There is evidence of an important role of immune system changes in the triggering and maintenance of idiopathic nephrotic syndrome (INS). The aim of this study was to investigate the expression of cytokines in lymphocyte populations of patients with INS in comparison to healthy individuals, according to proteinuria.
MethodsThis cross-sectional study included 44 patients with INS and eight healthy children, matched for age and sex (controls). Patients were subdivided according to proteinuria: persistent proteinuria or partial remission (PP≥300mg/24h, n=17) and low proteinuria or complete remission (LP<300mg/24h, n=27). Ex vivo analysis of peripheral blood leukocytes by flow cytometry was performed using surface markers for T-lymphocytes, TCD4, TCD8, natural killer (NK) cells, NKT, and B-lymphocytes. Frequencies of intracellular cytokines were analyzed in these cells.
ResultsThe frequencies of B-lymphocytes, NK cells, and NKT cells were lower in INS than in controls, whereas INS patients had a higher frequency of CD4+tumor necrosis factor (TNF)-α+ cells than controls. Cytotoxic-T-lymphocytes expressing IFN-γ were lower in INS than in controls. Patients with PP showed higher frequencies of CD4-T-lymphocytes expressing IFN-γ and TNF-α than controls. CD8-lymphocytes expressing TNF-α were increased in PP group when compared with LP and controls, while CD8+interferon (IFN)-γ+ cells were lower than in LP and in controls.
ConclusionRegardless the level of proteinuria, INS patients had increased expression of TNF-α in CD4-lymphocytes and reduced expression of IFN-γ in CD8-lymphocytes. Persistence of proteinuria was associated with higher levels of inflammatory markers.
Há comprovação do importante papel das alterações no sistema imunológico no desencadeamento e manutenção da síndrome nefrótica idiopática (SNI). O objetivo deste estudo foi investigar a expressão das citocinas em populações de linfócitos de pacientes com SNI em comparação a indivíduos saudáveis e de acordo com a proteinúria.
MétodosEste estudo transversal incluiu 44 pacientes com SNI e oito crianças saudáveis, pareados por idade e sexo (controles). Os pacientes foram subdivididos de acordo com a proteinúria: proteinúria persistente ou remissão parcial (PP≥300mg/24h, n=17) e proteinúria baixa ou remissão completa (PB<300mg/24h, n=27). A análise ex vivo de leucócitos no sangue periférico por citometria de fluxo foi feita utilizando marcadores de superfície para linfócitos T, TCD4, TCD8, células natural killer (NK), linfócitos NKT e B. As frequências das citocinas intracelulares foram analisadas nessas células.
ResultadosA frequência dos linfócitos B, células NK e células NKT foi menor em pacientes com SNI do que nos controles, ao passo que os pacientes com SNI apresentaram maior frequência de células CD4+fator de necrose tumoral (TNF)-α+ do que nos controles. Os linfócitos T citotóxicos que expressam interferon (IFN)-γ foram menores nos pacientes com SNI do que nos controles. Os pacientes com PP mostraram maiores frequências de linfócitos T CD4 que expressam IFN-γ e TNF-α que os controles. Os linfócitos CD8 que expressam TNF-α apresentaram aumento no grupo com PP, em comparação aos com PB e os controles, apesar de as células CD8+IFN-γ+ serem mais baixas nos pacientes com PB e nos controles.
ConclusãoCom relação ao nível de proteinúria, os pacientes com SNI apresentaram aumento na expressão de TNF-α nos linfócitos CD4 e expressão reduzida de IFN-γ nos linfócitos CD8. A persistência da proteinúria foi associada a maiores níveis de marcadores inflamatórios.
Nephrotic syndrome (NS) is a very common glomerulopathy in children, characterized by massive proteinuria, hypoalbuminemia, generalized edema, and hyperlipidemia. NS can be caused by primary renal lesion, referred as idiopathic nephrotic syndrome (INS), or be related with systemic illnesses.1
There is evidence of an important role of immune system changes in the triggering and maintenance of INS.2–5 These changes include abnormal T-lymphocyte response in patients with INS,2 a possible contribution of cytokines in INS physiopathology,3 the possibility of a circulating permeability factor that has been related with the recurrence of the illness after renal transplant,3 and the clinical improvement of patients after treatment with corticosteroids and immunosuppressive medications.4 However, apart from the advances on INS research over the past decades, especially on the immunology field, its physiopathology remains to be fully addressed.5
Several immunological alterations have been detected in patients with INS.5 There are reports of altered expression of cytokines/chemokines, including interleukin-2 (IL-2), IL-10, IL-4, and IL-8, as well as changes in TCD8+ and in TCD4+ cells of INS patients.2,6–10 IL-2 and tumor necrosis factor alpha (TNF-α) are considered possible pathogenic factors underlying the mechanisms of renal lesion in INS.11,12 For instance, the infusion of TNF-α in mice with NS caused a dose-dependent increase of proteinuria in parallel with impairment of clinical state.13 Importantly, the increase of plasma levels of TNF-α in INS patients associated with increased plasma levels of IL-2, its soluble receptor (sIL-2R), and IFN-γ during NS relapses in steroid-sensitive patients suggests a pro-inflammatory or T-helper 1 (Th1) immune response pattern.14 However, an imbalance between Th1 and Th2 immune response patterns has been reported in INS, with a tendency to shift to the Th2 profile.3,15
Based on the concept that INS and its progression might be associated with immune response unbalance, this study investigated the expression of pro- and anti-inflammatory cytokines in different leukocyte subpopulations from the peripheral blood of INS patients. The study also evaluated whether these changes in immune cells profile were related to the persistence of proteinuria.
Patients and methodsStudy designThe present cross-sectional study used a convenience sample of 44 pediatric patients with INS and eight healthy sex- and age-matched children as a control group. INS patients were regularly followed-up at the Pediatric Nephrology Unit of this institution from 2014 to 2016. Diagnostic criteria for INS were based on the KDIGO Clinical Practice Guidelines for Glomerulonephritis.16 The Pediatric Nephrology Unit was established in 1980 and has followed-up approximately 300 children with nephrotic syndrome, according to a systematic protocol that includes definition of disease etiology, assessment of clinical course and laboratory alterations, institution of treatment, and indication of renal biopsy based on clinical (corticosteroid unresponsiveness) and laboratory findings.
Patients with INSInclusion criteria included children and adolescents with well-established INS and still-preserved renal function, followed-up from 2014 to 2016, whose parents gave their consent to participate in the study protocol. Children and adolescents with congenital or secondary forms of nephrotic syndrome and INS patients at stages 2–5 of chronic kidney disease were automatically excluded from the study.
Control groupThe control group consisted of healthy sex- and age-matched subjects from the Pediatric Primary Care Center. Healthy status was determined through the subjects’ medical history and either a parental report or self-report to rule out the presence of chronic or acute diseases.
Ethical aspectsThe Ethics Committee of the institution approved the study. Informed consent was obtained from parents of all included subjects. The research protocol did not interfere with any medical recommendations or prescriptions. Blood samples in control group were only drawn simultaneously with other routine blood exams. The follow-up of the INS patients and healthy controls was guaranteed even in cases of refusal to participate in the study.
Study protocolBased on the levels of proteinuria at the moment of blood sampling, the patients with INS were subdivided into two groups. Patients with 24-h urine protein excretion equal or superior to 300mg/24h were considered in partial remission and allocated to the group named persistent proteinuria (PP, n=17), and those with proteinuria lower than 300mg/24h were in complete remission and classified as low proteinuria (LP, n=27). This cut-off point for proteinuria was based on the KDIGO Clinical Practice Guidelines for Glomerulonephritis.16
Clinical characteristics and casual measurements were obtained at the same time as blood and 24-h urine collection. The clinical variables analyzed were age, gender, height, weight, body mass index, and systolic and diastolic blood pressure. In INS patients, serum levels of urea, creatinine, albumin, cholesterol, triglycerides, and uric acid were assessed using the same blood sample obtained for the measurements of immune system cells. Urinary determination of 24-h protein excretion was also performed simultaneously to blood measurements. GFR was estimated using the conventional Schwartz et al.17 formula in INS patients and in healthy controls. Renal biopsy results and medications used at the time of blood sampling were also considered (Table 1).
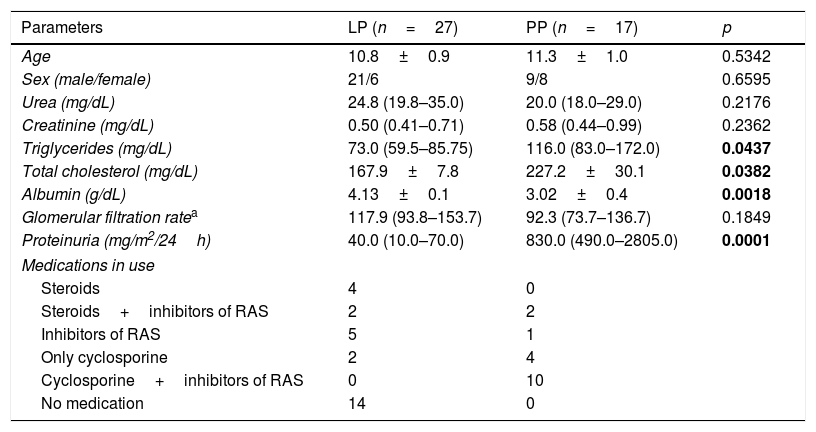
Clinical and laboratory features of patients with idiopathic nephrotic syndrome divided in two groups, according to 24-h urine protein excretion: persistent proteinuria (PP) when proteinuria≥300mg/24h and low proteinuria (LP) when proteinuria<300mg/24h.
| Parameters | LP (n=27) | PP (n=17) | p |
|---|---|---|---|
| Age | 10.8±0.9 | 11.3±1.0 | 0.5342 |
| Sex (male/female) | 21/6 | 9/8 | 0.6595 |
| Urea (mg/dL) | 24.8 (19.8–35.0) | 20.0 (18.0–29.0) | 0.2176 |
| Creatinine (mg/dL) | 0.50 (0.41–0.71) | 0.58 (0.44–0.99) | 0.2362 |
| Triglycerides (mg/dL) | 73.0 (59.5–85.75) | 116.0 (83.0–172.0) | 0.0437 |
| Total cholesterol (mg/dL) | 167.9±7.8 | 227.2±30.1 | 0.0382 |
| Albumin (g/dL) | 4.13±0.1 | 3.02±0.4 | 0.0018 |
| Glomerular filtration ratea | 117.9 (93.8–153.7) | 92.3 (73.7–136.7) | 0.1849 |
| Proteinuria (mg/m2/24h) | 40.0 (10.0–70.0) | 830.0 (490.0–2805.0) | 0.0001 |
| Medications in use | |||
| Steroids | 4 | 0 | |
| Steroids+inhibitors of RAS | 2 | 2 | |
| Inhibitors of RAS | 5 | 1 | |
| Only cyclosporine | 2 | 4 | |
| Cyclosporine+inhibitors of RAS | 0 | 10 | |
| No medication | 14 | 0 | |
RAS, renin angiotensin system.
Values are expressed as mean standard error of mean or median and interquartile ranges according to variable distribution. Sex and medications in use are expressed as absolute values.
Bold values of P are for p<0.05.
Blood samples were collected into sterile tubes containing EDTA and heparin. Blood samples collected in tubes with EDTA were used immediately for ex vivo analysis of populations and subpopulations of leukocytes by flow cytometry.
Blood samples collected in tubes containing heparin were immediately used for cell culture and subsequent analysis of intracellular cytokines.
Peripheral blood leukocyte countBlood leukocytes were counted using a CELM CC-550 cell counter (CELM – Barueri, SP, Brazil). Differential white blood cell counts were performed in blood smears using an optical microscope (Olympus-BX41 TF, Japan) after May–Grünwald–Giemsa staining.
Cell surface staining and flow cytometry analysisPeripheral blood samples (50μL/sample) were incubated with monoclonal antibodies for the following surface markers: anti-CD3, anti-CD4, anti-CD8 (Becton & Dickinson – San Jose, CA, USA), anti-CD80, and anti-CD18 (Caltag-Medsystems Limited – Buckingham, UK) conjugated with fluorescein phycoerythrin (PE), isothiocyanate (FITC), or biotin in the dark for 30min at room temperature. After incubation, the erythrocytes were lysed with Optilyse-B solution (Immunotec – USA). The cells were washed twice in 1mL of cold phosphate buffered saline (PBS, pH 7.4). The biotinylated antibodies were revealed using streptavidin–FITC (Becton & Dickinson – San Jose, CA, USA). The following antibodies against human proteins were used: anti-CD3 FITC (clone: UCHT1) and anti-CD8 FITC (clone: HIT8a) (Bio-Legend, CA, USA); anti-CD4 FITC (clone: RPA-T4) and anti-CD19 FITC (clone: HIB19) from BD Pharmigen; anti-CD56 PE (clone: B159), anti-CD19 PE (clone: HIB19), anti-CD4 PE (clone: L120), and anti-CD8 PE (clone: HIT8a) provided by BD Biosciences (Becton & Dickinson – San Diego, CA, USA). Data acquisition was performed using FACScan (Becton & Dickinson – San Diego, CA, USA). Cell acquisition was processed and analyzed using the Cell Quest software (Becton & Dickinson – San Jose, CA, USA). Total T lymphocytes (CD3+), T helper lymphocytes (CD3+CD4+), cytotoxic T lymphocytes (CD3+CD8+), B lymphocytes (CD3+CD19+), cells with phenotype of natural killer (NK, CD3−CD56+), and cells with phenotype of T natural killer (NKT, CD3+CD56+) were analyzed using fluorescence dot plots after the selection of the cell population of interest based on cell size and granulosity (SSC vs. FSC graph). These cells were then analyzed for their expression (frequency and mean fluorescent intensity [MFI]) of a given marker using histograms with markers set based on negative isotype controls.
Intracellular cytokine analysis in leukocytesTo perform the cytokine profile analysis in different populations of leukocytes, 500μL of peripheral blood was added to 500μL of RPMI-1640 (Sigma–Aldrich®, USA) culture medium in two separate tubes. The first tube had non-stimulated culture called control culture and the second tube contained culture nonspecifically stimulated with phorbol myristate acetate (PMA) at 25ng/mL and 1.0μL of ionomycin at 1ng/mL. In both tubes, 10μL of Brefeldin-A at 1mg/mL were added in order to keep cytokines in the intracellular compartment. The samples were then incubated for 4h at 37°C in a humidified incubator containing 5% CO2. After incubation, EDTA was added (20nM) followed by re-incubation for 15min. The samples were washed with PBS (0.015M PBS, pH 7.4, with 0.5% bovine serum albumin and 0.1% sodium azide) and incubated for 30min, hidden from light, at room temperature, with specific monoclonal antibodies to cell surface markers: FITC-anti-CD4, FITC anti-CD8, and FITC anti-CD19.
After this step, the erythrocytes were lysed with 2mL of Optilyse-B solution (Immunotec – United States) for 10min. Then, samples were centrifuged at room temperature and subsequently leukocytes were permeabilized by PBS solution (0.015M PBS, pH 7.4, containing 0.5% serum albumin bovine, 0.1% sodium azide, and 0.5% saponin). After permeabilization, these cells were incubated for 30min at room temperature in the dark, with specific monoclonal antibodies to cytokines: TNF-α anti-PE (clone: MAb11), anti-IL10 PE (clone: JES3-9D7), anti-IFN-γ PE (clone: 4S.B3), and anti-IL13 PE (clone: JES10-5A2) (BioLegend, CA, USA); anti-IL17 PE (clone: eBio64DEC17) from Biosciences; anti-IL6 PE (clone: 1936) (R&D Systems, MN, USA), and anti-IL4 PE (clone: 8D4-8 BD) (BD Pharmingen, CA, USA). The samples were evaluated for cell phenotype and cytokine production parameters by the acquisition of 30,000 events. Analyses were performed using the Cell Quest software (Becton & Dickinson – San Jose, CA, USA). Cytokine profile was expressed as the difference (delta) between the percentage of cells stained for intracellular cytokines obtained in non-stimulated cultures and cultures stimulated with PMA.
Data analysis and statistical evaluationResults obtained were presented as mean±standard error of the mean (SEM) or median and interquartile ranges. All data were tested for normality by the Shapiro–Wilk test. For normally distributed variables, differences were compared by unpaired Student's t-test or analysis of variance (ANOVA). Bonferroni's post-test was used for multiple comparisons. In case of variables with non-Gaussian distribution, differences were analyzed by the Mann–Whitney U-test or the Kruskal–Wallis non-parametric test. All statistical tests were two tailed and were performed using a significance level of α=0.05. Statistical analyses were performed using SPSS software (IBM SPSS Statistics for Windows, version 22.0. NY, USA) and GraphPad Prism version 5.0 (GraphPad Software, Inc., La Jolla, CA, USA).
ResultsSubjects’ characteristics and casual measurementsThe control group (n=8) included five boys and three girls ranging in age from 6.1 to 13.5 years. The mean values of weight, height, body mass index, systolic and diastolic pressures, and renal function parameters were within normal range (data not shown).
INS patients were divided according to the level of proteinuria at the time of blood sampling (PP or LP). The clinical and laboratorial features of each subgroup at the time of blood sampling are shown in Table 1. No differences were detected in age, sex distribution, and nitrogen waste levels (urea and creatinine). However, the PP group had increased serum levels of cholesterol and triglycerides and significant reduction of serum albumin when compared with LP group (p<0.05). As expected, the PP group had significantly higher proteinuria, and 15 among 17 patients in the PP group (88.2%) exhibited values of proteinuria above 600mg/24h. As previously mentioned, the disease was in remission in all patients of LP group. However, all patients of the PP group had relapsed (88.2%) or partially remitted disease (11.8%), since the levels proteinuria were always above 300mg/24h in all of them.
In regard to treatment, 14 of 27 patients (51.8%) of the LP group were not receiving any medication, whereas in PP groups all patients needed at least one medication as an attempt to control proteinuria. In addition, 25 of 27 patients of the LP group (88.2%) were steroid-sensitive. Only two patients with LP were treated with cyclosporine due to steroid dependence. In sharp contrast, 14 of 17 patients (82.3%) in the PP group were receiving cyclosporine, isolated or associated with inhibitors of the renin angiotensin system (RAS), due to steroid resistance. Only three patients of the PP group exhibited partial response to steroid administration. As expected, all patients of the PP group were submitted to renal biopsies, which showed focal segmental sclerosis in 13 patients (76.5%) and diffuse mesangial proliferation in the remaining four (23.5%). In the LP group, only two patients were biopsied due to steroid dependence; the histologic pattern evidenced minimal-change nephrotic syndrome in one and diffuse mesangial proliferation in the other.
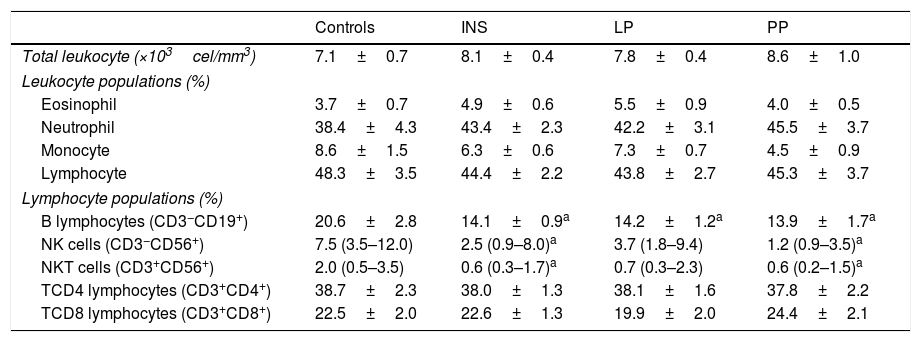
Peripheral blood leukocyte countAs shown in Table 2, no significant differences were found in the total white blood cell counts or in the specific leukocyte populations (monocytes, neutrophils, and lymphocytes) of INS patients when compared with control group. In addition, no differences were detected in the comparison between PP and LP groups. Similar results were obtained for the comparisons between the percentages of T-CD4+ and T-CD8+ lymphocytes in these groups (Table 2). On the other hand, patients with INS had lower frequencies of B-lymphocytes, NK cells, and NKT cells when compared to controls. In addition, INS patients with PP presented lower frequencies of B lymphocyte NK and NKT cells in comparison to the control group, whereas in patients with LP only B-lymphocytes were significantly reduced in comparison to controls (Table 2).
Populations and subpopulations of leucocytes in healthy subjects (controls), in the entire group of patients with idiopathic nephrotic syndrome (INS) and in INS patients divided in two groups according to 24-h urine protein excretion: persistent proteinuria (PP) when proteinuria≥300mg/24h and low proteinuria (LP) when proteinuria<300mg/24h.
| Controls | INS | LP | PP | |
|---|---|---|---|---|
| Total leukocyte (×103cel/mm3) | 7.1±0.7 | 8.1±0.4 | 7.8±0.4 | 8.6±1.0 |
| Leukocyte populations (%) | ||||
| Eosinophil | 3.7±0.7 | 4.9±0.6 | 5.5±0.9 | 4.0±0.5 |
| Neutrophil | 38.4±4.3 | 43.4±2.3 | 42.2±3.1 | 45.5±3.7 |
| Monocyte | 8.6±1.5 | 6.3±0.6 | 7.3±0.7 | 4.5±0.9 |
| Lymphocyte | 48.3±3.5 | 44.4±2.2 | 43.8±2.7 | 45.3±3.7 |
| Lymphocyte populations (%) | ||||
| B lymphocytes (CD3−CD19+) | 20.6±2.8 | 14.1±0.9a | 14.2±1.2a | 13.9±1.7a |
| NK cells (CD3−CD56+) | 7.5 (3.5–12.0) | 2.5 (0.9–8.0)a | 3.7 (1.8–9.4) | 1.2 (0.9–3.5)a |
| NKT cells (CD3+CD56+) | 2.0 (0.5–3.5) | 0.6 (0.3–1.7)a | 0.7 (0.3–2.3) | 0.6 (0.2–1.5)a |
| TCD4 lymphocytes (CD3+CD4+) | 38.7±2.3 | 38.0±1.3 | 38.1±1.6 | 37.8±2.2 |
| TCD8 lymphocytes (CD3+CD8+) | 22.5±2.0 | 22.6±1.3 | 19.9±2.0 | 24.4±2.1 |
Results are expressed as mean±standard error of mean or median and interquartile ranges, when appropriate.
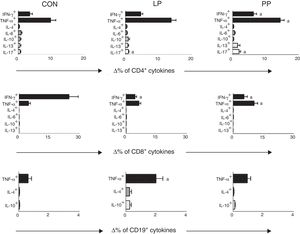
Percentages of TCD4+, TCD8+, and B-lymphocyte expressing cytokines after stimulation with PMA were compared in patients with INS and in the control group. The cytokine analysis in T-CD4+ lymphocytes revealed that INS patients had a significant increase in the frequency of cells expressing TNF-α when compared to controls (INS: 13.94±1.06% vs. controls: 8.74±1.92%, p<0.05). Patients with INS also exhibited a decrease in the percentage of TCD8+ lymphocytes expressing IFN-γ in comparison to controls (INS: 5.61±0.83% vs. controls: 19.46±3.91%, p<0.05). No other differences were detected.
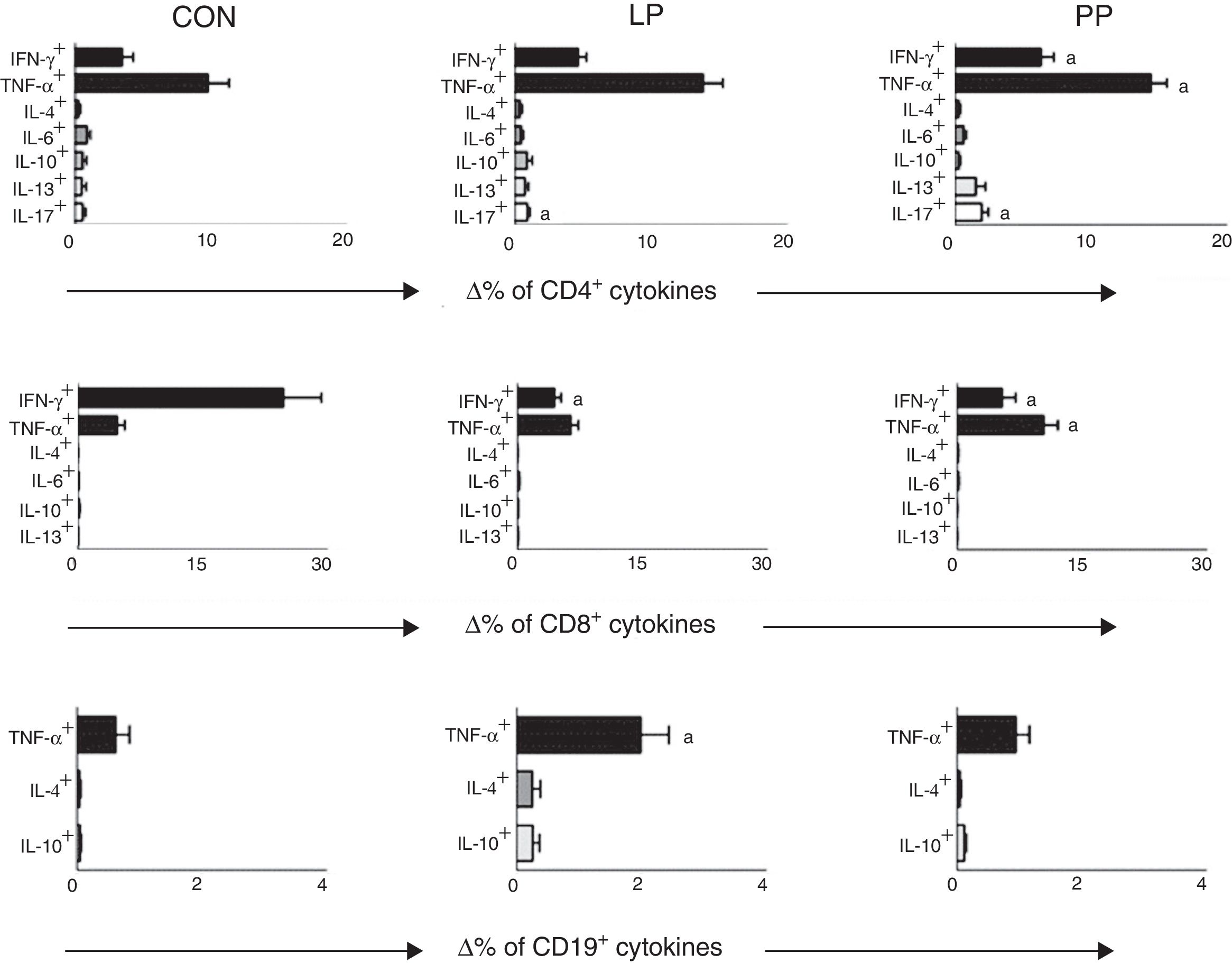
Comparisons between the percentage of TCD4+, TCD8+ and B-lymphocytes expressing cytokines after stimulation with PMA in control group and in INS patients with PP and with LP are displayed in Fig. 1. INS patients with PP had significantly higher percentages of TCD4+ cells expressing IFN-γ (6.58±0.89%), TNF-α (14.43±1.18%), and IL-17 (1.58±0.32%) when compared to the control group (3.47±0.82, 8.74±1.92%, and 0.55±0.18, respectively, Fig. 1). Patients of the PP group also presented a significant increase in the percentage of TCD8+ cells expressing TNF-α (10.37±1.75%) in comparison with patients of the LP group (6.43±0.91%) and controls (4.72±0.90%) (Fig. 1). Nonetheless, the percentage of TCD8+ cells expressing IFN-γ was significantly lower in the LP (4.75±0.78%) and PP groups (7.12%±1.8%) when compared to the controls (19.46±3.91%) (Fig. 1).
Percentage variation analysis (Δ%) of cells expressing anti- and pro-inflammatory cytokines after unspecific in vitro stimulation with PMA in patients with idiopathic nephrotic syndrome exhibiting low proteinuria (LP), persistent proteinuria (PP), and in healthy controls (CON). Results are expressed as bar graphs with mean values and standard deviation.
Δ, difference between cultures with and without PMA stimulation.
ap<0.05.
The molecular and cellular mechanisms underlying INS remains unclear. However, emerging evidence has been pointing out a role for the immune system in the pathogenesis of this condition.5 In the current study, INS patients presented a significant reduction of B-lymphocytes, NK cells, and NKT cells in peripheral blood when compared to healthy age and sex matched controls. These results are in line with previous studies showing the involvement of these immune cells in INS.18–20 In order to compare the relapsed disease or the lack of response to medication with the disease in remission, patients with INS were divided in PP or LP groups based on the levels of proteinuria at the time of blood sampling. Importantly, this study showed for the first time that decrease of B-lymphocytes is independent of proteinuria levels, whereas reduction of the prevalence of NK and NKT cells was associated with relapsed or partially remitted INS. The findings indicate that these cells population might be markers of the disease activity.
It has been reported that inflammatory mediators, including cytokines, play a role in INS pathophysiology and may contribute to proteinuria and glomerular damage.3,13 There is evidence that T cells mediate renal damage through the release of these inflammatory mediators.3,21 Accordingly, it was found that patients with INS presented higher frequencies of TCD4+ cells expressing the inflammatory cytokine TNF-α compared with healthy children. Interestingly, they also presented lower TCD8+ lymphocytes expressing IFN-γ. Over the past years, emerging evidence has supported a role for an imbalance in Th1/Th2 inflammatory responses in INS pathophysiology, with a shift toward the Th2 profile. For instance, increased expression of Th2 cytokines, like IL-4, IL-10, and IL-13, preceded disease occurrence in clinical and experimental studies.15,22,23 However, it is worth noting that Th1 cytokines, including IL-2, IFN-γ, and TNF-α, have been also associated with NS, corroborating the present findings.12,14,24 Methodological differences, including patient population and techniques used to measure the levels of cytokine, may account for the conflicting results.3
It was also shown that a nonspecific stimulation with PMA of the immune cells from patients with PP revealed a significant increase of TCD4+ cells expressing the inflammatory cytokines IL-17, IFN-γ, and TNF-α, as well as of TCD8+ lymphocytes expressing TNF-α. Corroborating these findings, increased mRNA expression for IL-17 in the peripheral blood mononuclear cells and kidney as well as higher serum levels of the inflammatory cytokines IL1-ß and IL-6 were previously reported in pediatric patients with INS.25 However, those authors did not evaluate the influence of the measured cytokines in the levels of proteinuria.26 Inflammatory mediators, especially TNF-α, are known to increase urine protein excretion in rodent models of INS.27,28 However, data in patients are still missing. To the best of the authors’ knowledge, this study is the first to provide evidence that increased expression of inflammatory cytokines in immune system cells may be associated with enhanced proteinuria in INS patients, which, in turn, may influence disease activity and prognosis.
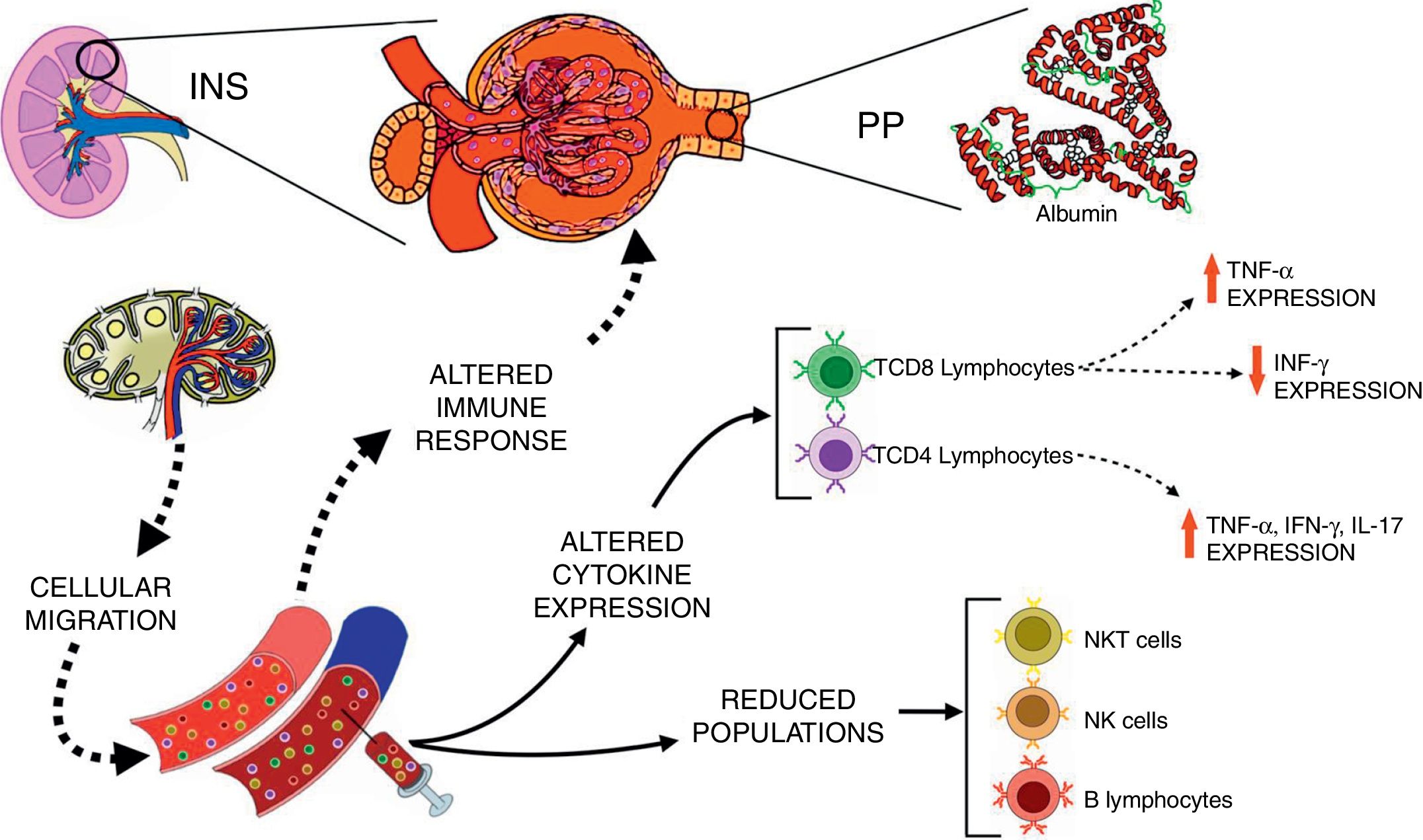
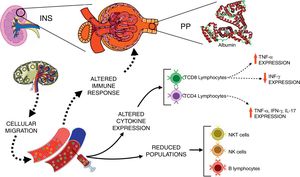
Corticosteroids are the first line of immunosuppressive medications for the treatment of INS.28 Pediatric patients with INS treated with corticosteroid presented an early and reversible suppression in T cell population, followed by a persistent depression in B cell numbers even with drug discontinuation.29 It has been also reported that immunosuppressive therapies improved renal function and decreased proteinuria in patients with focal segmental glomerulosclerosis.30 Interestingly, herein corticosteroids and cyclosporine were not able to completely suppress the expression of inflammatory cytokines by T lymphocytes, especially in patients with PP. The inflammatory profile of immune cells of patients with INS may contribute, at least in part, to the persistence and/or recurrence of proteinuria, and ultimately to inadequate response to steroids, as hypothesized in Fig. 2.
Schematic view of the interactions between immunological changes in the blood stream and renal alterations in patients with idiopathic nephrotic syndrome (INS). The figure emphasizes the main alterations in peripheral blood leukocyte populations and cytokine expression after in vitro stimulation with PMA in INS patients with persistent proteinuria (PP) in comparison to healthy controls.
The authors recognize the limitations associated with the cross-sectional design of this study. The main possible weakness was the use of a convenience sample, which makes homogeneity among the selected groups very difficult to obtain, since the presence and previous use of immunosuppressive drugs may interfere with cytokine and chemokine measurements. In addition, the size of the control group was reduced due to the difficulty in recruiting healthy patients for blood sampling simultaneously to blood collections from the patients. Nevertheless, some aspects of the study may increase the strength of its findings, including the utilization of strictly defined inclusion and exclusion criteria and of a well-established protocol for flow cytometry measurements.
In conclusion, this study provides the first evidence that pediatric patients with INS have an inflammatory profile, despite the use of steroid or cyclosporine therapy, which was associated with increased levels of proteinuria. Finally, immune cells expressing inflammatory cytokines, especially TNF-α, were identified as potential markers of disease activity, paving the way for the development of new therapeutic targets.
FundingThis study was partially supported by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil – grant number 470472/2014-6 and grant number 460334/2014-0) and FAPEMIG (Fundação de Amparo à Pesquisa do Estado de Minas Gerais, Brazil – grant number PPM-00555-15).
Conflicts of interestThe authors declare no conflicts of interest.
This work was supported by grant from FAPEMIG (Fundação de Amparo à Pesquisa do Estado de Minas Gerais, Brazil), CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil).
Please cite this article as: Guimarães FT, Melo GE, Cordeiro TM, Feracin V, Vieira ER, Pereira WF, et al. T-lymphocyte-expressing inflammatory cytokines underlie persistence of proteinuria in children with idiopathic nephrotic syndrome. J Pediatr (Rio J). 2018;94:546–53.