To translate and cross-culturally adapt the Childhood Bladder and Bowel Dysfunction Questionnaire (CBBDQ) for use in Brazilian Portuguese. The CBBDQ is an 18-item tool covering 10 bladder and 8 bowel symptoms that was developed for use with children of 5 to 12 years of age with bowel and bladder dysfunction (BBD). The instrument has already been validated for use in Dutch and English.
MethodIn the process of translation and cultural adaptation from English to Portuguese, the CBBDQ was submitted to undergo the required steps as established by the international methodological criteria: forward translation, synthesis, back-translation, expert panel review and pre-testing.
ResultsNinety-three parents of children with lower urinary tract dysfunction answered the questionnaire. The mean age of the children was 7.6±2.1 years and 54 were female. Internal consistency was excellent, with a Cronbach’s alpha of 0.91 to 0.96. Additionally, reliability was high, with an intraclass correlation coefficient of 0.94 (95%CI: 0.85-0.93; p<0.0001).
ConclusionThe translation and cultural adaptation of the CBBDQ enabled a quantitative evaluation of bladder and bowel symptoms to be performed in Brazilian children. The scores achieved allow the severity of BBD to be evaluated, as well as the patient’s progress during treatment. The use of this questionnaire in clinical practice and research will allow more consistent data on BBD to be obtained.
The International Children’s Continence Society (ICCS) defines bladder and bowel dysfunction (BBD) as a spectrum of bladder and bowel symptoms due to an association of lower urinary tract dysfunction (LUTD) and bowel dysfunction in children with no anatomical or functional abnormalities.1 BBD is a common clinical condition in pediatrics and is a cause of high morbidity, principally due to its association with urinary tract infections (UTIs), vesicoureteral reflux and increased renal scarring.2,3 Early diagnosis and treatment prevent comorbidities, particularly kidney damage.4 BBD is a clinical condition with limited knowledge available and, unfortunately, it is often diagnosed late and usually following recurrent UTIs. The symptoms that characterize LUTD include urinary urgency, holding maneuvers, daytime urinary incontinence, enuresis, vulvar dermatitis, abdominal pain, constipation and fecal incontinence.
The introduction of a validated questionnaire for use in clinical practice is interesting, not only to evaluate the severity of BBD through the application of scores, but also to monitor the patient’s response to treatment. The questionnaire most commonly used is the Dysfunctional Voiding Scoring System (DVSS).5 However, the DVSS contains only two questions on constipation, which is insufficient to enable a more complete evaluation of this symptom to be performed. Moreover, the way the intensity of symptoms in DVSS is graded (almost never, less than half the time, about half the time and almost every time) often makes comprehension difficult, particularly in the segment of the population with a lower level of schooling.
The Childhood Bladder and Bowel Dysfunction Questionnaire (CBBDQ) is an 18-item tool that covers 10 bladder symptoms and 8 bowel symptoms.6 It was constructed in accordance with the COSMIN (COnsensus-based Standards for the selection of health status Measurement INstruments) checklist, which is an internationally accepted tool for the evaluation of the psychometric criteria of viability, content and structural validity of measurement instruments.7 The internal consistency of the CBBDQ was found to be satisfactory with respect to the bladder and bowel subscales when completed by Dutch parents of children 5 to 12 years of age. In this questionnaire, symptoms can be evaluated as a function of their weekly or monthly frequency; therefore, it is more intuitive than the DVSS. Furthermore, there are more questions on bowel symptoms, resulting in a more complete evaluation of constipation.
The objective of the present study was to translate and transculturally adapt the English version of the CBBDQ to a version in Brazilian Portuguese using standardized and internationally applied methodology.8,9 According to the relevant guidelines, translations should first be evaluated in terms of conceptual equivalence so that any grammatical changes required will be conceptually similar in another culture. With respect to the transcultural adaptation, cultural factors such as the habits and activities of a population must be taken into consideration, since an activity that is uncommon to a certain population could invalidate the adaptation of the instrument.8
MethodsAfter receiving permission from the authors of the original study, the process was initiated to translate the English version of the CBBDQ into Brazilian Portuguese using the recommended methodology.8,9 In the process of transcultural adaptation, 93 parents of children 5 to 12 years of age with LUTD were included in the study. All the participating children were undergoing treatment in the institute where the study was conducted.
The ethics committee of Inspirar College approved the study protocol and all participating parents signed an informed consent form.
The institute’s internal review board approved the study protocol and all participating parents signed an informed consent form.
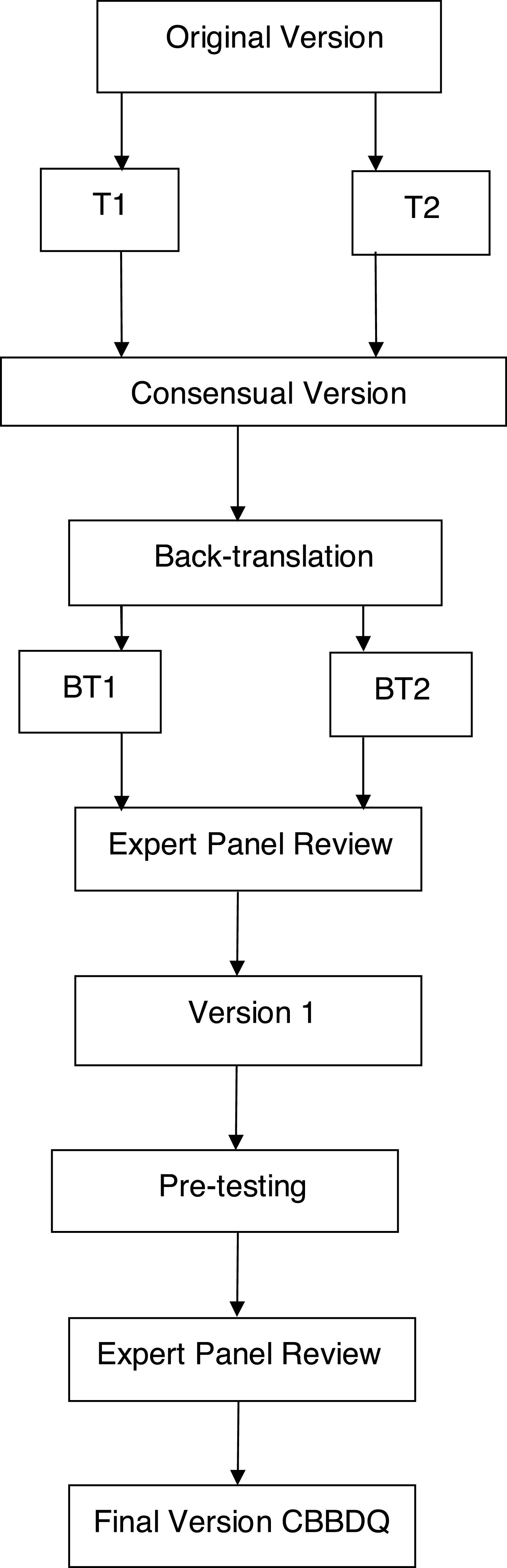
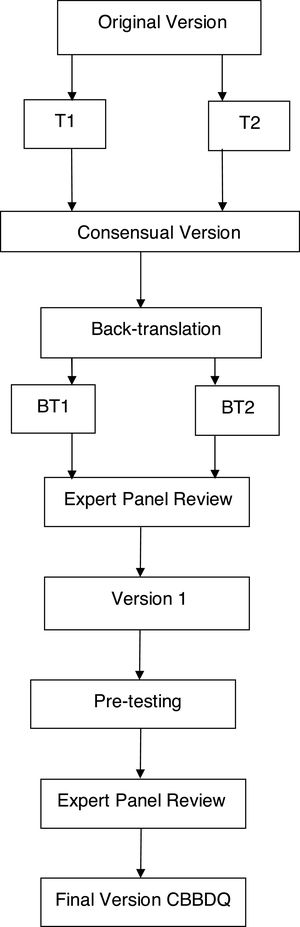
The study followed these recommended steps (Fig. 1):
- 1
Two bilingual translators, native to Brazil, performed the initial forward translation to Brazilian Portuguese, individually and with prior knowledge of the study objectives. This resulted in two preliminary translated versions in the Portuguese language (T1 and T2).
- 2
A group formed of three specialists in LUTD in children and a professional unfamiliar with the questionnaire analyzed T1 and T2 with the objective of minimizing the differences and preserving the cultural context and concepts of the original questionnaire. This synthesis step resulted in a consensual version.
- 3
Two bilingual English teachers who had no knowledge of the study but were well acquainted with Brazilian culture worked individually to translate the Portuguese consensual version back into English. Two back-translated versions were obtained (BT1 and BT2).
- 4
An expert panel evaluated the semantic, cultural and conceptual equivalence between the original questionnaire and the back-translated versions. A pre-final version was then prepared and sent to the original authors for their approval.
- 5
Pre-testing was performed in order to verify the cross-cultural adaptation of the questionnaire in Portuguese. During this step, the parents of children with LUTD were asked to complete the questionnaire twice, with an interval of one week between the two moments.
- 6
The expert review panel conducted a final analysis that resulted in the final version of the questionnaire translated into Brazilian Portuguese.
The Statistical Package for the Social Sciences (SPSS), version 22, was used for the data analysis. Cronbach’s coefficient alpha was used to analyze test-retest reliability, with values >0.70 indicating satisfactory internal consistency. The intraclass correlation coefficient, which evaluates reliability in relation to the stability of the instrument, was also used, with values of 0.61 to 0.80 being indicative of good reliability and values of 0.81 to 1.00 reflecting high reliability. Test-retest agreement was also evaluated using Bland-Altman plots (Medcalc statistical software, version 18). Significance was set at p<0.05.
ResultsOf 106 patients and parents eligible for inclusion in the study, 13 were excluded from analysis because they failed to repeat the evaluation within the seven-day period stipulated in the methodology. Therefore, 93 children with a mean age of 7.6±2.1 years who were undergoing treatment for some form of LUTD were included. Of these, 54 were female and 39 were male.
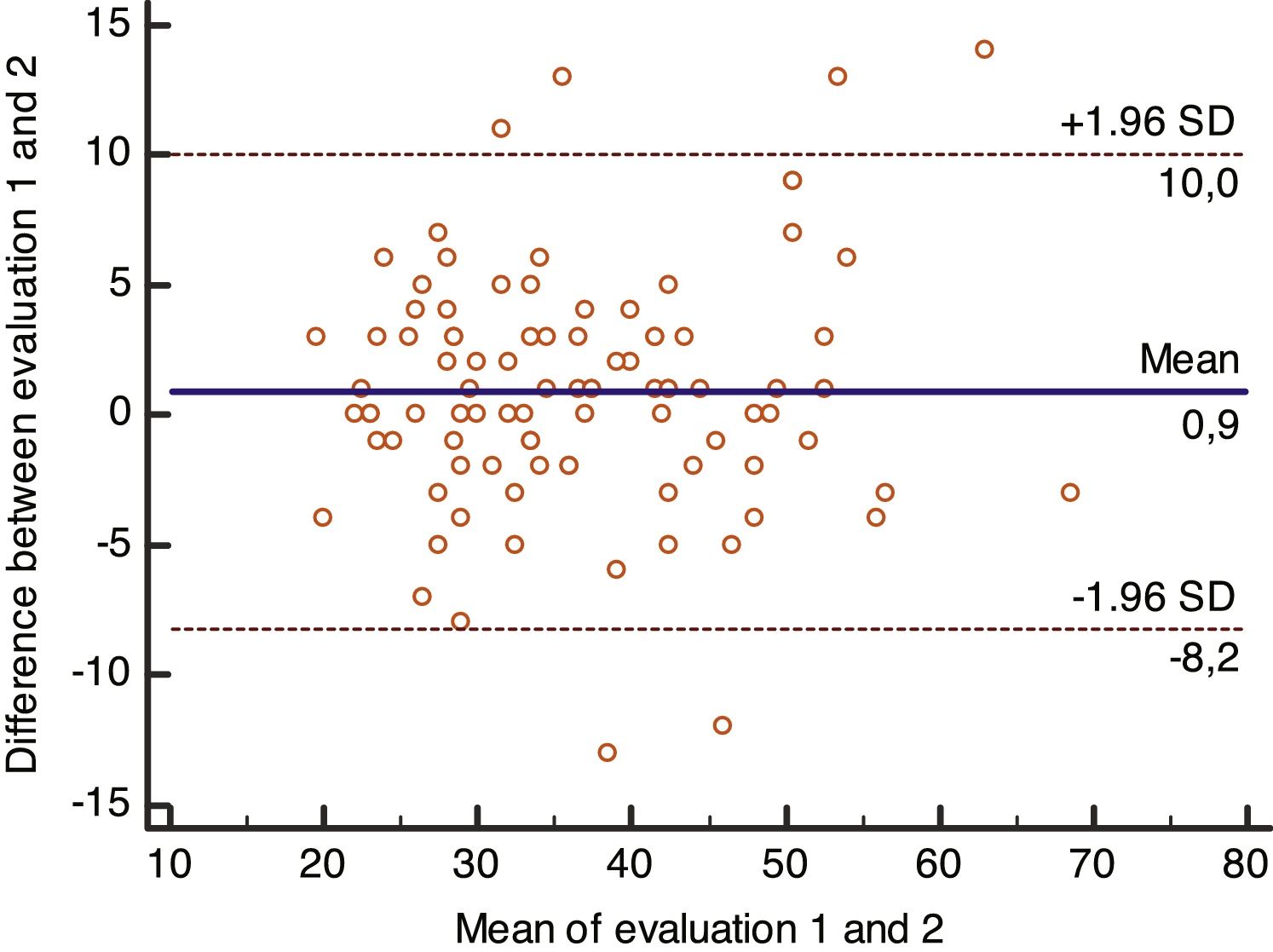
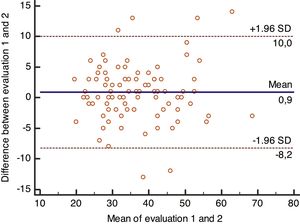
Data analysis showed satisfactory reliability. According to Cronbach’s alpha, internal consistency in the test-retest was satisfactory, with values of 0.91 and 0.96. The intraclass correlation coefficient showed a high degree of stability, with a value of 0.94 (95%CI: 0.85-0.94; p<0.001). Agreement between the evaluations is illustrated in the Bland-Altman plots (Fig. 2), with a low bias of -0.1 and limit of agreement between 5.9 and -6.6.
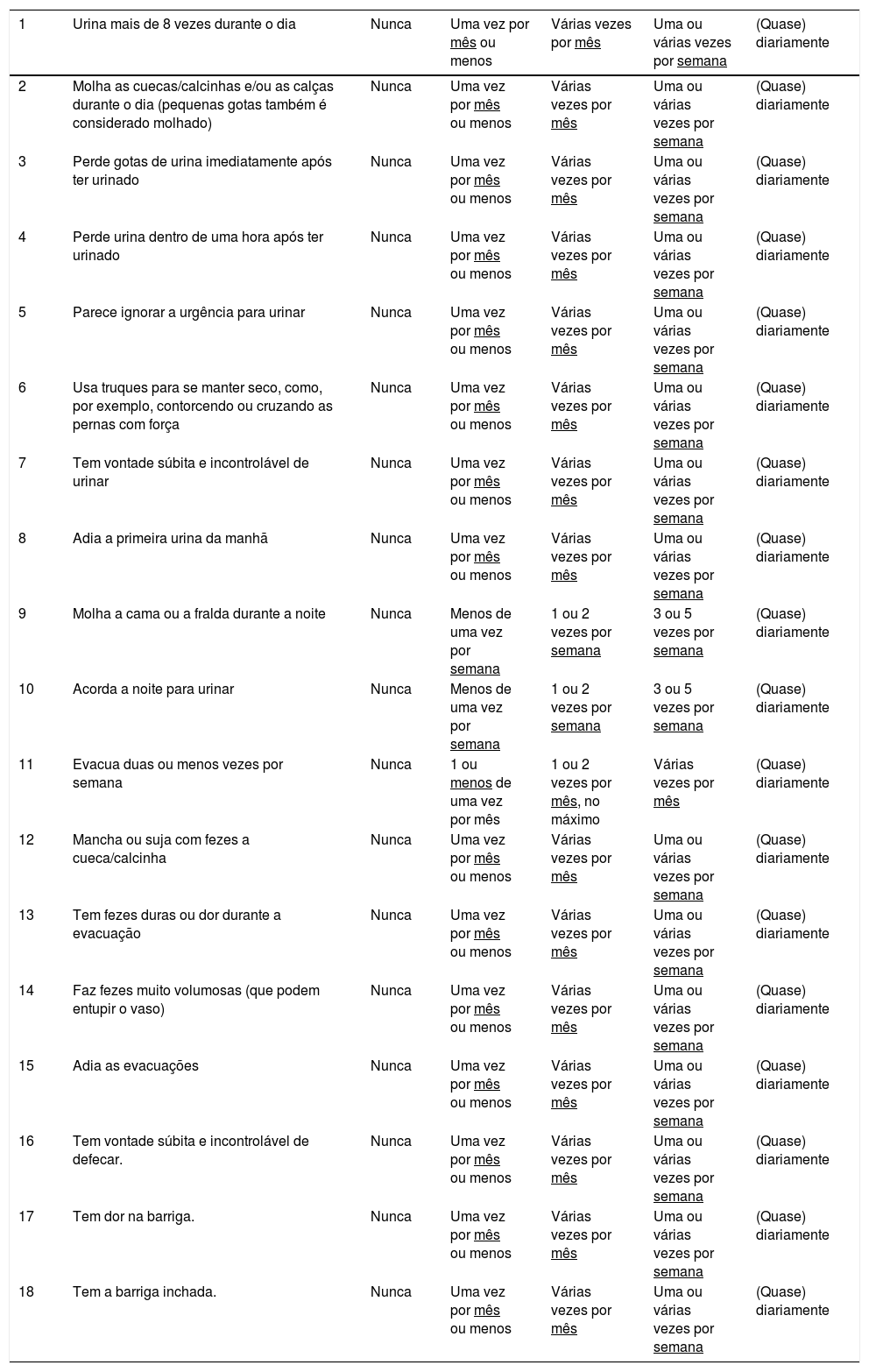
The final Brazilian Portuguese version of the CBBDQ is shown in Table 1.
Childhood Bladder and Bowel Dysfunction Questionnaire (CBBDQ5-12y) translated into Brazilian Portuguese: Questionário sobre problemas urinários e de evacuação em crianças de 5 a 12 anos.
| 1 | Urina mais de 8 vezes durante o dia | Nunca | Uma vez por mês ou menos | Várias vezes por mês | Uma ou várias vezes por semana | (Quase) diariamente |
|---|---|---|---|---|---|---|
| 2 | Molha as cuecas/calcinhas e/ou as calças durante o dia (pequenas gotas também é considerado molhado) | Nunca | Uma vez por mês ou menos | Várias vezes por mês | Uma ou várias vezes por semana | (Quase) diariamente |
| 3 | Perde gotas de urina imediatamente após ter urinado | Nunca | Uma vez por mês ou menos | Várias vezes por mês | Uma ou várias vezes por semana | (Quase) diariamente |
| 4 | Perde urina dentro de uma hora após ter urinado | Nunca | Uma vez por mês ou menos | Várias vezes por mês | Uma ou várias vezes por semana | (Quase) diariamente |
| 5 | Parece ignorar a urgência para urinar | Nunca | Uma vez por mês ou menos | Várias vezes por mês | Uma ou várias vezes por semana | (Quase) diariamente |
| 6 | Usa truques para se manter seco, como, por exemplo, contorcendo ou cruzando as pernas com força | Nunca | Uma vez por mês ou menos | Várias vezes por mês | Uma ou várias vezes por semana | (Quase) diariamente |
| 7 | Tem vontade súbita e incontrolável de urinar | Nunca | Uma vez por mês ou menos | Várias vezes por mês | Uma ou várias vezes por semana | (Quase) diariamente |
| 8 | Adia a primeira urina da manhã | Nunca | Uma vez por mês ou menos | Várias vezes por mês | Uma ou várias vezes por semana | (Quase) diariamente |
| 9 | Molha a cama ou a fralda durante a noite | Nunca | Menos de uma vez por semana | 1 ou 2 vezes por semana | 3 ou 5 vezes por semana | (Quase) diariamente |
| 10 | Acorda a noite para urinar | Nunca | Menos de uma vez por semana | 1 ou 2 vezes por semana | 3 ou 5 vezes por semana | (Quase) diariamente |
| 11 | Evacua duas ou menos vezes por semana | Nunca | 1 ou menos de uma vez por mês | 1 ou 2 vezes por mês, no máximo | Várias vezes por mês | (Quase) diariamente |
| 12 | Mancha ou suja com fezes a cueca/calcinha | Nunca | Uma vez por mês ou menos | Várias vezes por mês | Uma ou várias vezes por semana | (Quase) diariamente |
| 13 | Tem fezes duras ou dor durante a evacuação | Nunca | Uma vez por mês ou menos | Várias vezes por mês | Uma ou várias vezes por semana | (Quase) diariamente |
| 14 | Faz fezes muito volumosas (que podem entupir o vaso) | Nunca | Uma vez por mês ou menos | Várias vezes por mês | Uma ou várias vezes por semana | (Quase) diariamente |
| 15 | Adia as evacuações | Nunca | Uma vez por mês ou menos | Várias vezes por mês | Uma ou várias vezes por semana | (Quase) diariamente |
| 16 | Tem vontade súbita e incontrolável de defecar. | Nunca | Uma vez por mês ou menos | Várias vezes por mês | Uma ou várias vezes por semana | (Quase) diariamente |
| 17 | Tem dor na barriga. | Nunca | Uma vez por mês ou menos | Várias vezes por mês | Uma ou várias vezes por semana | (Quase) diariamente |
| 18 | Tem a barriga inchada. | Nunca | Uma vez por mês ou menos | Várias vezes por mês | Uma ou várias vezes por semana | (Quase) diariamente |
Para cada questão, selecione a resposta que melhor se aplica ao seu filho no último mês.
Se você não souber a resposta, por favor, pergunte ao seu filho (Ou complete o questionário junto com o seu filho).
Atenção às perguntas, nem todas tem as mesmas opções de respostas.
Meu filho….
The results of the present study show a high level of reliability in the translation of the CBBDQ, which can now be made available for use with children in Brazil. This recently developed questionnaire consisting of 18 questions, 10 on urinary symptoms and 8 on bowel symptoms, meets the psychometric criteria of viability, content and structural validity. The internal consistency of this instrument was found to be satisfactory when completed by parents of Dutch children of 5 to 12 years of age.6 The Dutch and English versions of the CBBDQ are already available for clinical use.
In general, there are several advantages in the use of questionnaires. First, they direct the collection of data on the patient’s clinical history through the use of pertinent questions validated for usage in the patient’s native language, then evaluating the symptoms in a clear and objective manner. Secondly, they allow a clearer distinction to be made regarding the intensity of symptoms. Third, they enable comparisons to be made between studies reporting the presence and intensity of symptoms. Fourth, they allow symptoms to be evaluated over time, monitoring different types of treatment. In the particular case of the CBBDQ, in addition to defining the severity of BBD, improvement in scores throughout treatment has a motivating effect on the children and their families.8,9
Farhat et al.5,10 developed the first symptom scoring system in this field of study, the Dysfunctional Voiding Scoring System (DVSS), an instrument with 10 questions on urinary symptoms and only 2 questions for the evaluation of constipation, which is insufficient for the assessment of BBD. The DVSS was translated into several different languages, including Brazilian Portuguese.11 Specificity was found to be good for voiding abnormalities in children and there was a correlation with the resolution of vesicoureteral reflux and with compliance to treatment.5 A Canadian questionnaire previously developed for children with bladder and bowel dysfunction, referred to at the time as dysfunctional elimination syndrome, proved to be a reliable tool, validated for the diagnosis of BBD in a sample of children in the English language.12
The two most important advantages of the CBBDQ over the DVSS are the following: (1) The CBBDQ is more comprehensive for the evaluation of bowel symptoms, thus enabling a more accurate diagnosis of BBD to be reached. It is generally known that instruments should be used to evaluate constipation and that constipation is characterized by the presence of symptoms that are not always present. For this reason, a more complete analysis with different questions is essential. The treatment of BBD should involve both dysfunctions. The application of questionnaires has been recommended not only to define the severity of the dysfunction, but also to monitor the outcome of treatment through the scores obtained.13 2) Understanding questions that involve temporality is often difficult and some criticism has been made of the DVSS with respect to the grading of answers as: almost never, less than half the time, about half the time and almost every time. With the CBBDQ, the frequency of symptoms, classified as monthly, weekly and daily tends to be more comprehensible. In view of these advantages, we believe this questionnaire will now be able to benefit patients who are native Portuguese speakers; hence the importance of the present study.
The answering options of the 5-point Likert scale of each item ranged from 0 (never) to 4 ([almost] daily). Therefore, the bladder subscale ranged from 0 to 40 and the bowel subscale from 0 to 32. The scores of the 2 subscales can be combined in 1 overall score for concomitant bladder and bowel symptoms (range 0–72).6 A cutoff point was not defined for diagnostic purposes, but a higher score defines the severity of the dysfunction, and the subsequent reduction during treatment allows for an evaluation of the outcome.
In conclusion, the translation and transcultural validation of the CBBDQ questionnaire was carried out successfully. Using the recommended methodology, including the evaluation of test-retest reliability with the participation of the families of children with LUTD, results were obtained that attested to the high reliability of the process. The availability of a version of the CBBDQ translated into Brazilian Portuguese provides a range of different professionals in Brazil with access to this tool. This may improve the quality of patient care, motivate children and their parents, and enable the results of studies conducted in different countries to be compared.
Conflicts of interestThe authors declare no conflicts of interest.
Study conducted at Clínica Nefrokids, Curitiba, PR, Brazil.