Obesity is a late effect in survivors of childhood cancer and correlates with chronic complications. Survivors of leukemia, brain tumors, and hematopoietic stem cell transplantation are more likely to develop obesity resulting from treatment modalities such as radiotherapy and glucocorticoids. This paper analyzes and integrates the current data available to health professionals in order to clarify strategies that can be used to treat and prevent obesity in childhood cancer survivors.
SourcesThis is a literature review from on scientifically reliable electronic databases. We selected articles published in the last five years and earlier articles of great scientific importance.
Data synthesisThe mechanisms involved in the pathophysiology of obesity in cancer survivors are not completely understood, but it is believed that damage to the hypothalamus and endocrine disorders such as insulin resistance, leptin resistance, and hormone deficiency may be involved. The body composition of this group includes a predominance of adipose tissue, especially in those undergoing hematopoietic stem cell transplant and total body irradiation. The use of body mass index in these patients may lead to an underestimation of individuals’ risk for metabolic complications.
ConclusionEarly identification of groups using accurate anthropometric assessments, interventional treatment, and/or preventative measures and counseling is essential to minimize the adverse effects of treatment. Physical activity and healthy eating to promote adequacy of weight in the whole population should be encouraged.
A obesidade é um efeito tardio em sobreviventes do câncer na infância e está correlacionada a complicações crônicas. Os sobreviventes da leucemia, tumores cerebrais e transplante de células-tronco hematopoiéticas têm maior probabilidade de desenvolver obesidade como resultado das modalidades de tratamento, como radioterapia e glicocorticoides. Este artigo analisa e integra os dados atuais disponíveis a profissionais da saúde para esclarecer as estratégias que podem ser utilizadas para tratar e prevenir a obesidade em sobreviventes do câncer na infância.
FontesEsta é uma análise da literatura de bases de dados eletrônicas cientificamente confiáveis. Selecionamos artigos publicados nos últimos cinco anos e artigos mais antigos de grande importância científica.
Resumo dos dadosOs mecanismos envolvidos na fisiopatologia da obesidade em sobreviventes do câncer não são completamente entendidos, porém acredita-se que o dano no hipotálamo e disfunções endócrinas, como resistência à insulina, resistência à leptina e deficiência hormonal, possam estar envolvidos. A composição corporal desse grupo inclui uma predominância de tecido adiposo, principalmente em pacientes submetidos a transplante de células-tronco hematopoiéticas e irradiação de todo o corpo. O uso do índice de massa corporal nesses pacientes pode subestimar indivíduos em risco de complicações metabólicas.
ConclusãoA identificação precoce de grupos por meio de avaliações antropométricas precisas, tratamento intervencional e/ou medidas preventivas e aconselhamento é fundamental para minimizar os efeitos colaterais do tratamento. A atividade física e alimentação saudável devem ser incentivadas para promover a adequação do peso na população em geral.
Obesity is an acknowledged late effect in childhood cancer survivors that is especially observed in certain groups, such as survivors of brain tumors or leukemia, since these cancers are associated with other chronic diseases including diabetes, hypertension, depression, cardiovascular disease, and dyslipidemia.1,2 Obesity in these groups of individuals is well described in the literature; among all survivors, cranial radiotherapy (CRT), chemotherapy, and steroids have been documented to contribute to an alteration in the body composition of patients already discharged from oncologic treatment.3
Endocrine and metabolic alterations have been reported as highly prevalent in cancer survivors who have received a hematopoietic stem cell transplant (HSCT). Contributing factors may include an intense or prolonged immunosuppressive treatment, post-transplant endocrine dysfunction, and insulin or leptin resistance. Although there are no studies suggesting HSCT as causative, prevention and early treatment of cardiovascular risks in these patients might diminish the incidence of late complications after the transplant.4
The early identification of high-risk groups, followed by a treatment plan based on metabolic and nutritional assessments that includes increased physical activity, is an essential component in the prevention of obesity.5 With this knowledge, it is important to study the causes of obesity in all survivors of childhood cancer, addressing not only causes well documented in the literature as influential, but also the seldom explored causal factors in this group. The authors also question the efficacy of body mass index (BMI) as an indicator to identify patients who are overweight, since these individuals experience changes in body composition, and therefore patients at risk for metabolic complications may be overlooked. Thus, the goal of this review is to promote discussion on the theme through data compilation and understanding possible causes, and to alert health professionals of the importance of this topic. Promotion of debate in the academic community is essential to encourage the implementation of future public health policies to prevent obesity in children and adolescents with cancer and in future survivors.
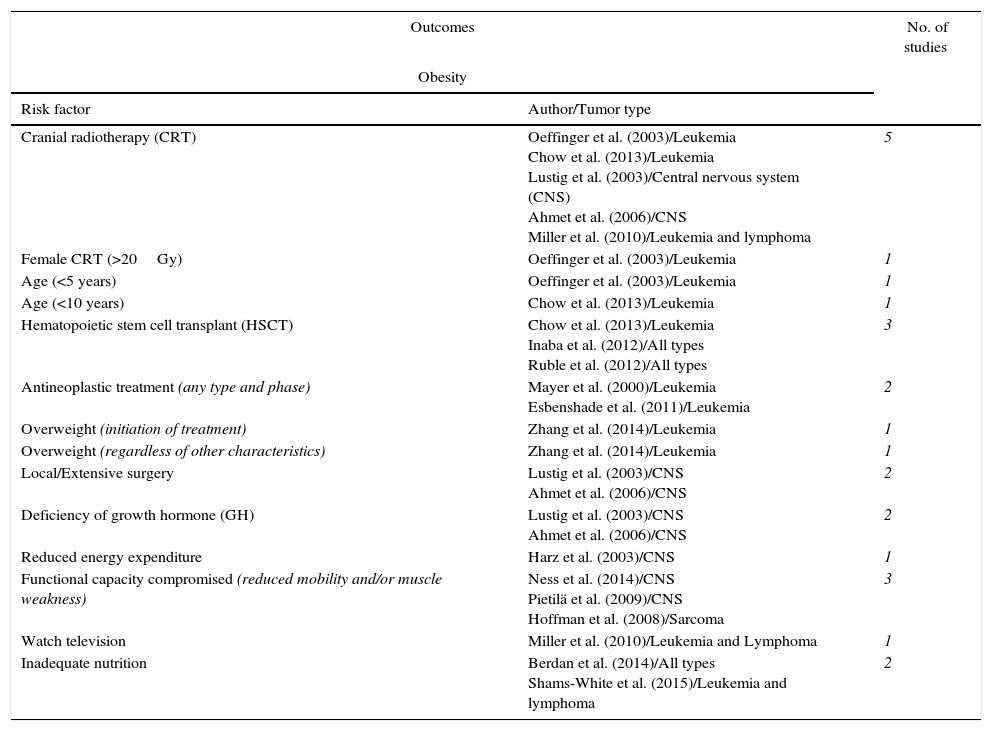
MethodsThis is a literature review from on scientifically reliable electronic databases and conducted in the second half of 2014 and first half 2015 using the following descriptive terms related to the topic: “Obesity”; “Survivor”; “Antineoplastic agents/adverse effects”; “Neoplasms”; “Childhood”. Articles published in the last five years and earlier articles of great scientific importance were selected, with exclusion of those that did not fit the proposed work, and were stratified according to study design, number of participants, type of tumor, aim of the study, and results obtained. Below is a summary of major studies that will be addressed and described in this review (Table 1).
Summary of studies on the main risk factors related to obesity and metabolic syndrome in childhood cancer survivors.
| Outcomes | No. of studies | |
|---|---|---|
| Obesity | ||
| Risk factor | Author/Tumor type | |
| Cranial radiotherapy (CRT) | Oeffinger et al. (2003)/Leukemia Chow et al. (2013)/Leukemia Lustig et al. (2003)/Central nervous system (CNS) Ahmet et al. (2006)/CNS Miller et al. (2010)/Leukemia and lymphoma | 5 |
| Female CRT (>20Gy) | Oeffinger et al. (2003)/Leukemia | 1 |
| Age (<5 years) | Oeffinger et al. (2003)/Leukemia | 1 |
| Age (<10 years) | Chow et al. (2013)/Leukemia | 1 |
| Hematopoietic stem cell transplant (HSCT) | Chow et al. (2013)/Leukemia Inaba et al. (2012)/All types Ruble et al. (2012)/All types | 3 |
| Antineoplastic treatment (any type and phase) | Mayer et al. (2000)/Leukemia Esbenshade et al. (2011)/Leukemia | 2 |
| Overweight (initiation of treatment) | Zhang et al. (2014)/Leukemia | 1 |
| Overweight (regardless of other characteristics) | Zhang et al. (2014)/Leukemia | 1 |
| Local/Extensive surgery | Lustig et al. (2003)/CNS Ahmet et al. (2006)/CNS | 2 |
| Deficiency of growth hormone (GH) | Lustig et al. (2003)/CNS Ahmet et al. (2006)/CNS | 2 |
| Reduced energy expenditure | Harz et al. (2003)/CNS | 1 |
| Functional capacity compromised (reduced mobility and/or muscle weakness) | Ness et al. (2014)/CNS Pietilä et al. (2009)/CNS Hoffman et al. (2008)/Sarcoma | 3 |
| Watch television | Miller et al. (2010)/Leukemia and Lymphoma | 1 |
| Inadequate nutrition | Berdan et al. (2014)/All types Shams-White et al. (2015)/Leukemia and lymphoma | 2 |
| Metabolic syndrome | No. of studies | |
|---|---|---|
| Risk factor | Author/Type of tumor | |
| HSCT (including total body irradiation [TBI]) | Van Waas M et al. (2010)/Leukemia and CNS Oudin et al. (2011)/Leukemia Taskinen et al. (2000)/All types Frisk et al. (2011)/All types Scott Baker et al. (2007)/All types | 5 |
n, number of studies evaluated.
It was decided to divide the discussion topics according to the tumor and treatment because they are the main types described in the literature correlated with obesity.
Results and discussionAcute lymphoblastic leukemia (ALL)Acute lymphoblastic leukemia (ALL) is the most common malignant childhood cancer and it has been considered an important model for the study of metabolic disorders and their correlation with cardiovascular events, such as strokes and chronic health conditions among survivors of ALL.6
Excessive weight gain occurring during treatment for ALL is usually related to the use of steroids, appetite regulation disorders, radiotherapy to the central nervous system (CNS), and reduced energy expenditure due to physical inactivity.3 Post-treatment weight gain conditions have also been associated with CRT, and women are more affected than men according to an analysis of 1765 adult survivors of childhood cancer enrolled in the Childhood Cancer Survivor Study (CCSS). Among these, female survivors treated with 20Gy had a two-to-three times greater probability of being obese compared to their male siblings. Those treated before the age of five years old were almost four times more likely to be obese than their siblings. In addition, boys treated with 20Gy had an almost two times increased chance of being obese compared to their male siblings.7,8
In agreement with this observed influence of radiotherapy on weight, Mayer et al. report that the prevalence of obesity was significantly higher after ALL therapy when the factors of ALL post-therapy obesity were evaluated during a transversal study with 39 survivors of the disease. In comparison with non-irradiated patients, those who had undergone therapy had highly reduced basal metabolic rates (BMR) as well as a reduction in their physical activity levels, and lower concentrations of insulin-like growth factor binding protein 3 and free thyroxin.9
In its analysis of the differential effects of CRT, spinal radiotherapy, and total body irradiation (TBI) on growth and endocrine function in 3467 ALL survivors, the CCSS found that patients treated with TBI were significantly more likely to report themselves as underweight than those who had not received any dose of radiotherapy. It was also found that those treated with CRT were significantly more likely to be overweight or obese. The study showed that age <10 years at diagnosis and HSCT are independent characteristics associated with BMI alterations and an increased risk of being overweight or obese. Other findings from the study include the following: supplementation with growth hormone (GH) was associated with being underweight, and female survivors, contrary to previous studies, were less likely to be overweight or obese than male survivors. The authors concluded that patients treated with any of the aforementioned therapies had an increased risk of alterations in body composition.10
Despite ample evidence for the association between obesity and RT, studies have also shown that patients present a risk of becoming overweight during all phases of treatment, regardless of the treatment modality. When the BMI of 183 ALL patients was evaluated during all phases of treatment (diagnosis, induction, consolidation, and maintenance), it has been observed that, at diagnosis, 36% of the patients were overweight and 19% were obese. The median BMI z-score increased during the induction phase and then later returned to base line; however, it increased again throughout the first 22 months of maintenance. By the end of the treatment, 49% of the patients were overweight and 21% were obese. The increase in the BMI z-score during the diagnosis was associated with the z-score increase during the maintenance phase. The study concluded that patients are at risk of an elevation in BMI at all phases of treatment, which places them at greater risk for adverse health conditions in their future.11
Another similar study examined the BMI of 83 pediatric survivors of ALL and observed that weight at diagnosis and BMI z-score were two important predictors of overweight and obesity at the end of the treatment. Patients who were overweight or obese at diagnosis were 11.9 times more likely to be overweight or obese by the end of their treatment than those who were underweight or normal weight at diagnosis. An increase of one unit in the BMI z-score at diagnosis was associated with a tripling in the risk of being overweight or obese by the end of the treatment. The study finally concludes that ALL survivors are at risk of becoming overweight at the beginning of treatment and that their weight increase is maintained throughout treatment, making early intervention necessary.12 Reaffirming these findings, the same author found in a meta-analysis of ALL survivors that obesity is prevalent in this group and is independent of patient characteristics such as sex or age at diagnosis, and treatments, such as cranial irradiation. Thus, health professionals may need to pay special attention to these survivors.13
Besides obesity, metabolic syndrome (MetS) is also experienced by childhood cancer survivors as a consequence of anti-neoplastic treatments. MetS can be defined as a group of interrelated risk factors of metabolic origin that contribute directly to the development of cardiovascular disease (CVD) and/or diabetes mellitus type 2. Well-known risk factors for MetS include atherogenic dyslipidemia, high blood pressure, and high plasmatic glucose levels. Individuals with these characteristics commonly present with a pro-thrombosis and pro-inflammatory state. Atherogenic dyslipidemia comprises a series of alterations in levels of lipoproteins, including a rise in triacylglycerol, apolipoprotein B (apo B), and low-density lipoprotein (LDL), and a decrease in high-density lipoprotein (HDL). Abdominal obesity, insulin resistance (IR), physical inactivity, aging, and hormonal imbalance can promote metabolic risk factors.14
Childhood cancer survivors, especially those treated with cranial irradiation, face a higher risk of developing MetS as adults,15 particularly those who had ALL and CNS cancers and presented with risk factors such as visceral adiposity, dyslipidemia, IR, and hypertension.16,17 The authors of one study determined that the prevalence of MetS in French ALL survivors was 9.2%, which was double the rate in the general population of French young adults. There was no association found between the prevalence of MetS and gender, age at diagnosis, leukemia subtype, or treatment with steroids or CNS radiation.18
Endocrine disorders observed in survivors who received an HSCT are multifactorial and are correlated to the age of the patient, base disease, and type of transplant (autologous or allogeneic). The HSCT conditioning regimen that uses high doses of chemotherapy agents alone or in combination with radiotherapy, which is intended to eliminate active and residual malignant cells, may be a contributing factor in the development of endocrine disorders. Another contributing factor could be administration of immunosuppressive agents, which can produce either early or late hematopoietic toxicity, as prophylaxis against graft-versus-host disease (GVHD).19,20
Central nervous systemHormonal deficiencies and obesity are common complications after treatment of CNS tumors. Currently, the main risk factors that seem to predict the development of obesity in survivors of CNS tumors are age at diagnosis, irradiation of the hypothalamus greater than 51Gy, location of the tumor, extensiveness of the surgery, histology of the tumor, and the presence of endocrine diseases, particularly GH deficiency.21,22
Craniopharyngiomas are rare tumors accounting for 2%–5% of CNS tumors and originate from remnants of the squamous epithelium of Rathke's pouch.23 Survivors of craniopharyngioma have a substantially increased risk of extreme obesity due to the location of their brain tumor and the hypothalamic damage resulting from surgical resection. These individuals experience a state of hypothalamic obesity, defined as any damage to the energy control centers of the hypothalamus, which is responsible for the regulation of body weight through balancing food ingestion, energy expenditure, and amount of adipose tissue.24 These lesions, which may be present on the ventromedial hypothalamus, paraventricular nucleus, arcuate nucleus, and lateral hypothalamus, can result from surgical procedures, anti-neoplastic treatments, or the tumor itself. The damage may be structural, functional, or genetic in nature, and ultimately compromises hypothalamic functions, leading to a state of severe obesity.25
The resulting morbidity from radical tumor resection performed to reduce the chances of relapse is explained by the intimate anatomic relation of craniopharyngiomas with the neurohypophysis and particularly with the hypothalamus. Hypothalamic-hypophysis disorder — characterized by pan-hypopituitarism, obesity, hyperphagia, obsession with the pursuit of food, and neuropsychological disorders — dramatically affects the quality of life of affected children and their families.26
Although the dose and the duration of treatment with peri-surgical dexamethasone has a short-term influence on post-surgical weight gain of craniopharyngioma patients, it cannot be said to affect long-term morbid obesity. Other factors can be correlated to the weight gain in this group, as observed by Harz and collaborators, who found that their obese craniopharyngioma patients had nearly normal caloric ingestion but reduced energy expenditure in comparison to the control group with a similar BMI. The authors hypothesize that this phenomenon may be related to neurological and visual deficits, as well as to an uncontrolled production of melatonin leading to an increase in daytime sleepiness.27
Treatments have been proposed to minimize the hypothalamic obesity present in this group of patients. In a double-blind randomized controlled study using octreotide therapy for pediatric hypothalamic obesity, it was observed that, analogous to somatostatin, it resulted in stabilization of weight and BMI. Furthermore, the placebo treatment did not result in any alteration in weight gain index, suggesting beneficial effects of octreotide in this group.28 Another study evaluated the effect of combined therapy with diazoxide-metformin for hypothalamic obesity, and observed a reduction in BMI and area under the insulin curve, which is a predictor of the efficacy of the treatment.29
Besides medicinal therapies, gastric bypass has been proposed as an efficient and safe method to treat hypothalamic obesity, as it has been described in a case study of an adult man who had undergone craniopharyngioma resection at the age of eight. Eighteen months after the bypass there was a reduction in BMI from 52kg/m to 31.9kg/m, as well as full remission of type 2 diabetes and express improvement of his apnea.30
Other CNS tumor types can also affect metabolism in an important way. Heikens et al. investigated risk factors for CDV in a mixed group of long-term survivors of CNS tumors and described an altered risk profile for this group of patients due to dyslipidemia, central obesity, and elevated systolic blood pressure, especially for those suffering from a GH deficiency.31 Additional mechanisms contribute to excess weight in this group, including reduced mobility, muscle weakness, and reduced tolerance for physical activity, compromising their ability to fully participate in a normal daily routine.32,33
Other types of cancerExcess weight has not only been demonstrated in survivors of ALL or CNS tumors. Other studies exploring isolated groups of tumor types, in addition to grouped studies on survivors of childhood cancer, have shown changes in the BMI patterns of survivors.
When analyzing survivors of childhood sarcoma — predominantly Ewing sarcoma — in the United States, survivors were more likely to have two or more criteria for MetS when compared with their controls. An analysis of individual MetS criteria revealed a greater prevalence of hypertension and hypertriglyceridemia. Regarding nutritional status based on the BMI, 22% of survivors were obese and 28% were overweight. Both male and female survivors had increased total body fat as measured by dual-energy X-ray absorptiometry (DEXA). Other parameters, such as testosterone levels and physical activity, were also assessed, revealing that among male survivors, total testosterone was reduced. In addition, free testosterone and mean activity z-scores had a strong tendency to decline as the number of MetS indicators increased. The authors concluded that this group of patients had an increase in the prevalence of MetS, especially among those younger than 40 years of age. The development of MetS in this population was associated with reduced levels of testosterone and functional capacity.34
Both changes in body composition of survivors and issues related to the use of BMI have been described. A study of 170 ALL and lymphoma survivors sought to compare body fat indicators and metabolic factors associated with body fat, and to determine the risk of adiposity in survivors of childhood cancer who had received a diverse range of treatments. The authors concluded that total body and abdominal fat are higher in male survivors of pediatric cancer than in sibling controls, and that BMI is not a sensitive indicator of adiposity. For both male and female survivors, CRT and the habit of watching television were associated with adiposity.35
Cancer treatments may have an impact on the hypothalamic-pituitary brain region, causing long-term changes in consumption and binge eating, as suggested by authors using a modified version of the Food Craving Inventory in 22 survivors of pediatric leukemia and lymphoma. Compared to survivors diagnosed at a younger age (<4.5 years), those diagnosed at an older age had significantly greater total cravings and cravings for fast-foods, sweets, carbohydrates, and fat; therefore, age at diagnosis may potentially affect food cravings in childhood cancer survivors.36
Healthier habits, such as adequate nutrition, a modifiable and preventive factor of chronic diseases, have not been demonstrated in survivors, as pointed out in a study by Berdan and collaborators in 2014 that aimed to assess the adherence of childhood cancer survivors to the guidelines of the American Cancer Society (ACS) in comparison with a control group. Only 35.8% of the surviving patients fell within a healthy BMI range, while 2.9% were underweight, 28.9% were overweight, and 32.4% were classified as obese. Hispanic survivors had a higher BMI than Caucasians. In regards to food consumption, only 4.8% of survivors fully adhered to the recommendations of the ACS. Only 10.2% complied with the recommended intake of dietary fiber and 17.7% consumed five fruits and vegetables per day, while 46.2% met the recommendation for processed red meat.37
Hematopoietic stem-cell transplant (HSCT)HSCT, including autologous and allogeneic stem cell transplants from bone marrow, peripheral blood, and umbilical cord blood, has increased considerably in recent years. HSCT is applied mainly in the treatment of leukemia and solid tumors, but it is also effective in the treatment of hematological, immunological, and genetic disorders, and in the cure of non-hematological metabolic diseases. Advances in transplant technology and treatment support practices have led to progressive improvements in the survival of HSCT recipients; however, due to patients living longer after the transplant, the risk of developing late complications related to pre-, peri-, and post-transplant exposures has increased. These complications may cause substantial morbidity, worsen patients’ quality of life, and contribute to late mortality in these individuals. The most common adverse effects, which generally result from pre-transplant conditioning regimes, include growth and thyroid disorders, metabolic changes, gonadal insufficiency, and osteoporosis.4,38
When the frequency of late effects on glucose and lipid metabolism after childhood transplantation was evaluated, 52% of the patients with transplants were observed to have IR. The main indicators of MetS (combined hyperinsulinemia and hypertriglyceridemia) were found in 39% of transplant patients, compared to 8% in a control group of ALL survivors treated only with chemotherapy (no HSCT) and 0% in healthy controls. The frequency of IR increased with time after the HSCT was performed. Abdominal obesity was common among patients with IR. The authors concluded that long-term survivors who have undergone HSCT are at substantial risk of IR, glucose intolerance, and type 2 diabetes mellitus, even with normal weight and at a young age.39 The authors suggest that the decrease in insulin sensitivity can be explained by adverse body composition after HSCT, as assessed in a cross-sectional study of 18 survivors of blood cancer (leukemia and lymphoma) in childhood who underwent transplantation and completed 10 years without treatment. Using DEXA, it was shown that fat mass was significantly higher and lean body mass significantly lower in the survivors than in control patients, and these parameters correlated inversely with insulin sensitivity. The median BMI of the survivors was 21.6kg/m2. BMI tended to be lower in the survivor group due to reduced lean body mass, but their higher percentages of fat mass were associated with a decrease in insulin sensitivity.40
In addition to the HSCT itself, TBI as a preparative therapy for HSCT has been described as playing an essential role in the genesis of IR, as was pointed out by a large multicenter study that included adult and pediatric survivors of transplantation. The risk of developing type 2 diabetes mellitus was three times higher in patients who underwent HSCT, and this risk was associated with TBI.41
Although endocrine complications in patients who have received a HSCT have been well-described in the literature, the causes of excess weight in this group have remained controversial. Such controversy possibly exists due to the compromised specificity of using BMI as a marker of MetS in childhood cancer survivors, especially in those who have undergone transplantation. One study measured longitudinal weight and body composition changes in survivors of malignant hematologic diseases in childhood and observed a significant decrease in BMI in survivors after HSCT, mainly due to a reduction in lean body mass. According to the authors, the findings of the study may be attributable to TBI and/or the degree of chronic GHVD. Furthermore, they suggest that health professionals should be alert to losses in not only BMI, but also in lean mass in these survivors in order to ensure early and appropriate intervention based on nutritional education and physical activity.42
Corroborating these findings, Rubles and collaborators observed that 54% of the survivors had body fat percentages (measured by DEXA) that exceeded the recommendations for a healthy body composition, and 31% qualified as having central obesity. Previous treatment with TBI was associated with a greater percentage of body fat and central obesity, and GHVD was associated with a lower percentage of body fat. The BMI criteria did not correctly identify the HSCT survivors with a high body fat percentage. The study concluded that survivors who received a HSCT in childhood are at risk of obesity and central obesity, which is not easily identified by the standard BMI criteria.43
ConclusionSurvivors of ALL and CNS tumors are more prone to developing obesity due to the employed treatment modalities, but antineoplastic treatments influence the appearance of excess weight in all survivors of childhood cancer, regardless of tumor type. As in the general population, other factors contribute to weight gain in survivors, given that obesity is a multi-factorial disease; these factors include poor nutrition, physical inactivity, and individual genetic characteristics. In addition, the administration of HSCT and TBI affect body composition by increasing adiposity, reducing lean body mass, and increasing metabolic complications. Clearly there is need for assistance in this group. Strategies for early identification of high-risk patients and development of interventional models, including nutritional counseling, should be offered by health professionals in order to minimize and/or prevent this chronic complication of antineoplastic therapy.
Conflicts of interestThe authors declare no conflicts of interest.
This study was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES. The role of the funding source is to encourage education and research development in Brazil.
Please cite this article as: Teixeira JF, Maia-Lemos PS, Cypriano MS, Pisani LP. The influence of antineoplastic treatment on the weight of survivors of childhood cancer. J Pediatr (Rio J). 2016;92:559–66.