To assess the applicability of salivary C-reactive protein, mean platelet volume, neutrophil-lymphocyte ratio, and platelet lymphocyte ratio in the diagnosis of neonatal sepsis.
MethodsProspective case-control study of 70 full-term neonates, 35 with sepsis (20 with proven sepsis and 15 with clinical sepsis) and 35 healthy controls. Serum and salivary C-reactive protein concentrations were measured by enzyme-linked immunosorbent assay while mean platelet volume, neutrophil-lymphocyte ratio, and platelet lymphocyte ratio were measured by automated blood cell counter.
ResultsThis study showed statistically significant difference of mean salivary C-reactive protein between septic neonates and controls (12.0±4.6ng/L vs. 2.8±1.2ng/L) respectively. At a cut-off point of 3.48ng/L, salivary C-reactive protein showed 94.3% sensitivity and 80% specificity. Salivary C-reactive protein also showed good predictive accuracy for predicting elevated serum C-reactive protein values in septic neonates. Mean platelet volume and neutrophil-lymphocyte ratio showed significant difference between septic neonates and controls (10.2±1.2fL vs.8.0±0.5fL; 2.9±1.7 vs. 1.6±0.4, respectively). At a cut-off point of 10.2fL, mean platelet volume presented 80% sensitivity and specificity. At a cut-off point of 2.7, neutrophil-lymphocyte ratio presented 80% sensitivity and 57.1% specificity.
ConclusionThis study provides support for further studies on the usefulness of salivary C-reactive protein, mean platelet volume, and neutrophil-lymphocyte ratio as diagnostic markers for neonatal sepsis.
Avaliar a aplicabilidade da proteína C reativa (PCR) salivar, do volume médio de plaquetas (VMP), a proporção de neutrófilos-linfócitos (PNL) e a proporção de plaquetas/linfócitos (PPL) no diagnóstico de sepse neonatal.
MétodosEstudo caso-controle prospectivo de 70 neonatos a termo, 35 com sepse (20 com sepse comprovada e 15 com sepse clínica) e 35 controles saudáveis. As concentrações de PCR no soro e salivar foram medidas por ensaio imunossorvente ligado a enzima (ELISA), ao passo que o VMP, PNL e PPL foram medidos por contador de células sanguíneas automatizado.
ResultadosEste estudo mostrou uma diferença estatisticamente significativa da média de PCR salivar entre os neonatos com sepse e os controles (12,0±4,6ng/L em comparação a 2,8±1,2ng/L), respectivamente. Um ponto de corte 3,48ng/L na PCR salivar mostrou sensibilidade de 94,3% e especificidade de 80%. A PCR salivar mostrou, ainda, boa precisão preditiva para prever altos valores de PCR no soro em neonatos com sepse. O VMP e a PNL mostraram diferença significativa entre os neonatos com sepse e os controles (10,2±1,2fL em comparação a 8,0±0,5fL), (2,9±1,7 em comparação a 1,6±0,4), respectivamente. O VMP no ponto de corte 10,2fL apresentou 80% de sensibilidade e especificidade. A PNL no ponto de corte 2,7fL apresentou 80% de sensibilidade e 57,1% de especificidade.
ConclusãoEste estudo fornece uma base para outros estudos na utilidade da PCR salivar, VMP e PNL como marcadores de diagnóstico de sepse neonatal.
Sepsis is a serious life-threatening condition and a leading cause of morbidity and mortality in full and pre-term infants, especially in the developing countries. It is broadly defined as a systemic inflammatory response occurring in the first 28 days of life as a result of a suspected or proven infection.1
There is an urgent call for detecting reliable biomarkers to differentiate between infected and non-infected neonates. Blood culture has been considered as the gold standard for diagnosis, but this analysis is still slow and limited by false negative results.
C-reactive protein (CRP), a major acute phase protein, is a member of the pentraxin family and plays a central role in innate and adaptive immunity.2 Despite the development of new biomarkers, to date CRP is one of the most studied and most used laboratory tests for diagnosis of neonatal sepsis.3 CRP takes 10–12h to significantly rise after the onset of infection.4 As CRP shows an increase in several conditions, it is preferably used in combination with other biomarkers.3
A relevant problem in the current clinical approach for sick neonates is the limited availability of simple, safe, noninvasive diagnostic tools with high diagnostic accuracy. Neonatal saliva overcomes many of the hurdles associated with neonatal research and offers investigators a new, exciting, and non-invasive sample source for exploring neonatal biology. CRP is detectable in neonatal saliva and can predict abnormal serum CRP thresholds.5
Recently, certain parameters of complete blood count (CBC) were used as markers of inflammation and infections. Mean platelet volume (MPV) has been used in diagnosis, follow-up, and prediction of neonatal sepsis severity in term and preterm neonates.6–8 The neutrophil–lymphocyte ratio (NLR) is a simple biomarker of inflammation.9 In addition, platelet lymphocyte ratio (PLR) is also a useful marker of systemic inflammation.9,10
The present study aimed to assess the applicability of salivary CRP, MPV, NLR, and PLR as diagnostic markers in full-term neonates with neonatal sepsis.
MethodsThe present study was conducted at the neonatal intensive care unit (NICU) of the Suez Canal University Hospital, Ismailia, Egypt between January 2016 and June 2016. During these six months, 320 neonates were admitted to the unit. Thirty-five neonates with either early or late onset neonatal sepsis and 35 healthy control neonates were enrolled.
Full term infants of both genders from birth to the 28th day of life who were admitted to the Suez Canal University NICU with clinical features of either early- or late-onset neonatal sepsis were enrolled in this study. The diagnosis of clinical sepsis was made by history, clinical findings, laboratory findings, and blood culture. The clinical findings included the presence of three or more of the following: (1) temperature instability (hypothermia, hyperthermia); (2) respiratory alterations (grunting, intercostal retractions, apnea, tachypnea, cyanosis); (3) cardiovascular alterations (bradycardia, tachycardia, poor perfusion, hypotension); (4) neurologic alterations (hypotonia, lethargy, seizures); and (5) gastrointestinal alterations (feeding intolerance, abdominal distension). CRP values >10mg/L were considered to be positive.3,11
Preterm neonates and neonates with confirmed pneumonia or other inflammatory conditions, CNS malformations, metabolic disorders, chromosomal abnormalities, intrauterine growth restriction, or birth asphyxia were excluded from the study.
Thirty-five healthy neonates with no symptoms, signs, or risk factors for infections who had been hospitalized for physiological jaundice in the neonatal unit, and all neonates without infectious risk factors admitted to outpatient clinics of the hospital were prospectively enrolled as controls. Serum CRP level for neonates in this group was negative (CRP<10mg/L).
Samples were collected from septic neonates within 4–12h of clinically indicated serum CRP levels measurement and before starting antibiotic treatment.
For CBC measurement, peripheral blood was collected into an EDTA Vacutainer tube and analyzed by an automated blood cell counter (Cell-Dyn 3700, Abbott Laboratories, IL, USA). The NLR and PLR were calculated as the ratio of neutrophils to lymphocytes and platelets to lymphocytes respectively. Their levels were obtained from the same blood samples. For accurate MPV calculation, samples were analyzed within 60minutes after collection to avoid platelet swelling and false increase of MPV value.
For determination of serum CRP level, the fully automated auto-analyzer Cobas c501 (Roche Diagnostics, Manheim, Germany) was used. For salivary CRP, samples were collected nearly 1h before feeding to avoid milk contamination. Saliva was collected by tilting the head forward to pool saliva in the floor of the mouth. Samples were then obtained by using a 1-mL syringe attached to low wall suction (<20mm Hg), ensuring that the saliva remained in the syringe and did not enter into the tubing or suction trap. Saliva was suctioned from under the neonates’ tongues and in their gingival crevices. Suctioning was maintained for approximately 10–15s, collecting around 0.5mL. The syringe was then detached from suction and the plunger was placed back into the syringe.
After collection, samples were put in polypropylene tubes to avoid contamination and analytic retention faults. Samples were stored at −20°C until use. Salivary CRP was measured by ELISA. Blood cultures were performed for the septic group.
This study was approved by the local institutional ethics committee and an informed consent was obtained from the parents of all the neonates throughout the study.
Statistical analysesStatistical analyses were performed using SPSS for Windows statistical package, version 20 (SPSS, Chicago, IL, USA). The differences between groups regarding non-parametric quantitative data were assessed by Mann–Whitney's U-test. The chi-squared test was used for testing significant differences of qualitative variables. The sensitivity, specificity, and optimal salivary CRP cut-point were determined using the receiver operating characteristic (ROC) curve. For all statistical analysis, the level of statistical significance was set at p<0.05.
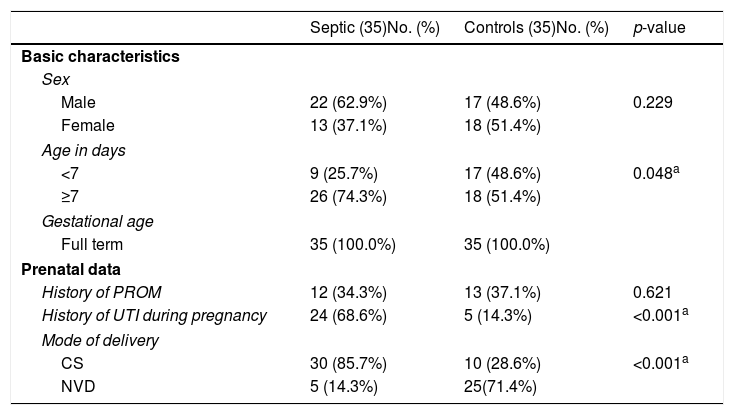
ResultsDemographic and prenatal dataStatistical differences were observed between the two groups regarding age, history of maternal urinary tract infection (UTI), and mode of delivery. Late-onset sepsis (LOS) was diagnosed in 74.3% of the septic neonates, while early onset sepsis (EOS) was diagnosed in only 25.7% of them (Table 1).
Demographic and prenatal data for each group.
| Septic (35)No. (%) | Controls (35)No. (%) | p-value | |
|---|---|---|---|
| Basic characteristics | |||
| Sex | |||
| Male | 22 (62.9%) | 17 (48.6%) | 0.229 |
| Female | 13 (37.1%) | 18 (51.4%) | |
| Age in days | |||
| <7 | 9 (25.7%) | 17 (48.6%) | 0.048a |
| ≥7 | 26 (74.3%) | 18 (51.4%) | |
| Gestational age | |||
| Full term | 35 (100.0%) | 35 (100.0%) | |
| Prenatal data | |||
| History of PROM | 12 (34.3%) | 13 (37.1%) | 0.621 |
| History of UTI during pregnancy | 24 (68.6%) | 5 (14.3%) | <0.001a |
| Mode of delivery | |||
| CS | 30 (85.7%) | 10 (28.6%) | <0.001a |
| NVD | 5 (14.3%) | 25(71.4%) | |
PROM, premature rupture of membrane; UTI, urinary tract infection; CS, cesarean section; NVD, normal vaginal delivery.
At time of presentation of septic neonates, 19 (54.3%) of them presented with poor activity and refusal of feeding, while nine (25.7%) presented with fever and poor activity, and seven (20%) presented with refusal of feeding in addition to respiratory distress. There were significant differences between the two groups in CBC indices. Anemia was observed in 12 (34.3%) septic neonates and in four (11.4%) controls. A difference was observed in white blood cell count between septic and control infants; leucocytosis was observed in 11 (31.4%) septic neonates and in one (2.9%) control. Thrombocytopenia was found in nine (25.7%) septic neonates and in one (2.9%) control.
In the septic group, blood culture was positive in 25 (71.5%). Coagulase-negative staphylococci were the most frequent isolated pathogens, followed by E. coli, Klebsiella pneumonia, and Serratia marcescens.
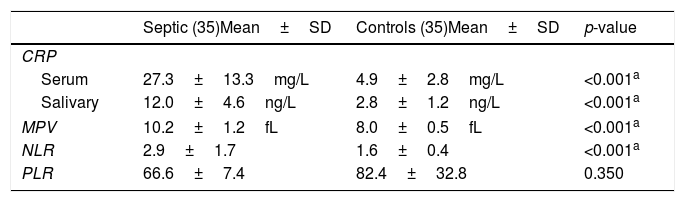
Serum CRP, salivary CRP, MPV, NLR, and PLR valuesThere were significant differences between both groups in serum CRP, salivary CRP, MPV, and NLR. Serum CRP values showed significant differences between both groups (mean 27.3±13.3mg/L vs. 4.9±2.8mg/L, respectively). The mean serum CRP for neonates with EOS was 23.2±11.3, while its mean in neonates with LOS was 29.4±13. Salivary CRP also showed a significant difference between both groups (mean 12.0±4.6ng/L vs.2.8±1.2ng/L, respectively). MPV showed a significant difference between both groups (mean 10.2±1.2fL vs.8.0±0.5fL, respectively). NLR also showed a significant difference between both groups (mean 2.9±1.7, vs.1.6±0.4, respectively). The difference in PLR between both groups was not significant (Table 2).
Serum CRP, salivary CRP, NLR, PLR, and MPV values.
| Septic (35)Mean±SD | Controls (35)Mean±SD | p-value | |
|---|---|---|---|
| CRP | |||
| Serum | 27.3±13.3mg/L | 4.9±2.8mg/L | <0.001a |
| Salivary | 12.0±4.6ng/L | 2.8±1.2ng/L | <0.001a |
| MPV | 10.2±1.2fL | 8.0±0.5fL | <0.001a |
| NLR | 2.9±1.7 | 1.6±0.4 | <0.001a |
| PLR | 66.6±7.4 | 82.4±32.8 | 0.350 |
CRP, C-reactive protein; NLR, neutrophil–lymphocyte ratio; PLR, platelet lymphocyte ratio; MPV, mean platelet volume.
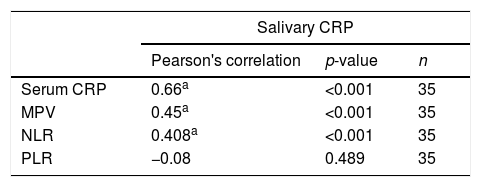
There were statistically significant correlations between salivary CRP and serum CRP (0.66, p<0.001), MPV (r=0.45, p<0.001), and NLR (r=0.408, p<0.001). The correlation between salivary CRP and PLR was not statistically significant (Table 3).
Correlation between salivary CRP and serum CRP, MPV, NLR, and PLR in the septic group.
| Salivary CRP | |||
|---|---|---|---|
| Pearson's correlation | p-value | n | |
| Serum CRP | 0.66a | <0.001 | 35 |
| MPV | 0.45a | <0.001 | 35 |
| NLR | 0.408a | <0.001 | 35 |
| PLR | −0.08 | 0.489 | 35 |
CRP, C-reactive protein; MPV, mean platelet volume; NLR, neutrophil–lymphocyte ratio; PLR, platelet lymphocyte ratio.
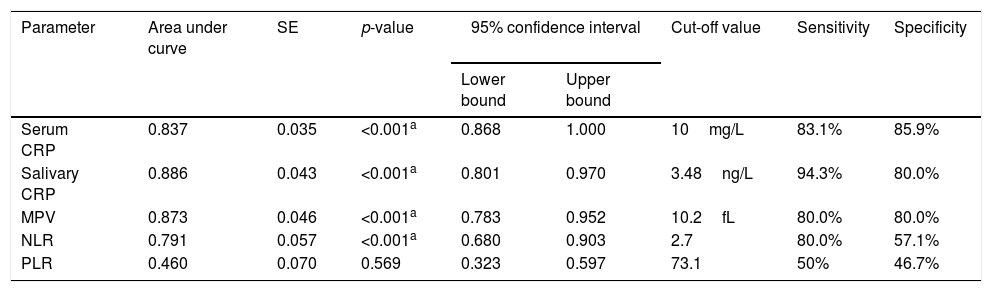
The analysis of the ROC curves showed that serum CRP, at a cut-off value of 10mg/L, had sensitivity of 83.1% and specificity of 85.9%. Salivary CRP, at a cut-off value of 3.48ng/L, had sensitivity of 94.3% and specificity of 80%. MPV showed 80% sensitivity and 80% specificity at a cut-off value of 10.2fL. NLR, at a cut-off value of 2.7, had sensitivity of 80% and specificity of 57.1%. PLR showed low sensitivity and specificity in predicting neonatal sepsis (Table 4).
ROC curves results for serum CRP, salivary CRP, MPV, NLR, and PLR.
| Parameter | Area under curve | SE | p-value | 95% confidence interval | Cut-off value | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||||
| Serum CRP | 0.837 | 0.035 | <0.001a | 0.868 | 1.000 | 10mg/L | 83.1% | 85.9% |
| Salivary CRP | 0.886 | 0.043 | <0.001a | 0.801 | 0.970 | 3.48ng/L | 94.3% | 80.0% |
| MPV | 0.873 | 0.046 | <0.001a | 0.783 | 0.952 | 10.2fL | 80.0% | 80.0% |
| NLR | 0.791 | 0.057 | <0.001a | 0.680 | 0.903 | 2.7 | 80.0% | 57.1% |
| PLR | 0.460 | 0.070 | 0.569 | 0.323 | 0.597 | 73.1 | 50% | 46.7% |
ROC, receiver operating characteristic; CRP, C-reactive protein; MPV, mean platelet volume; NLR, neutrophil–lymphocyte ratio; PLR, platelet lymphocyte ratio; SE, standard error.
Salivary CRP at a cut-off value of 3.4ng/L showed sensitivity of 94% and specificity of 91.3% in predicting serum CRP ≥10mg/L in septic neonates.
DiscussionNeonatal sepsis is a major cause of morbidity and mortality in neonatal intensive care units.12 Furthermore, it has long-term morbidities.13,14 In turn, simple practices, such as the use of hand sanitizer and training in hand hygiene could reduce LOS.15
Worldwide, infections account for two-thirds of the 6 million annual deaths in children younger than 5 years. The neonatal period has the highest lifetime risk of serious infections, with an estimated 400,000 newborn deaths annually.16 In Egypt, rates of sepsis exceed 50% in neonatal intensive care units.17
Recently, analysis of saliva for clinical monitoring and biomarker detection in neonates holds great promise for improving the healthcare of this fragile population. Saliva represents an ideal and easily accessible bio-fluid for non-invasive screening of common neonatal morbidities, such as prematurity, infections, and brain insults.18–20
To date, CRP is the most extensively studied acute-phase reactant; despite the detection of new markers of infection, its wide availability and its fast, simple, and cost-effective determination makes it one of the preferred indices in many NICUs.
The literature, serum CRP showed different kinetics and cut-off values ranging from 1.5 to 20mg/L, with wide-ranging sensitivities and specificities (from 74% to 98% and from 71% to 94%, respectively) for either serial CRP determinations or a single measurement at least 12h after the onset of neonatal sepsis symptoms.3,11,21–23
These differences in cut-off values can be explained by different gestational ages, birth weights, and the physiologic kinetics of CRP after birth in the studied neonates.3 There is a debate about the cut-off point for CRP in cases of EOS and LOS, with some agreement about the use of 10mg/L as a reliable cut-off, especially in EOS.3,11 In the present study, the mean serum CRP was 27.3±13.3mg/L in septic neonates, with 83.1% sensitivity and 85.9% specificity at a cut off-value of 10mg/L.
Serial measurements of CRP level may also be useful for identification of neonates with bacterial infection, monitoring the response to treatment in infected neonates and determining the duration of antibiotic therapy.21,24 Nonetheless, serial CRP monitoring in the neonatal population puts these patients in medical risks from frequent sampling.
Salivary CRP detection is a new method for diagnosis in neonatal population. In 2014, Iyengar et al. published the first article about detection and utility of salivary CRP in neonates, which was considered the first study to detect, quantify, and demonstrate that salivary CRP is a good index for clinically relevant serum CRP thresholds.5
To the best of the authors’ knowledge, this was the first study to investigate the role of salivary CRP as non-invasive diagnostic marker in septic neonates. The difference in mean salivary CRP between septic neonates and controls was statistically significant, with mean of 12.0±4.6ng/L in septic neonates versus 2.8±1.2ng/L in controls. At a cut-off point of 3.48ng/L, salivary CRP showed high sensitivity (94.3%) and specificity (80.0%). In the present study, salivary CRP showed good accuracy in predicting high serum CRP levels. It was observed that salivary CRP, at a cut-off point of 3.4ng/L, had a corresponding sensitivity (94%) and specificity (91.3%) in accurately predicting a serum CRP level of ≥10mg/L.
Iyengar et al. showed that raw salivary CRP concentration of 4.84ng/L had a sensitivity and specificity of 64% and 94%, respectively, for accurately predicting a serum CRP level of 5mg/L, and a sensitivity and specificity of 54% and 95%, respectively, for accurately predicting a serum CRP of 10mg/L.5 This discrepancy could return to the nature of disease under investigation.
CBC is a simple and routine workup to determine infection in neonates. Low white blood cell count, absolute neutrophil count, and high immature-to-total neutrophil ratio were the most commonly used indices for detection of infection in neonates.25
Recently, other CBC indices, such as MPV, NLR, and PLR, have been used as markers of systemic inflammation in children and adults. In neonates, very limited data are available about the usage of NLR and PLR for diagnosis of neonatal infection or other inflammatory conditions.
In the present study, MPV values showed a significant difference between septic neonates and controls, with a mean of 9.5±1.2fL and 8.0±0.5fL, respectively, and showed a significant correlation with serum and salivary CRP. Oncel et al. also reported a higher mean MPV in septic neonates when compared with controls(8.82±0.8fL and 8.44±0.5fL, respectively).6
MPV was able to predict diseased neonates and demonstrated diagnostic accuracy with 80% sensitivity and specificity at a cut-off point of 10.2fL. Aydın et al. reported that the diagnostic cut-off value for MPV in neonates with sepsis, 10.4fL, presented sensitivity of 54% and specificity of 82%.26 Recently, Yao et al. found that optimal cut-off point of MPV for the diagnosis of sepsis was 11.4fL, with sensitivity of 40.5% and specificity of 88.4%.27
NLR, which was recently considered as a new indicative marker of systemic inflammation, is simple and easy to calculate using routine laboratory data without an additional technique or cost.
To the best of the authors’ knowledge, this study was the first report of a statistically significant difference in mean NLR between septic neonates and controls (2.9±1.7 vs. 1.6±0.4, respectively) that also showed a good statistical significance in predicting and differentiating between septic neonates and controls with 80% sensitivity and 57.1% specificity at a cut-off point of 2.7. With regard to infections, NLR has been reported as a predictor of the severity and clinical outcome in patients with community- acquired pneumonia28 and bacteremia,29 being able to differentiate between bacterial and viral meningitis.30
PLR is a blood ratio that is largely used in chronic diseases and malignancies in adults. In the present study, PLR showed no significant accuracy in predicting septic neonates with low sensitivity (50%) and specificity (46.7%) at a cut-off point of 73.1.
The main limitations of the present study are the relatively small number of septic neonates, the small percentage of neonates with EOS, and the use of the same cut-off value for serum CRP in neonates with EOS and LOS.
In conclusion, this study provides initial evidence for the usefulness of salivary CPR, MPV, and NLR to be combined with other markers in the diagnosis of neonatal sepsis. However, these findings still need to be confirmed by other future studies aiming to examine larger numbers of neonates and different categories of infections using rigorous methods, in order to fully establish the role of salivary CRP and other tested markers in diagnosis of neonatal sepsis.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Omran A, Maaroof A, Mohammad MH, Abdelwahab A. Salivary C-reactive protein, mean platelet volume and neutrophil lymphocyte ratio as diagnostic markers for neonatal sepsis. J Pediatr (Rio J). 2018;94:82–7.