To describe the results of a long-term follow-up of Bartter syndrome patients treated with different drugs.
MethodPatients were diagnosed according to clinical and laboratory data. Treatment protocol was potassium supplementation, sodium, spironolactone, and non-steroidal anti-inflammatory drug. Patients who developed proteinuria were converted to angiotensin conversion enzyme inhibitor. The variables evaluated for each drug were Z-score for weight and stature, proteinuria, creatinine clearance, gastrointestinal complaints, amount of potassium supplementation, serum potassium and bicarbonate levels, and findings of upper digestive endoscopy.
Results20 patients were included. Follow-up was 10.1 ± 5.2 years. 17 patients received indomethacin for 5.9 ± 5.3 years; 19 received celecoxib, median of 35 months; and five received enalapril, median of 23 months. During indomethacin, a statistically significant increase was observed in the Z-score for stature and weight, without a change in the creatinine clearance. Seven of 17 patients had gastrointestinal symptoms, and upper digestive endoscopy evidenced gastritis in three patients and gastric ulcer in four patients. During celecoxib use, a significant increase was detected in the Z-score for stature and weight and a reduction of hyperfiltration; seven patients presented gastrointestinal symptoms, and upper digestive endoscopy evidenced mild gastritis in three. During enalapril use, no significant changes were observed in the Z-score for stature, weight and creatinine clearance. The conversion to enalapril resulted in a significant reduction in proteinuria.
ConclusionThe authors suggest starting the treatment with celecoxib, and replacing by ACEi if necessary, monitoring the renal function. The safety and efficacy of celecoxib need to be assessed in larger controlled studies.
Descrever os resultados de um acompanhamento de longo prazo de pacientes com síndrome de Bartter tratados com diferentes medicamentos.
MétodoPacientes diagnosticados segundo os dados clínicos e laboratoriais. Protocolo de tratamento: suplementação de potássio, sódio, espironolactona e medicamento anti-inflamatório não esteroidal. Os pacientes que desenvolveram proteinúria foram submetidos a inibidor da enzima de conversão da angiotensina. As variáveis avaliadas durante o uso de cada medicamento foram: escore Z para peso e estatura, proteinúria, depuração da creatinina, queixas gastrointestinais, quantidade da suplementação de potássio, níveis séricos de potássio e bicarbonato e achados da endoscopia digestiva alta.
ResultadosForam incluídos 20 pacientes. O acompanhamento foi de 10,1±5,2 anos. No total, 17 pacientes receberam indometacina por 5,9±5,3 anos, 19 receberam celecoxib por aproximadamente 35 meses e cinco receberam enalapril por aproximadamente 23 meses. Durante o uso de indometacina, observamos um aumento estatístico significativo no escore Z para estatura e peso, sem alteração na depuração da creatinina. 7/17 pacientes apresentaram sintomas gastrointestinais, e a endoscopia digestiva alta mostrou gastrite em três pacientes e úlcera gástrica em quatro. Durante o uso de celecoxib, detectamos um aumento significativo no escore Z para estatura e peso e uma redução da hiperfiltração; sete pacientes apresentaram sintomas gastrointestinais e a endoscopia digestiva alta mostrou gastrite leve em três pacientes. Durante o uso de enalapril, não observamos alterações significativas no escore Z para estatura, peso e depuração da creatinina. A mudança da medicação para enalapril resultou em uma redução significativa na proteinúria.
ConclusãoSugerimos iniciar o tratamento com celecoxib e, caso necessário, substituí-lo por ACEi, monitorando a função renal. A segurança e a eficácia do celecoxib precisam ser comprovadas com grandes estudos controlados.
Bartter syndrome (BS) encompasses a group of rare genetic, autosomal recessive, renal tubular diseases characterized by urinary loss of sodium, potassium, and chloride; hypokalemic metabolic alkalosis; high plasma levels of renin and aldosterone; and high levels of prostaglandins (PGs) in blood and urine as a secondary phenomenon. Clinically patients present polyuria, polydipsia, failure to thrive, life-threatening episodes of dehydration, episodes of fever, and normal or low blood pressure. Frequently, pediatricians are the first professionals to attend to these patients and it is therefore important to be aware of this condition, since prognosis is better with earlier diagnosis and treatment. There are different types of BS, and clinical and laboratorial variability depends on the affected tubular carrier.1,2 According to the affected region, some differences can be observed in the management of the disease, for instance, type II BS is associated with very mild hypokalemia, whereas in type IV BS, treatment with indomethacin is much less effective.3
The present study aimed to describe the results of a long-term follow-up of BS patients treated with different drugs.
Patients and methodsThis retrospective study, based on a prospective protocol, enrolled patients with clinical and laboratorial diagnosis of BS from 1993 until 2012, and adherent to the treatment, which was evaluated by adherence to scheduled clinic appointments and serum bicarbonate and potassium levels. Genetic analysis is not available in this service.
Treatment protocolThe protocol was initially based on electrolytes supplementation (potassium and, in some cases, sodium), spironolactone, and the non-selective non-steroidal anti-inflammatory drug (nsNSAID), indomethacin. However, during the period of indomethacin treatment (1993 to 2003), six of 12 (50%) patients presented significant gastrointestinal symptoms;4 and since 2003, it was decided to adopt a selective NSAID (sNSAID), celecoxib, in order to avoid gastrointestinal compromise.
Patients who developed proteinuria were converted to an angiotensin conversion enzyme inhibitor (ACEi), enalapril, in replacement to NSAID. This conversion was made during hospitalization, since patients can potentially develop serious hypotension with ACEi.
VariablesThe following variables were evaluated during treatment with each drug: Z-score for weight and stature, glomerular filtration rate (GFR) through creatinine clearance (using Schwartz's formula, since urinary collection of 24hours is difficult, especially in polyuric patients),5 average potassium supplementation, and serum levels of potassium and bicarbonate. The presence of proteinuria, gastrointestinal (GI) complaints, and findings of upper digestive endoscopy (UDE) were also evaluated.
In this study, hyperfiltration was defined as creatinine clearance ≥ 145mL/min/1.73m2BS (mL/min/1,73 m2Body Surface).6
Statistical analysisData with homogeneous distribution were shown as mean and standard deviation. All others were shown as median and range. Student's t-test for paired samples was employed to compare variables with normal distribution, and the Wilcoxon test was used to compare non-normal distribution variables. The latter was also employed to compare the microalbuminuria levels before and after the substitution of celecoxib to enalapril. The chi-squared test was used to compare the findings in UDE in symptomatic patients during indomethacin and celecoxib use.
The study was approved by local ethics committee.
ResultsTwenty patients were included, of whom 12 were females. Follow-up time was 10.1 ± 5.2 years, age at diagnosis had a median of 17.5 months (3–178) and at last evaluation was 14.0 ± 5.3 years. Five patients were born from consanguineous parents and two patients were siblings. Seven patients, five of whom females, presented with polyhydramnio and/or prematurity, characteristics of neonatal BS. Neurosensorial deafness was observed in two patients (one female), and they are classified as BS with deafness. Eleven patients showed characteristics of classic BS. No patient presented with hypocalciuria, thus excluding the diagnosis of Gitelman syndrome.
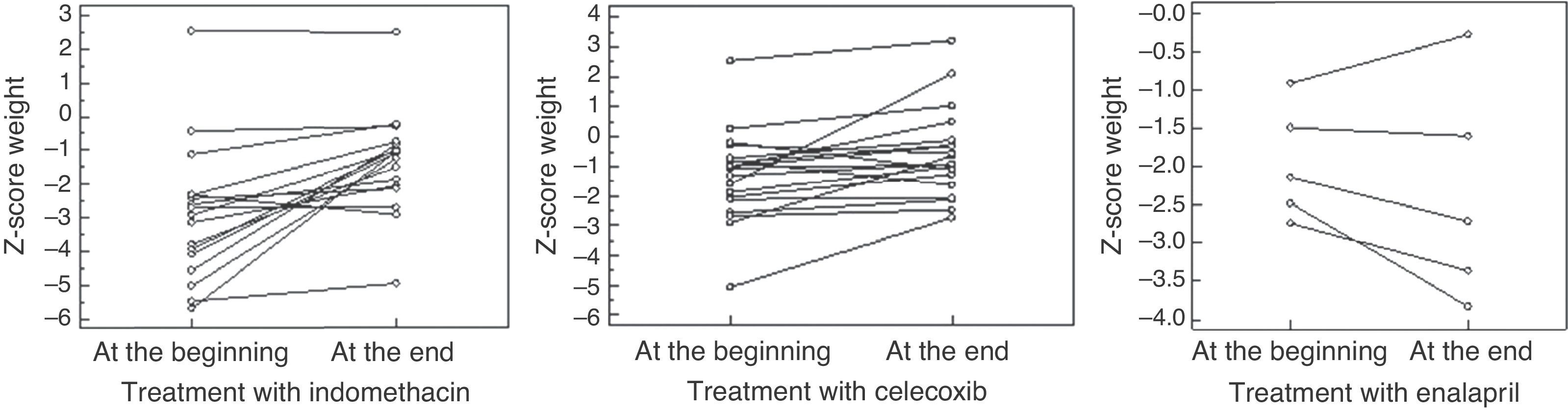
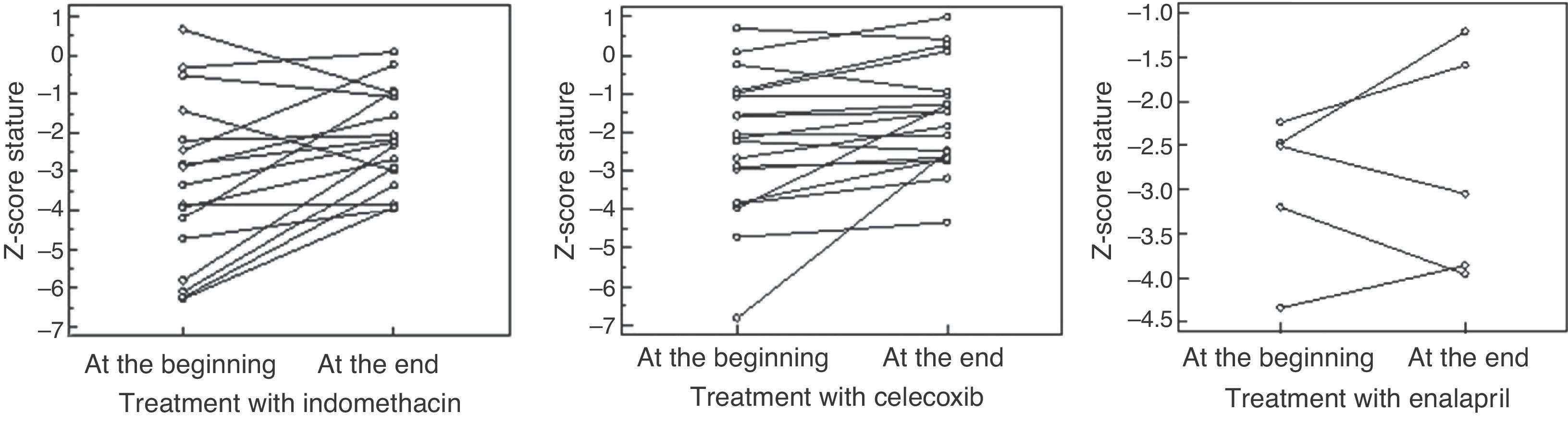
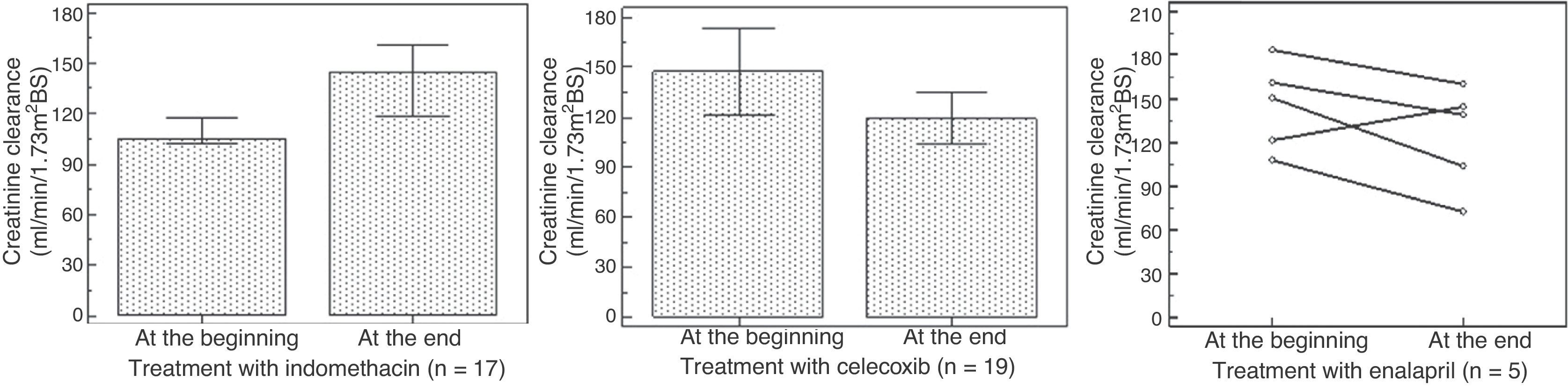
Period of indomethacin treatmentSeventeen patients received indomethacin for 5.9 ± 5.3 years in a dosage of 2.1 ± 0.6mg/Kg/day divided in three doses. An increase was observed in height-for-age Z-score, from -3.3 ± -2.1 to -2.2 ± 1.2 (p = 0.01), and in weight-for-age Z-score, from median = -2.9 (-5.7 – 2.5) to median = -1.05 (-4.9- 2.5) (p = 0.0004), without a significant change in the creatinine clearance, which varied from median = 105 (64-277) to median = 144 (71-279) mL/min/1.73m2BS (p = 0.34); and with metabolic and electrolyte stability. However, four patients had hyperfiltration at the beginning and eight presented hyperfiltration at the end of the treatment. Seven of 17 patients had GI symptoms, and UDE evidenced gastritis in three cases and gastric ulcer, a severe finding, in four.
Period of selective prostaglandin inhibitorNineteen patients received celecoxib, during a median of 35 months (8-144). An increase was observed in height-for-age Z-score from -2.4 ± -1.7 to 1.8 ±1.3 (p = 0.02) and in weight-for-age Z-score from -1.3 ± 1.5 to -0.81 ± 1.2 (p = 0.01), as well as a reduction in creatinine clearance from 147 ± 52 to 119 ± 31mL/min/1.73m2BS (p = 0.04). Nine patients presented with hyperfiltration at the beginning of treatment; at the end, hyperfiltration was detected in only two patients. Seven of 19 patients presented GI symptoms, but UDE evidenced mild gastritis in three cases and no case of ulcer.
Comparing indomethacin with celecoxib, positive findings in UDE were more present in indomethacin group, although not significant (p = 0.06); however, indomethacin was associated with more severe compromise. During the treatment with celecoxib, no patient developed gastric ulcer.
Period of ACEi treatmentFive patients received enalapril by median of 23 months (3-80) in a dosage of 0.2 ± 0.1mg/kg/d,ym divided in two doses, despite we have tried in 6; one patient presented severe hypotension and the drug was withdrawn. No significant changes were observed in height-for-age Z-score (median at the beginning of treatment = -2.5 [-4.3 to -2.2]; median at the end = -3.0 [-3.9 to -1.21]; p = 0.8), nor in the weight-for-age Z-score (median at the beginning of treatment = -2.1 [-2.7 to -0.9]; median at the end= -2.7 [-3.8 to -0.3]; p = 0.4). The creatinine clearance showed no statistically significant differences, from median 150 (107-183) to 138 (62-160) (p = 0.18); however, a decrease was observed in four patients, one of whom below 90mL/min/1.73m2BS. In this case, the dose of enalapril was reduced.
Fig. 1 presents the weight-for-age Z-score, Fig. 2 shows the height-for-age Z-score at the beginning and at the end of the treatment with each drug. Fig. 3 presents the creatinine clearance during the treatment with each drug.
Seven patients developed microalbuminuria during treatment with indomethacin; in five patients, the problem resolved when their treatment was converted to celecoxib; however, four patients developed microalbuminuria during the use of celecoxib. Patients with proteinuria had their treatment converted to ACEi, and presented a significant reduction: median was 76.2% (62.8%-80.6%).
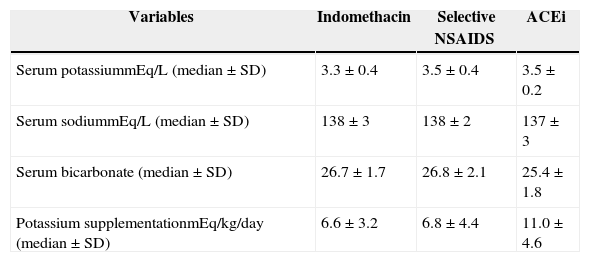
Table 1 presents the average of serum potassium, sodium, and bicarbonate levels of patients during each drug and the amount of potassium supplementation including data from each six months. No significant differences were observed in serum potassium, sodium, and bicarbonate levels during the use of the three drugs. No significant differences were observed between the amount of potassium supplementation during the use of indomethacin and celecoxib (p = 0.8). Although no significant differences were detected in the amount of potassium supplementation during indomethacin and ACEi (p = 0.09) and celecoxib and ACEi (p = 0.2), a tendency to supplement a larger amount of potassium during ACEi was observed, as seen in Table 1.
Serum potassium, sodium, bicarbonate, and potassium supplementation in 20 Bartter syndrome patients during different schedules of treatment.
| Variables | Indomethacin | Selective NSAIDS | ACEi |
|---|---|---|---|
| Serum potassiummEq/L (median ± SD) | 3.3 ± 0.4 | 3.5 ± 0.4 | 3.5 ± 0.2 |
| Serum sodiummEq/L (median ± SD) | 138 ± 3 | 138 ± 2 | 137 ± 3 |
| Serum bicarbonate (median ± SD) | 26.7 ± 1.7 | 26.8 ± 2.1 | 25.4 ± 1.8 |
| Potassium supplementationmEq/kg/day (median ± SD) | 6.6 ± 3.2 | 6.8 ± 4.4 | 11.0 ± 4.6 |
ACEi, angiotensin conversion enzyme inhibitor; NSAIDS, non-steroidal anti-inflammatory drugs; SD, standard deviation.
In BS, an over activation of renin-angiotensin-aldosterone system (RAAS) and an over production of PGs can be observed. Those findings are the result of sodium, chloride, and potassium urinary wasting. Therefore, the recommended treatment has been based on potassium supplementation, a PGs inhibitor;7,8 spironolactone1 is also an option, but with transient effect, and ACEi has been used in some studies.9
Indomethacin has been long employed in the treatment of these patients. Studies have demonstrated that with indomethacin, spironolactone, and potassium chloride supplementation and, sometimes, sodium chloride supplementation, patients experience improvement in growth speed, weight gain, and metabolic stability.10 However, there are significant GI effects resulting from inhibition of cyclooxygenase (Cox) 1.4 These data are in accordance with the present findings during the use of indomethacin.4 As an option, selective inhibitors of Cox (Cox-2 inhibitors more than Cox-1, such as celecoxib) have been employed, seeking a renal effect, with reduction of undesirable GI effects.11 The majority of studies have observed that sNSAIDs are associated with a lower risk of ulcers and complications than nsNSAIDs.12 In the present study, celecoxib promoted a better compliance to the treatment and a reduction in severe GI involvement (such as gastric ulcers), with metabolic and electrolyte stability and improvement in growth speed and weight gain.4 In addition, celecoxib was associated with a lower rate of hyperfiltration than indomethacin. Hyperfiltration is associated to focal glomerulosclerosis occurrence.
Thus, the use of celecoxib can be a good option for BS patients, although larger studies are needed in order to prove its safety and efficacy.
In the last decade, an increase in cardiovascular events has been observed during the use of COX-2 selective inhibitors. Those studies were made in patients with high cardiovascular risk, such as the elderly or those in use of acetylsalicylic acid. There are few long-term trials evaluating cardiovascular safety of celecoxib, but no conclusion can be drawn.13,14 The balance between the production of prostacyclin and thromboxane is thought to play a role in regulation of platelet aggregation and in vascular tone. BS is associated with overproduction of prostaglandins and thromboxane.15 It can thus be speculated that the side-effect on cardiovascular system is not a risk factor in BS patients. However, no such well-defined clinical trials have been conducted.
Long-term follow-up of patients under the use of sNSAID has demonstrated that alongside its beneficial effect, patients can develop proteinuria, which is an aggravating to the progression to end-stage renal disease.2 In this situatio,n an RAAS inhibitor can be used as a replacement drug to NSAID. Few reports have been published on the use of these drugs.16 Seyberth & Schlingmann2 stated that “only in the case of persistent hypokalemia (plasma potassium <3.0 mEq/L) that occurs despite adequate and tolerated inhibition of prostaglandin synthesis and salt and potassium supplementation, one might consider the use of drugs that interfere with the RAAS”. However, close monitoring of renal function and blood pressure is mandatory. This supplemental therapy might have an additional beneficial effect on proteinuria.17
In the present study, in patients who developed proteinuria during treatment with celecoxib, the replacement by enalapril provided electrolyte and metabolic balance with significant reduction of proteinuria. In addition, good tolerance and compliance were observed with this treatment. It is noteworthy that the administration of an inhibitor of RAAS in these patients can determine severe hypotension. Thus, the recommendation is that the transition from NSAID to a RAAS inhibitor should be performed in the hospital setting, under medical supervision.
NSAIDs, both indomethacin and celecoxib, are effective in treating BS. The latter has demonstrated benefits on severity of GI tract involvement and decreasing in hyperfiltration. However, the safety profile of celecoxib may, in the future, allow for its use as first drug for the treatment of BS. In patients who develop proteinuria, enalapril was effective in reducing it. Thus, it is suggested to start the treatment with celecoxib and if necessary replacing it by ACEi.
This study has some limitations, such as the small number of patients and lack of a genetic assessment. Randomized, larger and controlled studies are needed to confirm the present data. However, it is a rare disease, and the present study had one of the largest series in the literature.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Nascimento CL, Garcia CL, Schvartsman BG, Vaisbich MH. Treatment of Bartter syndrome. Unsolved issue. J Pediatr (Rio J). 2014;90:512–7.
Study conducted at Unidade de Nefrologia, Instituto da Criança, Hospital das Clínicas, Faculdade de Medicina, Universidade de São Paulo (HCFMUSP), São Paulo, SP, Brazil.