Characterize the role of human parainfluenza virus and its clinical features in Brazilian children under 2 years of age presenting with acute lower respiratory tract infections.
MethodsReal-time assays were used to identify strains of human parainfluenza virus and other common respiratory viruses in nasopharyngeal aspirates. One thousand and two children presenting with acute lower respiratory tract illnesses were enrolled from February 2008 to August 2010.
ResultsOne hundred and four (10.4%) patients were human parainfluenza virus positive, of whom 60 (57.7%) were positive for human parainfluenza virus-3, 30 (28.8%) for human parainfluenza virus-4, 12 (11.5%) for human parainfluenza virus-1, and two (1.9%) for human parainfluenza virus-2. Seven (6.7%) patients had more than one strain of human parainfluenza virus detected. The most frequent symptoms were tachypnea and cough, similar to other viral respiratory infections. Clinical manifestations did not differ significantly between human parainfluenza virus-1, -2, -3, and -4 infections. Human parainfluenza virus-1, -3, and -4 were present in the population studied throughout the three years of surveillance, with human parainfluenza virus-3 being the predominant type identified in the first two years.
ConclusionHuman parainfluenza viruses contribute substantially to pediatric acute respiratory illness (ARI) in Brazil, with nearly 30% of this contribution attributable to human parainfluenza virus-4.
Caracterizar o papel do VPH-4 e suas características clínicas em crianças brasileiras com menos de dois anos de idade com infecções agudas do trato respiratório inferior.
MétodosEnsaios em tempo real foram utilizados para identificar tipos de VPH e outros vírus respiratórios comuns em aspirados nasofaríngeos. Mil e duas crianças com doença aguda do trato respiratório inferior foram inscritas para participar de fevereiro de 2008 a agosto de 2010.
Resultados104 (10,4%) pacientes eram VPH positivos, dos quais 60 (57,7%) eram positivos para VPH-3, 30 (28,8%) para VPH-4, 12 (11,5%) para VPH-1 e dois (1,9%) para VPH-2. Sete (6,7%) pacientes apresentaram mais de um tipo de VPH detectado. Os sintomas mais frequentes foram tosse e taquipneia, semelhantes a outras infecções respiratórias virais. As manifestações clínicas não diferiram de forma significativa entre as infecções por VPH-1, -2, -3 e -4. Os VPH-1, -3 e -4 estavam presentes na população estudada ao longo dos três anos de vigilância, e o VPH-3 foi o tipo predominante identificado nos primeiros dois anos.
ConclusãoOs VPHs contribuem substancialmente para a DRA pediátrica no Brasil com quase 30% dessa contribuição atribuível ao VPH-4.
Viruses are the predominant cause of acute respiratory illness (ARI) worldwide, and are responsible for substantial morbidity and mortality in children between 1 and 5 years of age. Human parainfluenza viruses (HPIVs) account for a significant proportion of viral ARIs in children, representing the second most common cause of upper and lower respiratory tract infections, just after human respiratory syncytial virus (HRSV).1 The four HPIV serotypes, HPIV-1, -2, -3 and -4, and two subtypes, -4a and -4b, are estimated to cause up to 10% of childhood ARIs.2 HPIV1 and HPIV2 are the primary cause of croup in children aged 6–48 mons; HPIV3, and to a lesser extent HPIV1, are more often associated with bronchiolitis and pneumonia in children under 1 year. HPIVs also cause severe disease, including pneumonia and death in transplant recipients, as well as nosocomial infections and outbreaks, similar to HRSV and influenza virus.3
Little is known about the epidemiology and disease burden of HPIVs in the pediatric populations of Latin America, especially in Brazil.1,4–6 There are even less studies concerning HPIV-4 infection in America since few laboratories provide specific diagnoses of HPIV-4. Because of its apparently low prevalence and its difficulty of growth in cell culture.7
To better characterize the role of HPIV-4 and its clinical features in children under 2 years of age with ARI, real time reverse transcription polymerase chain reaction (rRT-PCR) analyses were used to identify four strains of HPIV and other common respiratory viruses in nasopharyngeal aspirates.
MethodsThe research ethics committee of Institute Biomedical Science of the University of São Paulo approved the study. From March 2008 to August 2010, nasopharyngeal aspirate samples were collected from patients under 2 years of age with ARI, attended to or hospitalized at the Santa Casa de Misericordia Hospital (São Paulo, Brazil), after written consent was obtained from the children's parents. The samples were placed in a viral transport tube and held up to 48h at 4°C. The samples were processed in a biosafety level 2 laboratory at the Institute of Biomedical Science, University of São Paulo. Total nucleic acids were automatically extracted from 300μL of fresh specimen and eluted in 110μL of RNase-free elution buffer using NucliSENS easyMAG (bioMérieux–Brazil) according to the manufacturer's instructions. Nucleic acids were kept frozen at −70°C until use. A multiple singleplex rRT-PCR assay panel was used to detect and identify HPIVs (types 1, 2, 3, and 4)8 and other human respiratory viruses, including respiratory syncytial virus, human metapneumovirus, adenovirus, and influenza viruses A and B.9–11 The statistical analyses was conducted with Statgraphics Centurion XV software; the chi-squared test for comparison of proportions was used for each symptom to verify the proportion of patients presenting the symptom in the serotypes analyzed. It also generated an analysis of means (ANOM) plot to determine which samples were significantly different from the grand mean. Since the p-value was greater than or equal to 0.05, there are no significant differences between the samples at the 95% or higher confidence level.
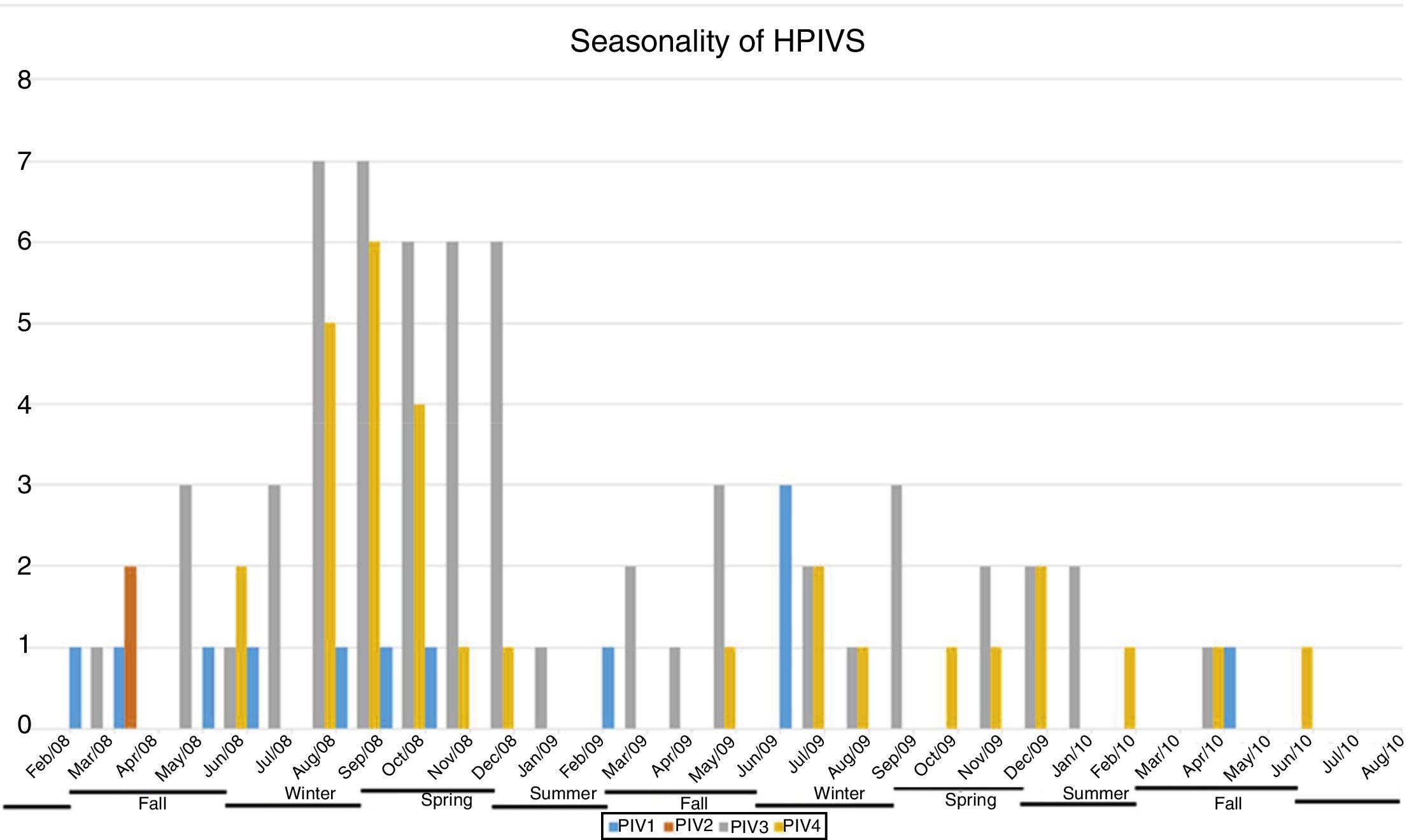
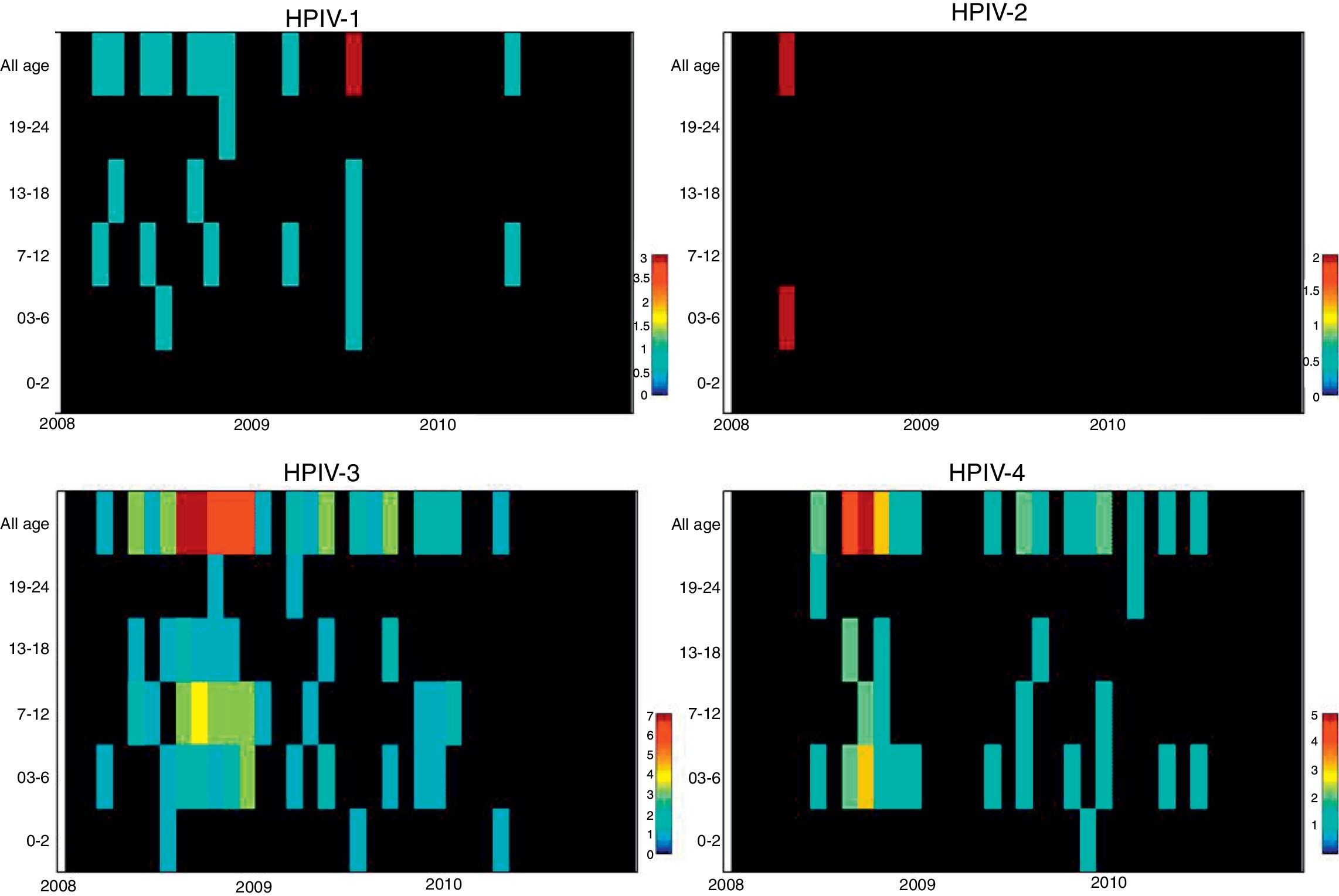
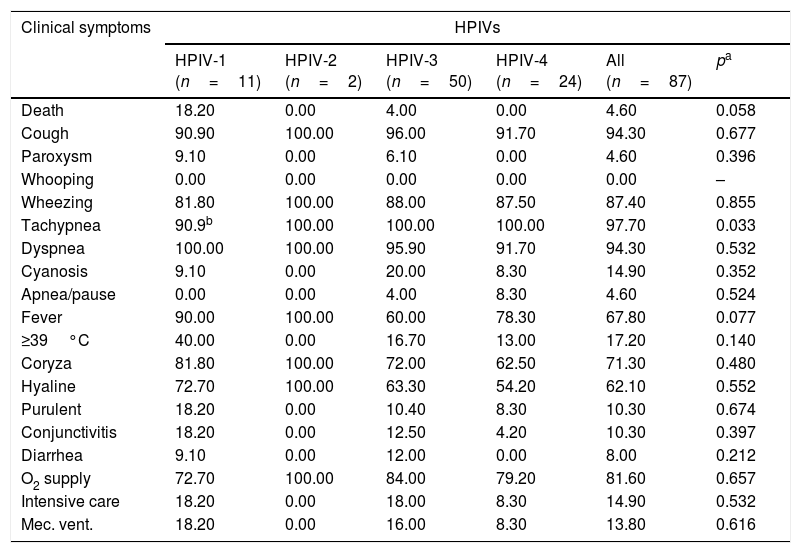
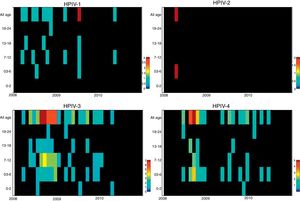
ResultsNasopharyngeal specimens were collected from 1002 patients with ARI. Parainfluenza virus infection was laboratory confirmed in 104 (10.4%) samples, of which 60 (57.7%) were positive for HPIV-3, 30 (28.8%) for HPIV-4, 12 (11.5%), for HPIV-1, and two (1.9%) for HPIV-2. Most laboratory-confirmed HPIV-4 specimens were collected from August 2008 to December 2008, but there were few cases collected in 2009 and 2010, as shown in Fig. 1. The median age of HPIV-4 infected patients was 7 months (ranging 0–24), 8 months (1–24) for HPIV-3, 5 months (3–6) for HPIV-2, and 11 months (6–22) for HPIV-1. In contrast, the HPIV-4 frequency was higher in the age group of 3–6 months. Somewhat lower to HPIV-3 and HPIV-1 detection, with greater number of cases between 7 and 12 months (Fig. 2). Medical records of 87 HPIV infected patients were available and the clinical data are summarized in Table 1. Prominent clinical symptoms included tachypnea (97.7%), cough (94.3%), dyspnea (94.3%), wheezing (87.4%), coryza (71.3%), and fever (67.8%). Thirteen (15%) of the infected patients required hospital admission (intensive care). In 23 cases, HPIV-4 was the sole HPIV type detected. However, in the seven remaining cases, HPIV-1 (one case) and HPIV-3 (six cases) were also detected.
Clinical symptoms of HPIV positive patients (%).
| Clinical symptoms | HPIVs | |||||
|---|---|---|---|---|---|---|
| HPIV-1 (n=11) | HPIV-2 (n=2) | HPIV-3 (n=50) | HPIV-4 (n=24) | All (n=87) | pa | |
| Death | 18.20 | 0.00 | 4.00 | 0.00 | 4.60 | 0.058 |
| Cough | 90.90 | 100.00 | 96.00 | 91.70 | 94.30 | 0.677 |
| Paroxysm | 9.10 | 0.00 | 6.10 | 0.00 | 4.60 | 0.396 |
| Whooping | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | – |
| Wheezing | 81.80 | 100.00 | 88.00 | 87.50 | 87.40 | 0.855 |
| Tachypnea | 90.9b | 100.00 | 100.00 | 100.00 | 97.70 | 0.033 |
| Dyspnea | 100.00 | 100.00 | 95.90 | 91.70 | 94.30 | 0.532 |
| Cyanosis | 9.10 | 0.00 | 20.00 | 8.30 | 14.90 | 0.352 |
| Apnea/pause | 0.00 | 0.00 | 4.00 | 8.30 | 4.60 | 0.524 |
| Fever | 90.00 | 100.00 | 60.00 | 78.30 | 67.80 | 0.077 |
| ≥39°C | 40.00 | 0.00 | 16.70 | 13.00 | 17.20 | 0.140 |
| Coryza | 81.80 | 100.00 | 72.00 | 62.50 | 71.30 | 0.480 |
| Hyaline | 72.70 | 100.00 | 63.30 | 54.20 | 62.10 | 0.552 |
| Purulent | 18.20 | 0.00 | 10.40 | 8.30 | 10.30 | 0.674 |
| Conjunctivitis | 18.20 | 0.00 | 12.50 | 4.20 | 10.30 | 0.397 |
| Diarrhea | 9.10 | 0.00 | 12.00 | 0.00 | 8.00 | 0.212 |
| O2 supply | 72.70 | 100.00 | 84.00 | 79.20 | 81.60 | 0.657 |
| Intensive care | 18.20 | 0.00 | 18.00 | 8.30 | 14.90 | 0.532 |
| Mec. vent. | 18.20 | 0.00 | 16.00 | 8.30 | 13.80 | 0.616 |
There were six co-detections of HPIV-3 and -4, and one co-detection of HPIV-1 and -4.
n, number of positive patients with clinical information available.
Other respiratory viruses were detected in 473 (47.2%) of the 1002 patient specimens. The most frequently detected viruses were RSV in 244 cases (24.3% of the total specimens), adenovirus in 121 cases (12.1%), influenza in 58 cases (5.8%), and human metapneumovirus (HMPV) in 50 cases (5%). Co-infections with two or more viruses were detected in 11.7% of studied samples.
DiscussionDespite associations between HPIV-4 infection and ARI having been proposed for more than half a century,12 it is not commonly included in molecular testing available in Brazil because it is not considered to be a major disease-causing virus.13 To the best of the authors’ knowledge, this is the first report of HPIV-4 infection in children with ARI in Brazil. This virus was circulating among pediatric patients with ARIs during entire period tested, along with other respiratory viruses including other HPIVs. These results corroborate studies from other countries in which HPIV-4 was identified as the cause of respiratory disease in institutionalized children, as well as community acquired pneumonia and bronchiolitis.14–16 HPIV-4 was the third most prevalent virus (5.8%) in cases of adult influenza-like illness over two consecutive seasons in USA during 1998 to 2000.3 In this study, HPIV-4 prevalence lagged more common respiratory pathogens (RSV, adenovirus, influenza, and HMPV), but was the second most prevalent HPIV, following HPIV-3. Overall, HPIVs were the third most common respiratory viral pathogen detected.
The analysis of age distribution according to viral infection shows that the largest number of positive cases of all HPIVs, like other respiratory viruses, occurs in children aged less than 1 year, which is consistent with the international literature.8,9,17
When physical symptoms and clinical diagnosis were compared with etiology, no association could be found, so it was impossible to identify the HPIV type based only on clinical signs; however, a relatively higher rate of mechanical ventilation and intensive care need was found in patients with HPIV-1 and 3, as well as a higher rate of cyanosis in patients with HPIV-3. Among HPIVs, a higher rate of deaths was observed in HPIV-1 cases (18.2%).
A limitation of this study was the end of sample collection before the end of last year studied, precisely in the higher expected incidence period, affecting possible inferences on the seasonality of HPIVs. Moreover, there was a lack of asymptomatic controls to help determine the prevalence of subclinical HPIV4 infections, and only one hospital was sampled. The restricted population complicates generalization of data to the broader community. Longitudinal studies should be performed to confirm the results obtained here.
There are no data describing HPIV-4 circulation in Brazil. Evidence was found of a moderate frequency of HPIV-4 detection (28.8% of HPIVs; 3% of all virus positive samples) in young children with ARI, equal to or higher than other HPIVs. Further studies are needed confirm these findings. HPIV-4 should be included with other respiratory pathogens routinely tested for in developing countries, especially in the group of high-risk infants, such as prematurity, congenital heart diseases, chronic lung disease, immune deficiencies, and co-infections (children with multiple viruses), who may develop more severe disease. Furthermore, other risk factors associated with severe disease are longer hospitalization, more frequent requirement of supplemental oxygen, mechanical ventilation, and intensive care.
DisclaimerThe findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
FundingCNPq, FAPESP.
Conflicts of interestThe authors declare no conflicts of interest.
The authors thank the FAPESP, and the CNPq (Y0204004040131) for their financial support. They are grateful to Wladimir Jimenez Alonso and the NIH team for the support in the use of EPIPOI software on the MISMS 2015 (Multinational Influenza Seasonal Mortality Study) (Taipei, Taiwan), and Rosana Prisco for the statistical support.
Please cite this article as: Thomazelli LM, Oliveira DB, Durigon GS, Whitaker B, Kamili S, Berezin EN, et al. Human parainfluenza virus surveillance in pediatric patients with lower respiratory tract infections: a special view of parainfluenza type 4. J Pediatr (Rio J). 2018;94:554–8.