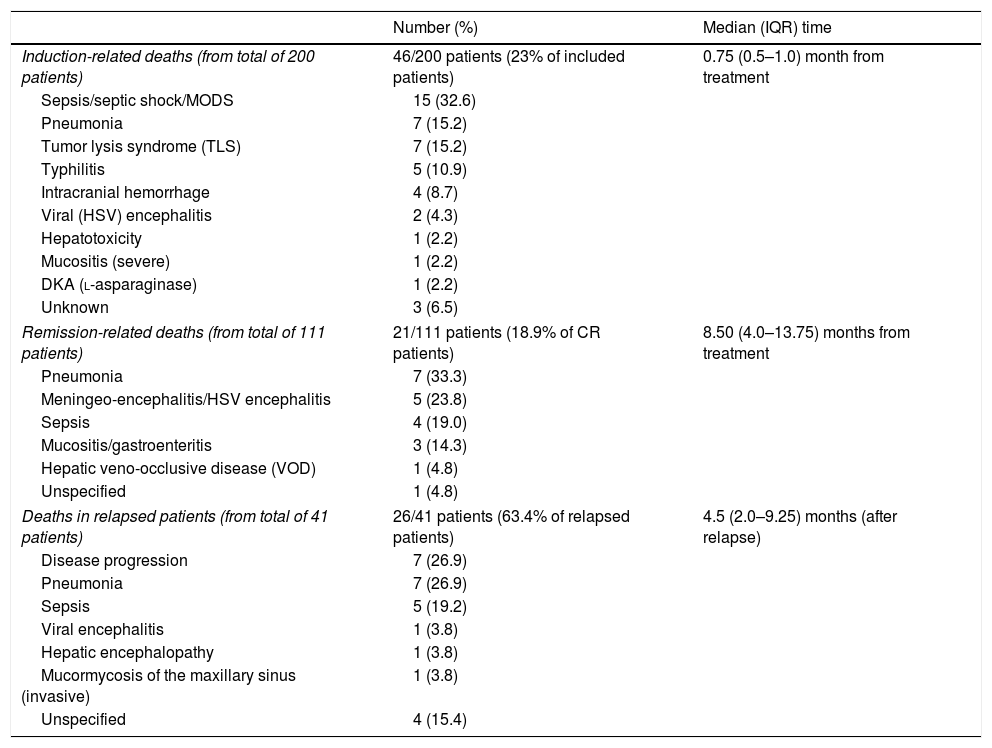
Table 2 documents the majority of the dead patients who underwent induction and unfortunately succumbed to infection-related maladies. The rates of death were unchanged over the observed period.
Comparison data of different causes and time of occurrence of deaths in relapsed and non-relapsed patients.
| Number (%) | Median (IQR) time | |
|---|---|---|
| Induction-related deaths (from total of 200 patients) | 46/200 patients (23% of included patients) | 0.75 (0.5–1.0) month from treatment |
| Sepsis/septic shock/MODS | 15 (32.6) | |
| Pneumonia | 7 (15.2) | |
| Tumor lysis syndrome (TLS) | 7 (15.2) | |
| Typhilitis | 5 (10.9) | |
| Intracranial hemorrhage | 4 (8.7) | |
| Viral (HSV) encephalitis | 2 (4.3) | |
| Hepatotoxicity | 1 (2.2) | |
| Mucositis (severe) | 1 (2.2) | |
| DKA (l-asparaginase) | 1 (2.2) | |
| Unknown | 3 (6.5) | |
| Remission-related deaths (from total of 111 patients) | 21/111 patients (18.9% of CR patients) | 8.50 (4.0–13.75) months from treatment |
| Pneumonia | 7 (33.3) | |
| Meningeo-encephalitis/HSV encephalitis | 5 (23.8) | |
| Sepsis | 4 (19.0) | |
| Mucositis/gastroenteritis | 3 (14.3) | |
| Hepatic veno-occlusive disease (VOD) | 1 (4.8) | |
| Unspecified | 1 (4.8) | |
| Deaths in relapsed patients (from total of 41 patients) | 26/41 patients (63.4% of relapsed patients) | 4.5 (2.0–9.25) months (after relapse) |
| Disease progression | 7 (26.9) | |
| Pneumonia | 7 (26.9) | |
| Sepsis | 5 (19.2) | |
| Viral encephalitis | 1 (3.8) | |
| Hepatic encephalopathy | 1 (3.8) | |
| Mucormycosis of the maxillary sinus (invasive) | 1 (3.8) | |
| Unspecified | 4 (15.4) | |
CR, complete remission; DKA, diabetic ketoacidosis; HSV, herpes simplex virus; IQR, inter-quartile range 25–75%; MODS, multiple organ dysfunction syndrome; TLS, tumor lysis syndrome; VOD, veno-occlusive disease.
The 5-year OS and EFS for all patients were 63.1% and 46%, respectively. The 5-year OS for patients who achieved remission, after excluding deaths related to induction and/or patients who had failed induction, was 82.2% for non-relapsed patients compared to 36.6% for relapsed patients (p<0.001), and is highlighted in Fig. S1 for comparison. Relapsed patients were more at risk, and four times more likely to die than non-relapse patients (p<0.001).
Outcome of non-relapse patientsNinety patients (81%) survived out of the 111 patients who achieved CR without exhibiting relapse, while the remainder, 21 patients (19%), died mainly due to infection-related complications, which are shown in Table 2.
Outcome of relapsed patientsFifteen patients (36.6%) of the 41 relapsed patients survived and were managed in the following manner: nine patients received re-induction chemotherapy, four patients were given chemotherapy and cranial irradiation, and two patients underwent allogeneic peripheral stem cell transplantation (PSCT). Unfortunately, twenty-six of the relapsed patients (63.4%) died. A large majority of these deaths occurred at the early stages of the second re-induction, with a median survival duration of 4.5 months. Once again, these deaths were mainly due to infection-related events such as pneumonia, sepsis, or disease progression (Table 2). Patients experiencing relapse were significantly older in age, and mainly hailed from the rural areas, exhibiting high-risk criteria with higher WBC counts on diagnosis.
Nineteen of the relapsed patients (46.3%) experienced ‘very early’ relapse, which was the most common type, followed by ‘early’ and then ‘late’ relapses. BM was the most frequent site of relapse, followed by isolated CNS (Fig. 1).
A significant difference was found in regards to the 5-year OS and relapse type (p=0.005); the 5-year OS was found to be 61.5% for CNS relapse patients, 27.3% for BM relapse patients, and no patient survived combined relapses. Fig. S2 shows survival in relation to relapse time, where ‘very early’ relapsers had a significantly (p<0.001) lower 5-year OS (15.8%), compared to ‘early’ and ‘late’ relapsers, whose 5-year OS were 46.2% and 66.7% respectively.
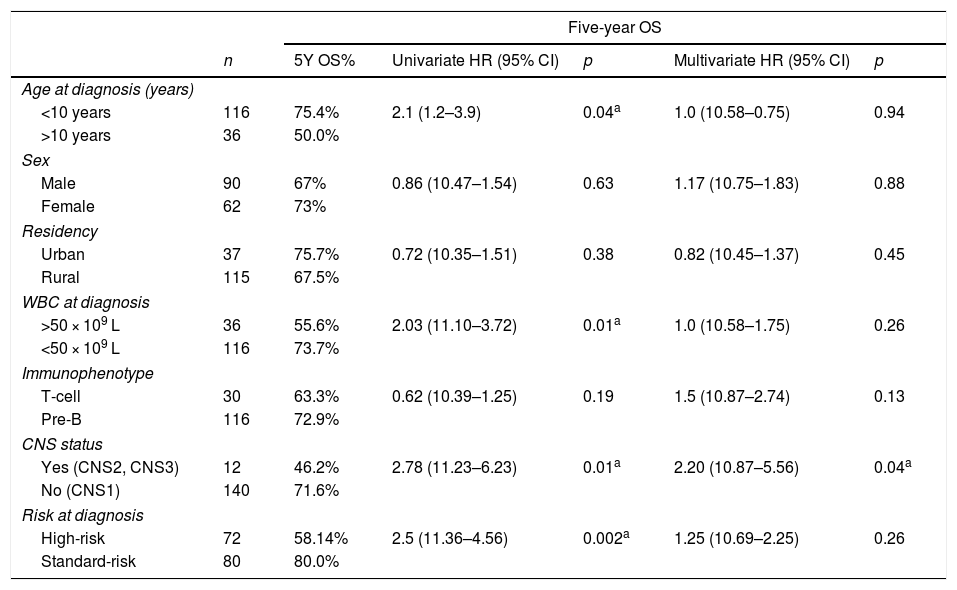
Predictors of deathEvaluating the risk of death was accomplished by utilizing the Cox proportional-hazard model, as shown in Table 3. Univariate analysis showed that patients >10 years of age who exhibited a high initial WBC count >50×109L−1, along with positive CNS status (CNS2 or CNS3) from a high-risk category on diagnosis and were significant predictors for lowering the 5-year OS. The multivariate Cox regression analysis highlighted that only CNS status (CNS2 or CNS3) were considered unfavorable independent prognostic factors in the 5-year OS, as it was significantly correlated to increased risks of death.
Hazard ratio (HR) for death by univariate and multivariate Cox regression analysis of clinical and laboratory characteristics, over a 5-year OS.
| Five-year OS | ||||||
|---|---|---|---|---|---|---|
| n | 5Y OS% | Univariate HR (95% CI) | p | Multivariate HR (95% CI) | p | |
| Age at diagnosis (years) | ||||||
| <10 years | 116 | 75.4% | 2.1 (1.2–3.9) | 0.04a | 1.0 (10.58–0.75) | 0.94 |
| >10 years | 36 | 50.0% | ||||
| Sex | ||||||
| Male | 90 | 67% | 0.86 (10.47–1.54) | 0.63 | 1.17 (10.75–1.83) | 0.88 |
| Female | 62 | 73% | ||||
| Residency | ||||||
| Urban | 37 | 75.7% | 0.72 (10.35–1.51) | 0.38 | 0.82 (10.45–1.37) | 0.45 |
| Rural | 115 | 67.5% | ||||
| WBC at diagnosis | ||||||
| >50 × 109 L | 36 | 55.6% | 2.03 (11.10–3.72) | 0.01a | 1.0 (10.58–1.75) | 0.26 |
| <50 × 109 L | 116 | 73.7% | ||||
| Immunophenotype | ||||||
| T-cell | 30 | 63.3% | 0.62 (10.39–1.25) | 0.19 | 1.5 (10.87–2.74) | 0.13 |
| Pre-B | 116 | 72.9% | ||||
| CNS status | ||||||
| Yes (CNS2, CNS3) | 12 | 46.2% | 2.78 (11.23–6.23) | 0.01a | 2.20 (10.87–5.56) | 0.04a |
| No (CNS1) | 140 | 71.6% | ||||
| Risk at diagnosis | ||||||
| High-risk | 72 | 58.14% | 2.5 (11.36–4.56) | 0.002a | 1.25 (10.69–2.25) | 0.26 |
| Standard-risk | 80 | 80.0% | ||||
OS, overall survival for all patients; HR, hazard ratio; 95% CI, 95% confidence interval.
Fourteen patients abandoned treatment immediately after starting the leukemia treatment and follow-up contact was lost, without justifiable cause or reason. None of these patients returned nor did they make contact with the hospital. There is a possibility that they sought treatment elsewhere, in another hospital, or died at home. Abandonment of the treatment occurred during the maintenance phase for seven patients (13–26 months), after the induction for five patients (1–1.5 months), and for another two patients after delayed intensification (4 months).
Poor compliance and/or treatment interruption were documented in 28.9% of the studied patients. The most common causes of non-compliance were the hospital not acquiring enough medication due to economic scarcity and inadequate budget, and the inability of patients to financially purchase medication from outside sources, which compounded the non-availability of medication. Few patients reported omission of doses, whether due to forgetfulness, patient's refusal to take medications, or parents stopping medications due to minor illnesses (common-cold) or fear of complications.
DiscussionThis study reported the treatment outcomes of children with ALL with attempts to explore areas requiring improvement in the implementation of cancer care policies. High-intensity treatment regimens without adequate supportive care usually leads to paradoxically lower EFS by increasing toxic death.10 Using HIC protocols for childhood ALL treatment, the Total XI protocol in the US resulted in 72% EFS, while Brazil reported 32% EFS.11 But interestingly, when the same regimen was used in El Salvador with some modifications/adaptations (without induction anthracyclines) the EFS improved from 10% to 48%.12
In the authors’ treatment protocols, an attempt was made to remain compliant with international standards and protocols that were difficult to adhere to; as such standards are becoming more complicated with extensive, constantly introduced requirements, expensive supportive care, and medications. Unfortunately in this study, the majority of the deaths were induction related, where infection/sepsis was the main cause of death. Treatment outcome studies of Egyptian children with ALL and from neighboring developing countries are shown in Table S3.6,13–17 Similarly, in studies from Mexico2 and India,5 deaths were reported to occur in 24% to 63.9% of patients, with sepsis being the main cause of death, in 53.3% and 71.4%, respectively. In contrast, Jastaniah et al.,6 from a developing country (Saudi Arabia) with adequate supportive care and financial resources, reported only 2.7% induction death in their patients.
Reduction of induction chemotherapy may be of benefit; more is not necessarily always better. Minor modifications/adaptations of treatment regimens, such as induction omission of two doses of doxorubicin or the use of prophylactic antibiotics, could result in great benefits in LIC/MIC.18 Prophylactic antimicrobials usage is an approach to decrease infection-related induction mortalities, especially in settings with poor resources and supportive care facilities. Oncologists are on occasion obligated to modify treatment regimens in the face of severe bacterial/viral or disseminated fungal infection, poor nutritional status, severe mucositis, and poor access to supportive care facilities. Modification of chemotherapy can usually be achieved by dosage reductions, regimen component removal, cycle shortening, or administration of fewer cycles.18–20 Messinger et al.21 reported no added improvement of EFS for standard-risk patients using anthracyclines with a three-drug induction therapy (prednisone, vincristine, and l-asparaginase). Also, in ALL-BFM 90, 25% reduced anthracycline induction dose had no adverse effects on survival.22 Interestingly, the ALL-BFM 95 trials, compared to previous trials, reported that a 50% reduction of the daunorubicin induction dose in standard-risk patients was safe, with an excellent EFS of 89.5%.23 Trials in the Dominican Republic by Hunger et al.19 reported that using reduced induction chemotherapy in standard-risk patients, administering a three-drug induction without anthracyclines, together with 4-week oral consolidation of 6-MP, resulted in an improvement of the 12- and 24-month OS, from 50% to 80%, and from 40% to 70%, respectively, and it reduced the death rate.
Decreasing or omitting anthracyclines given to ALL children who have low-risk criteria may be considered a good practice, i.e., those who are aged <10 years with WBC <50×109L−1, and with negative CNS involvement. Even possibly delaying anthracycline administration during the induction phase, where one would wait until after BM evaluation results at day 15 of induction, then consider appropriate later anthracycline administration. It may be worth a trial to give only two out of four doses of anthracyclines during the induction phase and re-evaluate BM at end of induction on day 29, and if necessary, compensate later during the re-induction/intensification phase if the patient has shown non-satisfactory response or has had delays in their treatment.
The usage of prophylactic antimicrobial medications is highly encouraged to decrease induction mortality related to infection and sepsis. Yeh et al.24 reported that ALL children undergoing induction chemotherapy who were placed on prophylactic oral ciprofloxacin and oral voriconazole or intravenous micafungin during neutropenia periods had fewer episodes of bloodstream infection, no invasive fungal infections, and experienced no patient deaths with severe infection. More significantly, it reduced episodes of fever and neutropenia, and decreased the ICU length of stay. They also reported significant lower therapy costs when utilizing prophylactic antibiotics/antifungal treatment compared to infection treatment costs; but such costs are still considered very expensive, and beyond reach for LIC. Additionally, these treatment streams were still considered risky due to increased toxicity (hepatotoxicity), with emerging and increased states of bacterial antibiotic resistance as well as the fear of drug interactions.
In the present study during the ALL induction phase, only Pneumocystis jirovecii prophylaxis with trimethoprim-sulfamethoxazole was utilized, which was continued throughout the whole treatment. The authors are currently evaluating the possibility of implementing prophylactic antimicrobial therapy regimens, but this was hindered due to limited financial resources, and insufficient and/or pending support from pharmaceutical companies.
Relapse is considered to be a major problem because it reduces ALL children's survival. A significant childhood ALL study reported that relapse rates tended to range between 12% and 20%.25 Yet in the present study, relapse occurred in 27% patients of those who achieved remission, with high mortality rates and a low 5-year OS. The majority of relapsed patients experienced ‘very early’ relapses. These results to some extent are in keeping with other studies from developing LIC/MIC (Mexico and Pakistan).2–4 They reported relapse rates of 21.5%, 26.2%, even up to 58.5%. ALL patients with ‘early’ relapses were the most frequently recorded, at 45.83%, while ‘very early/early relapse’ were recorded at 33.9%/50.4%, with 5-year OS of 48.9% and 65%. In turn, another study from a developing and well-resourced country (Saudi Arabia)6 reported a very good 5-year OS of 84.7% and a relapse rate of 15.1%.
Data from 20 cancer centers from all over Egypt reported a 5-year OS rate of 40% with treatment guidelines based on international protocols, with the absence of uniform national treatment plans.26 Governmental annual health-care expenditure per capita was an important factor that is highly correlated to 5-year OS. There is a strong need for increased government spending, as the service shortages and improper support to improve the healthcare infrastructure is evident.27 Adequate care support structures impact the survival rates of ALL children, and there is a need to significantly improve them in LIC/MIC.15 Difficulties in providing adequate and supportive care in Egypt were more evident after the Egyptian revolution period (2011–2016), which could negatively impacted all healthcare provisioning.
Improvements in the near future may be achieved by increasing healthcare budgets that will, at the bare minimum, keep essential medication stocks replenished. However, improving physician training will require international collaboration efforts. This will also encourage freedom of data exchange, which will promote collaborative evaluation of the children's requirements and promote the establishment of cancer registry that appropriately tracks and evaluates the needs of children for their benefit.
Management of children with cancer in Egypt is mainly provided by university hospitals, free of charge, yet with inadequate budgets, improper support networks, and inadequate care services. The patient often experiences and suffers from shortages of chemotherapy and supportive medications, which are only available through non-profit organizations or personal charitable donations. There is also the lack of infection control policies or proper adherence to hygiene practices by hospital staff and/or the patients’ families.14 Reducing infection is possible through careful corrections to the inadequate standards of infection practices, which include the necessary improvement of the patient's and their family's own knowledge to enhance their home hygiene practices, especially for those living in rural areas. Rural areas are often afflicted by a contaminated environment and poor water supply.28
Effective ALL treatment in LIC requires that therapy, including medication costs, be made free of charge or at the very minimum be highly subsidized, so that the patient's family can afford the minimal of contributions necessary for adequate treatment. Infrastructure must be in place to minimize therapy abandonment, such as funds for sustenance for patient and their family, as well as the provision of housing near the treatment hospital, for patients who have traveled far distances. There is a requirement to develop local fund raising foundations or accept funding from external agencies that are efficiently organized.18
In conclusion, this study found high induction deaths mostly related to infection/sepsis, with high percentages of very early/early relapses, and high mortality rates with low 5-year OS. These findings suggest the urgent need for the chemotherapy treatment regimen to be modified to suit local conditions, for adequate supportive care, and for implementation of infection control policies. There is also a need for induction-phase antimicrobial prophylaxis usage. Finally, there is an urgent need for the government of Egypt to increase healthcare spending, with the establishment of fundraising agencies to help improve our ALL children's survival while decreasing the complications encountered during their treatment.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Abdelmabood S, Fouda AE, Boujettif F, Mansour A. Treatment outcomes of children with acute lymphoblastic leukemia in a middle-income developing country: high mortalities, early relapses, and poor survival. J Pediatr (Rio J). 2020;96:108–16.