To evaluate phenylalanine plasma profile in preterm newborns fed different human milk diets.
MethodsTwenty-four very-low weight preterm newborns were distributed randomly in three groups with different feeding types: Group I: banked human milk plus 5% commercial fortifier with bovine protein, Group II: banked human milk plus evaporated fortifier derived from modified human milk, Group III: banked human milk plus lyophilized fortifier derived from modified human milk. The newborns received the group diet when full diet was attained at 15 ± 2 days. Plasma amino acid analysis was performedon the first and last day of feeding. Comparison among groups was performed by statistical tests: one way ANOVA with Tukey's post-test using SPSS software, version 20.0 (IBM Corp, NY, USA), considering a significance level of 5%.
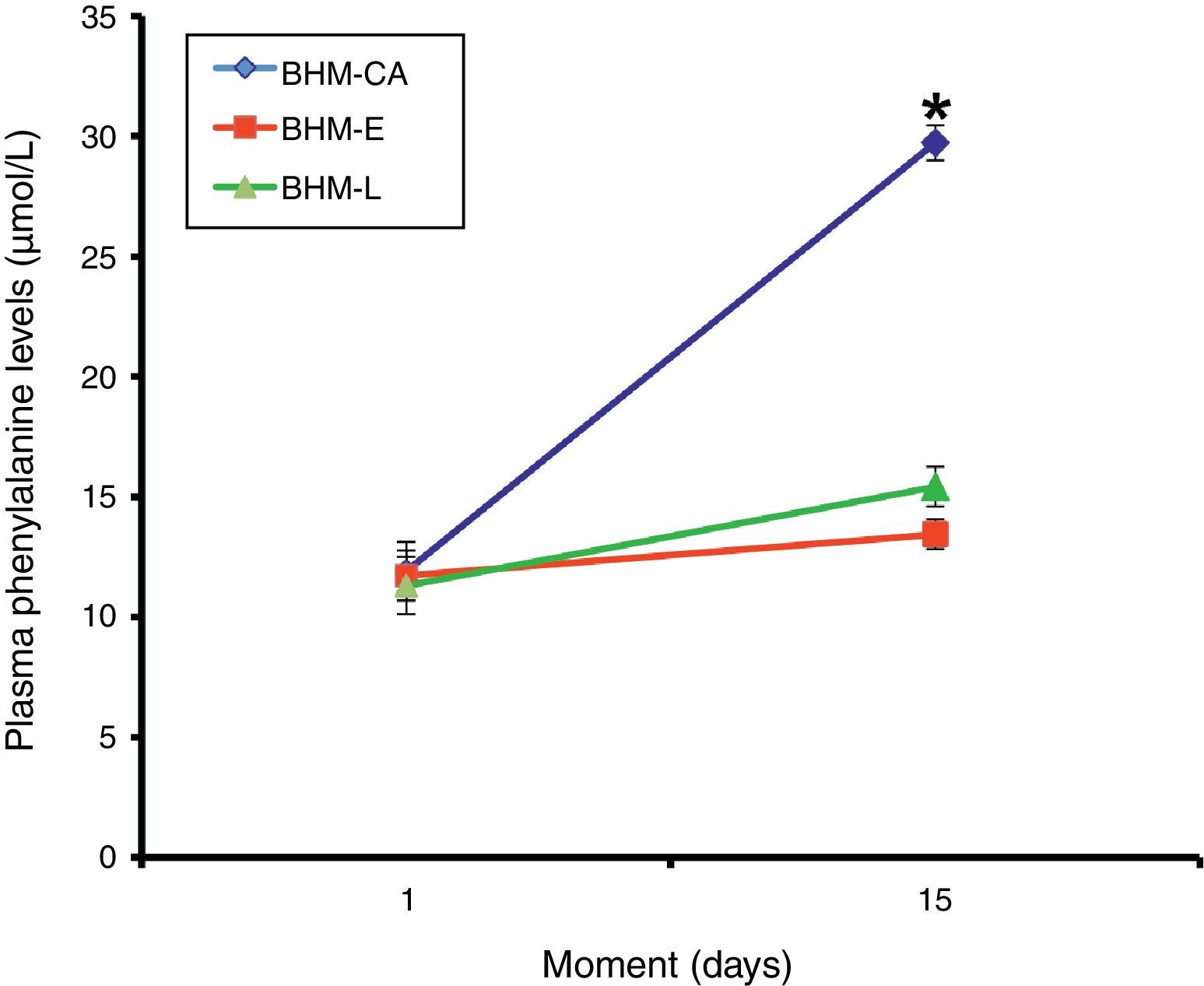
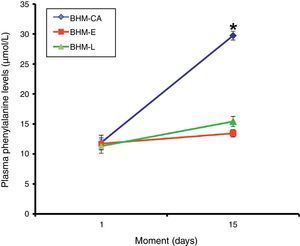
ResultsPhenylalanine levels in the first and second analysis were, respectively, in Group I: 11.9 ± 1.22 and 29.72 ± 0.73; in Group II: 11.72 ± 1.04 and 13.44 ± 0.61; and in Group III: 11.3 ± 1.18 and 15.42 ± 0.83μmol/L.
ConclusionThe observed results demonstrated that human milk with fortifiers derived from human milk acted as a good substratum for preterm infant feeding both in the evaporated or the lyophilized form, without significant increases in plasma phenylalanine levels in comparison to human milk with commercial fortifier.
Avaliar o perfil plasmático do aminoácido fenilalanina em recém-nascidos pré-termo alimentados com diferentes dietas de leite humano.
MétodosForam estudados 24 recém-nascidos pré-termo de muito baixo peso, distribuídos em três grupos com diferentes dietas: Grupo I: leite humano de banco com 5% de aditivo comercial para leite humano com proteína de origem bovina (LHB-AC); Grupo II: leite humano de banco com aditivo de leite humano modificado evaporado (LHB-E); e Grupo III: leite humano de banco com aditivo de leite humano modificado liofilizado (LHB-L). Os recém-nascidos receberam a dieta definida para o grupo quando alcançaram dieta plena por 15±2 dias. A análise do aminoácido plasmático foi feita no primeiro e último dias da dieta. A comparação entre os grupos foi realizada por meio do teste ANOVA de uma via, seguido pelo pós-teste de Tukey, utilizando-se o software SPSS, versão 20.0 (IBM Corp, NY, EUA), e considerando um nível de significância de 5%.
ResultadosAs concentrações plasmáticas do aminoácido fenilalanina na primeira e segunda análises foram, respectivamente, no Grupo I (LHB-AC) 11,9±1,22 e 29,72±0,73; no Grupo II (LHB-E) 11,72±1,04 e 13,44±0,61; e no Grupo III 11,3±1,18 e 15,42±0,83 umol/L.
ConclusãoOs resultados encontrados demonstram que o leite humano com aditivos do próprio leite humano comportou-se como um bom substrato para alimentação do recém-nascido pré-termo, tanto na forma evaporada como liofilizada, sem levar a aumentos significativos na concentração plasmática de fenilalanina em comparação ao leite humano com aditivo co-mercial.
The superiority of human milk (HM) feeding in preterm newborns (PNs) is well documented. HM has an important impact on brain growth and development, even when it does not promote great weight gain, supporting the concept that the optimal postnatal growth of PNs is not yet known.1–5
Regarding the supply of proteins, not only the quantity but also the quality is important for proper growth. The amino acid composition of formulas and additives to human milk using bovine protein has reduced quality in relation to HM,6–9 which is considered the gold standard.
The protein fraction of cow's milk has a predominance of casein, which has high content of the amino acid phenylalanine.10 Although it is an essential amino acid in children receiving cow's milk protein, plasma levels of this amino acid are high (close to those associated with metabolism defects).11–13
The increased intake and plasma levels of phenylalanine results in the inhibition of the enzyme tyrosinase, and subsequent conversion, through hydroxylation, of phenylalanine into tyrosine, increasing tyrosine availability. This increase can cause a deleterious effect on brain development, leading to consequences such as sleep disturbance, memory deficits, and attention and concentration deficits.14–17
While the optimal nutrition for PNs is unknown, neonatologists should be committed to what appears to be ideal, which does not result in changes in the short-term, and provides better long-term development. In this context, supplementing HM with an additive containing a protein homologous to that of HM appears to be a suitable alternative for protein supply, while maintaining safe plasma levels of phenylalanine.18–20
Considering this hypothesis, this study aimed to comparatively analyze plasma levels of phenylalanine in PNs fed banked human milk (BHM) plus the commercial additive FM85 (Fortified Milk 85, Nestlé, São Paulo, Brazil) and PNs fed with BHM plus an additive derived from the HM itself, after removal of fat and lactose in evaporated or lyophilized forms.
MethodsAfter approval of the Federal University of Mato Grosso do Sul (UFMS) Research Ethics Committee (Res. 17/2006), a non-blinded randomized clinical trial was performed from 2008 to 2010, in the neonatology section of the Núcleo do Hospital Universitário (NHU) of UFMS (Universidade Federal de Mato Grosso do Sul, Campo Grande, MS, Brazil).
A total of 24 PNs hospitalized in the neonatal sector, of both genders, were studied after being divided into three groups. Each group received a different HM-based diet. The groups were compared for plasma levels of phenylalanine. To confirm that the groups had similar characteristics and that the difference in plasma phenylalanine levels was associated with the diet they received, the PNs were compared regarding gender, gestational weight/age, respiratory distress syndrome (RDS), gestational age, birth weight, start of feeding, volume, calories, early minimal enteral nutrition, and days on ventilator.
The diets offered to each group were:
Group I: PNs fed BHM, plus 5% commercial additive FM 85® (Nestlé, São Paulo, Brazil), identified by the acronym: BHM-CA;
Group II: PNs fed BHM with modified HM supplement: 100mL of skimmed HM, evaporated at 20%, with lactose extraction and added 80mL of pasteurized HM, identified by the acronym: BHM-E;
Group III: PNs fed BHM, with modified HM supplement: 70mL of skimmed HM, evaporated at 20%, with lactose extraction, lyophilized, reconstituted in 100mL of BHM and pasteurized, identified by the acronym: BHM-L.
The additives obtained from HM were prepared according to the method described by Thomas et al.21
Of the 24 PNs, ten belonged to GI, five to GII, and nine to GIII. They were fed according to this order at different times. Although selection was not blinded, all PNs who met the inclusion criteria and who were hospitalized during the study period in NHU-UFMS were selected for the study.
The PNs included in the study had gestational age < 34 weeks; birth weight ≤ 1.500kg,whether or not adequate for gestational age; were clinically stable; had no congenital malformations; and their parents, after being informed of the nature of the study, signed the informed consent.
PNs were excluded from the study in the presence of congenital malformations, metabolic disorders, anemia, any active disease (respiratory disorders, central nervous system manifestations and gastrointestinal), periventricular hemorrhage ≥ grade 2, and those whose mothers had sufficient milk for feeding.
During the study, PNs that presented unfavorable conditions for the research development were substituted; these conditions were utterly related to worsening of infection level.
The PNs started receiving the specific diet of the group they belonged to only when they reached full (100mL/kg) and well-tolerated enteral diet and, therefore, the PNs were enrolled in the study as soon as they started enteral feeding by gavage.
The PNs were followed since they startedreceiving the modified milk for 15 ± 2 days.
Non-blinded analysis of levels of the amino acid phenylalanine in plasma was performed. For the analysis, a pre-prandial venous sample was collected (2.5 to 3hours after the last feeding) by percutaneous puncture with a syringe containing three drops of heparin (anticoagulant effect), packaged in a microfuge tube (Eppendorf do Brasil Ltda, São Paulo, Brazil).The plasma was then separated by centrifugation (2,500rpm for 10min), identified, and frozen at -20°C for subsequent amino acid analysis by high performance liquid chromatography. Blood collection was performed in each child of the three groups for comparison of the amino acid profile on the first day, before the newborns started the specific group diet, and on the last day they received this diet.
The evaluation of the association between diets offered to the PNs with the variables gender, gestational weight/age, and RDS was performed using the chi-squared test. The association between diets offered to the PNs and the variables gestational age, birth weight, early feeding, volume, calories, early minimal enteral nutrition, days of mechanical ventilation, and plasma levels of phenylalanine was performed by one-way ANOVA, followed by Tukey's post-test. The results of the other variables assessed in this study were shown as descriptive statistics or in tables and charts. Statistical analysis was performed using SPSS software program, release 20.0 (IBM Corp, NY, USA), considering a significance level of 5%.
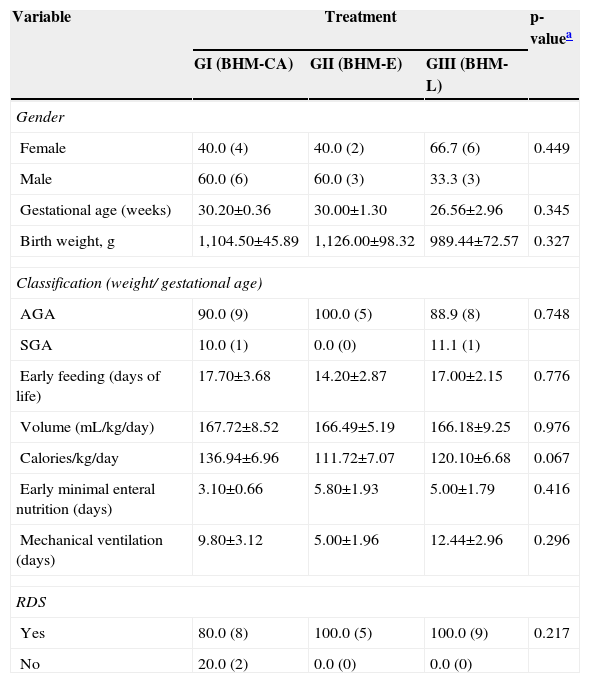
ResultsThe characteristics of the three groups regarding gender, gestational age, birth weight, adequate weight for gestational age, early minimal enteral feeding, early full enteral feeding, use of mechanical ventilation, respiratory distress syndrome, mean volume, and calories received in the daily diet are shown in Table 1.
Results for the variables assessed in this study, according to the feedingtype offered to preterm newborns.
| Variable | Treatment | p-valuea | ||
|---|---|---|---|---|
| GI (BHM-CA) | GII (BHM-E) | GIII (BHM-L) | ||
| Gender | ||||
| Female | 40.0 (4) | 40.0 (2) | 66.7 (6) | 0.449 |
| Male | 60.0 (6) | 60.0 (3) | 33.3 (3) | |
| Gestational age (weeks) | 30.20±0.36 | 30.00±1.30 | 26.56±2.96 | 0.345 |
| Birth weight, g | 1,104.50±45.89 | 1,126.00±98.32 | 989.44±72.57 | 0.327 |
| Classification (weight/ gestational age) | ||||
| AGA | 90.0 (9) | 100.0 (5) | 88.9 (8) | 0.748 |
| SGA | 10.0 (1) | 0.0 (0) | 11.1 (1) | |
| Early feeding (days of life) | 17.70±3.68 | 14.20±2.87 | 17.00±2.15 | 0.776 |
| Volume (mL/kg/day) | 167.72±8.52 | 166.49±5.19 | 166.18±9.25 | 0.976 |
| Calories/kg/day | 136.94±6.96 | 111.72±7.07 | 120.10±6.68 | 0.067 |
| Early minimal enteral nutrition (days) | 3.10±0.66 | 5.80±1.93 | 5.00±1.79 | 0.416 |
| Mechanical ventilation (days) | 9.80±3.12 | 5.00±1.96 | 12.44±2.96 | 0.296 |
| RDS | ||||
| Yes | 80.0 (8) | 100.0 (5) | 100.0 (9) | 0.217 |
| No | 20.0 (2) | 0.0 (0) | 0.0 (0) | |
The results are shown as mean ± standard error of the mean or relative frequency (absolute frequency).
RDS, respiratory distress syndrome; AGA, appropriate for gestational age; SGA, small for gestational age.
Regarding these characteristics, the groups showed no significant differences (Table 1).
Plasma levels of the amino acid phenylalanine (mean ± SEM) in the first and second analysis were, respectively: 11.9 ± 1.22 and 29.72 ± 0.73μmol/L in Group I (BHM-CA); 11.72 ± 1.04 and 13.44 ± 0.61μmol/L in Group II (BHM-E); and 11.3 ± 1.18 and 15.42 ± 0.83μmol/L in Group III (BHM-L). The results regarding the concentration of the essential amino acid phenylalanine in Groups I, II, and III are shown in Fig. 1.
There was no difference between treatments in relation to plasma levels of phenylalanine on the first day of full enteral feeding (one-way ANOVA, p = 0.931). Conversely, the treatments showed differences 15 days after the start of feeding (one-way ANOVA, p <0.001), with plasma phenylalanine levels in the group of PNs fed BHM-CA higher than that for the groups BHM-E and BHM-L (Tukey's post-test, p <0.05), albeit with no significant differences between the two latter groups (p > 0.05).
DiscussionIt is suggested that blood samples should be collected immediately before feeding when analyzing the amino acid profile, so they can be analyzed with less interference from the diet offered. This evidence justifies the choice of performing the pre-prandial collection of blood for amino acid analysis.22
When phenylalanine levels were compared between PNs fed different diets, it was observed that those fed commercial additives had higher plasma levels of this amino acid, with a significant difference when compared to those fed the evaporated and lyophilized additives.
Considering that the groups were similar regarding the characteristics shown in Table 1 and that the plasma phenylalanine levels were similar in the three groups at baseline, this difference in phenylalanine levels at the end of the study seems to be related to the quality of the protein in the additive used by each group and the lower degradation capacity of this amino acid in PNs compared to full-term newborns. The difference should not be associated to the total amount of protein consumed, as the mean protein content of the diet in the BHM-CA group (1.96 ± 0.01g/dL) is intermediate to those in groups BHM-E (1.81 ± 0.01g/dL) and BHM-L (2.38 ± 0.03g/dL).21
Although the caloric value of BHM-CA (81.65 ± 0.87kcal/dL) is greater than that of BHM-E (67.78 ± 2.01kcal/dL) and BHM-L (72.27 ± 2.56kcal/dL), a still unpublished clinical study observed that weight and length gain was similar in the three groups, with the advantage of increased head circumference in the BHM-L group in relation to the others.21 These growth characteristics show the good use of protein offered by homologous additives in comparison to the commonly used commercial additive.
Studies evaluating the amino acid blood profile of PNs fed HM with the commercial additive FM85® observed that better adequacy of the protein supplied by this supplement is necessary. The additive of heterologous origin resulted in biochemical macronutrient alterationsthat may affect the children's neurodevelopment.23,24
When analyzing plasma phenylalanine levels in groups of healthy newborns at 6 months fed breast milk, regular formula, two types of formulas with hydrolyzed casein, and formulas with hydrolyzed whey protein, the group fed breast milk had the lowest levels of the amino acid,25 which appears to occur even in full-term newborns, due to the higher content of phenylalanine in formulas based on cow's milk.
The same result was found in PNs fed HM with three different additives: HM protein, cow's milk whey protein, and a mixture of cow's milk whey protein, peptides, and amino acids, with an amino acid composition similar to that of HM. Phenylalanine levels were higher in the group who received HM with cow's milk whey protein additive. The other two groups showed no differences.26
Additionally, in PNs grouped according to gestational age and fed HM or four types of formula with protein extracted from cow's milk with different levels and proportion of whey protein/casein, those fed HM showed lower plasma levels of phenylalanine.27
Conversely, a study that offered PNs BHM, BHM evaporated at 70%, or BHM with the commercial additive FM85® found no significant differences in plasma levels of phenylalanine. In this case, neither the quantity nor the quality of protein offered had an effect on serum levels of this amino acid.23
A study evaluating the concentrations of the amino acids glycine, leucine, and phenylalanine in PNs on parenteral and enteral nutrition observed that the concentrationsof glycine is more affected by the route of administration, whereas leucine is little affected, and phenylalanine is more affected by the offered supply.28
Despite the significant increase in plasma phenylalanine, no significant metabolic effects were observed in babies fed bovine protein during the study; however,in the long term, it can be a negative factor for cognitive development. Therefore, the optimal plasma levels of phenylalanine in order to avoid effects on cognitive development in the long term are still questioned.
This investigation demonstrated that the HM with its own additives acted as a good substrate to feed PNs, whether in the evaporated or lyophilized forms, without leading to significant increases in plasma phenylalanine when compared to HM with commercial additive.
FundingResearch grant from Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul (FUNDECT).
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Thomaz DM, Serafin PO, Palhares DB, Tavares LV, Grance TR. Serum phenylalanine in preterm newborns fed different diets of human milk. J Pediatr (Rio J). 2014;90:518–22.
Study performed at Universidade Federal de Mato Grosso do Sul, Campo Grande, MS, Brazil.