To assess the performance of cerebrospinal fluid (CSF) lactate as a biomarker to differentiate bacterial meningitis from viral meningitis in children, and to define an optimal CSF lactate concentration that can be called significant for the differentiation.
MethodsChildren with clinical findings compatible with meningitis were studied. CSF lactate and other conventional CSF parameters were recorded.
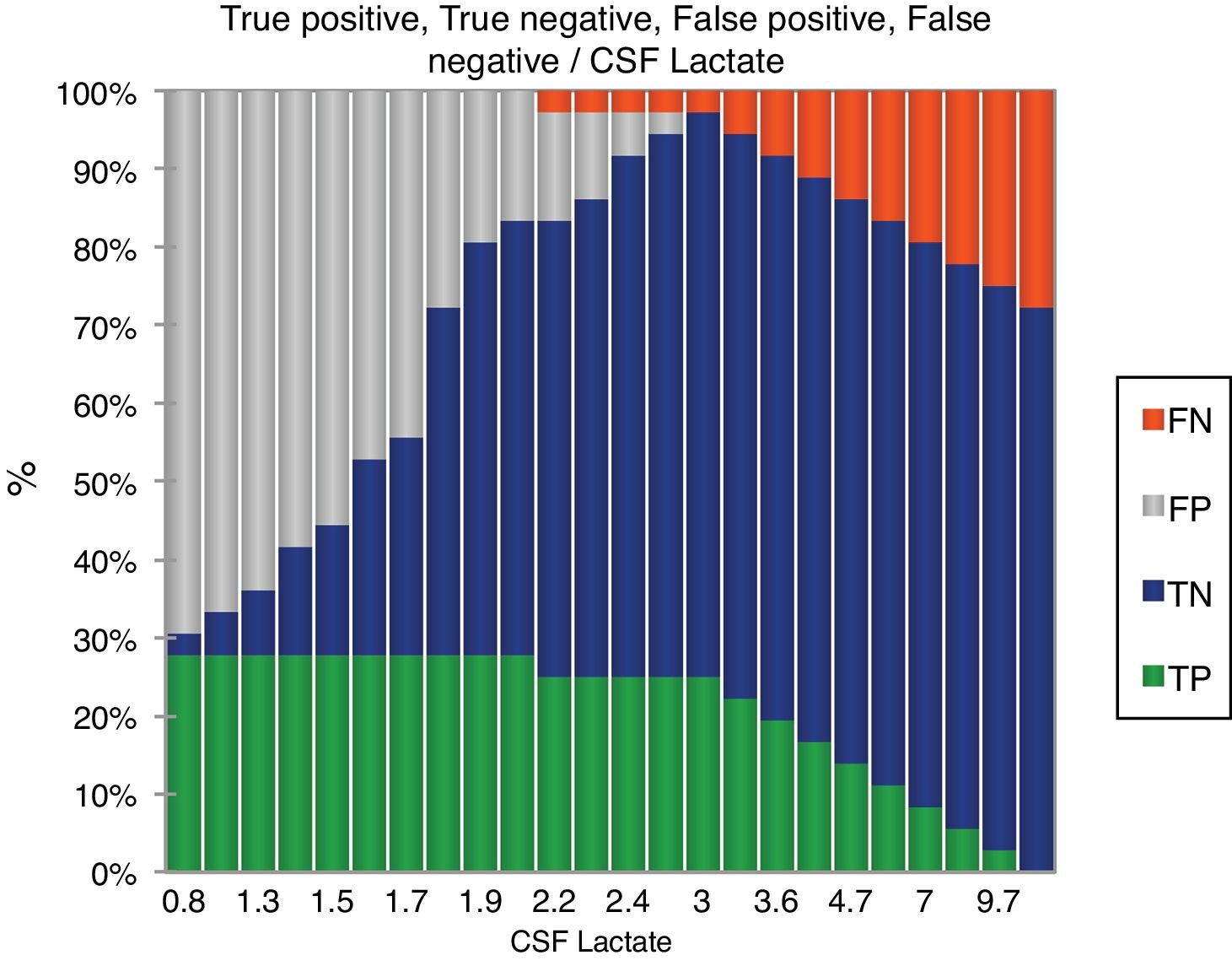
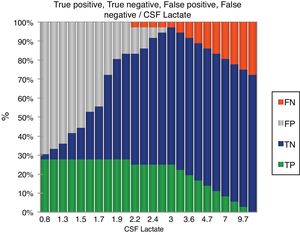
ResultsAt a cut-off value of 3mmol/L, CSF lactate had a sensitivity of 0.90, specificity of 1.0, positive predictive value of 1.0, and negative predictive value of 0.963, with an accuracy of 0.972. The positive and negative likelihood ratios were 23.6 and 0.1, respectively. When comparing between bacterial and viral meningitis, the area under the curve for CSF lactate was 0.979.
ConclusionsThe authors concluded that CSF lactate has high sensitivity and specificity in differentiating bacterial from viral meningitis. While at a cut-off value of 3mmol/L, CSF lactate has high diagnostic accuracy for bacterial meningitis, mean levels in viral meningitis remain essentially below 2mmol/L.
Estudar o desempenho do lactato no líquido cefalorraquidiano como biomarcador para diferenciar a meningite bacteriana da meningite viral em crianças, e definir uma concentração de lactato ótima no líquido cefalorraquidiano que possa ser significativa para a diferenciação.
MétodosForam estudadas crianças com achados clínicos compatíveis com meningite. O nível de lactato no líquido cefalorraquidiano e outros parâmetros convencionais do líquido cefalorraquidiano foram registrados.
ResultadosEm um valor de corte de 3 mmol/L, o lactato no líquido cefalorraquidiano apresentou uma sensibilidade de 0,90, especificidade de 1,0, valor preditivo positivo de 1,0, valor preditivo negativo de 0,963, com uma precisão de 0,972. Os índices de probabilidade positivo e negativo foram 23,6 e 0,1, respectivamente. Para comparação entre a meningite bacteriana e viral, a área abaixo da curva do lactato no líquido cefalorraquidiano foi 0,979.
ConclusõesConcluímos que o lactato no líquido cefalorraquidiano possui alta sensibilidade e especificidade na diferenciação da meningite bacteriana da meningite viral. Embora em um valor de corte de 3 mmol/L o lactato no líquido cefalorraquidiano possua alta precisão de diagnóstico da meningite bacteriana, os níveis médios na meningite viral permanecem basicamente abaixo de 2 mmol/L.
Cases of acute bacterial meningitis (BM) require prompt diagnosis and treatment due to significant mortality rates.1,2 A delay in starting appropriate therapy may worsen the prognosis.1 While BM causes significant morbidity and mortality despite advances in antibiotic therapy, aseptic meningitis is essentially a benign condition requiring only supportive care.3 Therefore, rapid differentiation between BM and aseptic meningitis is important to allow early initiation of appropriate therapy. Despite the availability of vaccines against prevalent organisms, BM continues to be a health problem with long-term sequelae in children and adults, especially in low-income countries.4,5
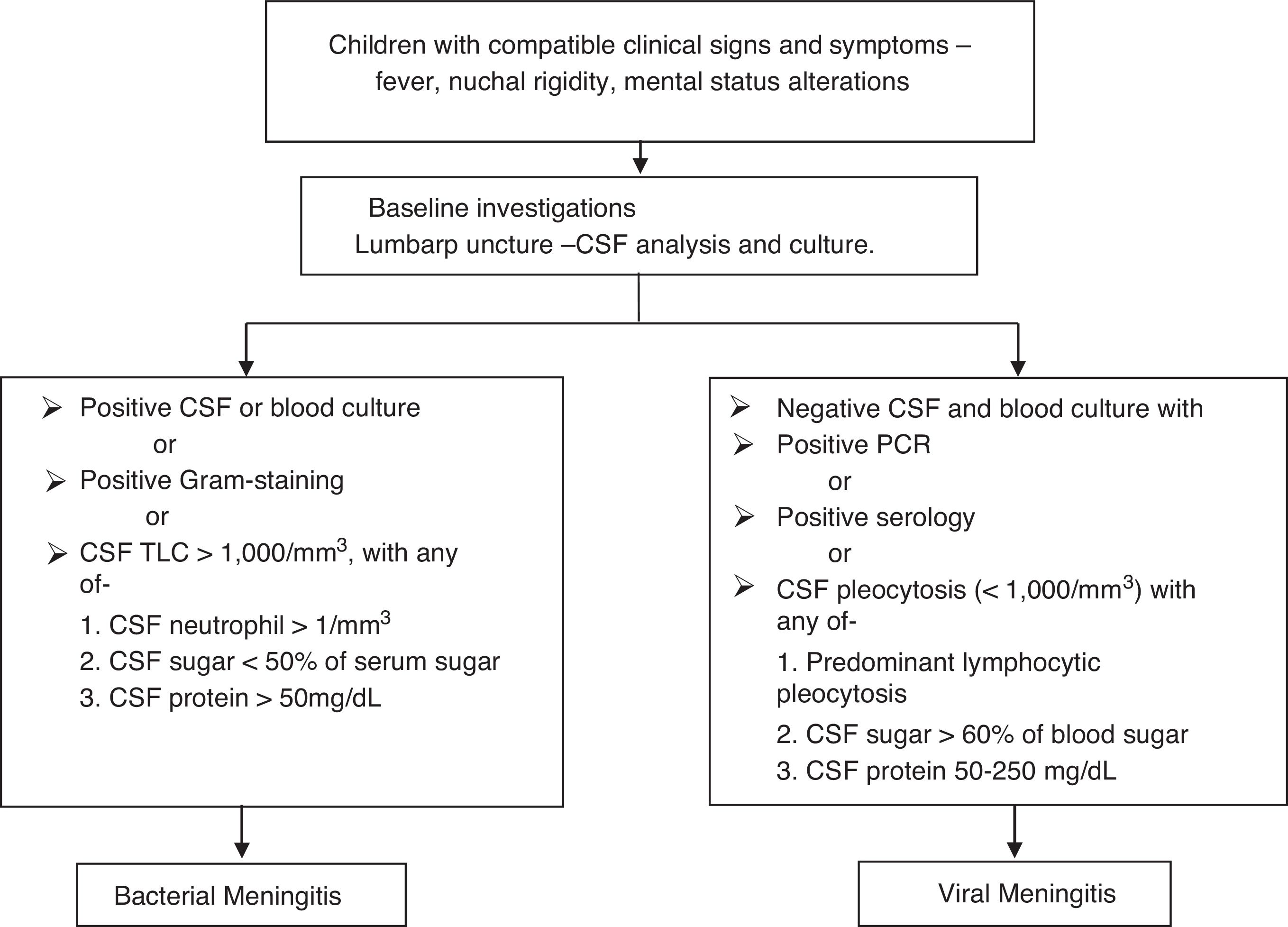
Although culture remains the gold standard for diagnosis, the results are only available after several days.6 Rapid diagnosis is carried out through assessment of conventional markers in cerebrospinal fluid (CSF): leukocyte counts, sugar, protein, and Gram-staining.7 However, meningitis sometimes presents with atypical CSF manifestations and cultures may not always be positive or available for early diagnosis.8 In recent years, it has been proposed that CSF lactate may be a good marker that can differentiate BM from partially treated meningitis and aseptic meningitis.9 However, other researchers have suggested that CSF lactate offers no additional clinically useful information over conventional CSF markers.10,11 The reported diagnostic accuracy of CSF lactate for the differential diagnosis of BM from aseptic meningitis has varied across studies.10,11 This prospective study aimed to evaluate the performance of CSF lactate as a biomarker to differentiate between BM and viral meningitis (VM) in children and to determine an optimal CSF lactate level that can be called significant for the differentiation.
MethodsThis was a prospective study including children with meningitis between 1 month and 15 years of age, who presented to the emergency department of the SKIMS hospital (Sher-I-Kashmir Institute of Medical Sciences), Kashmir, from January 2014 to December 2015. The study included children with clinical findings consistent with meningitis (e.g., fever, headache, vomiting, nuchal rigidity, and impaired consciousness). Blood samples were drawn and a lumbar puncture was performed after initial clinical assessment. Biochemical and cytological examinations of CSF samples were performed, including the measurement of leukocyte counts, neutrophil counts, glucose level, and protein and lactate concentration. CSF culture for BM, CSF polymerase chain reaction (PCR) for herpes simplex virus, and serology for viral meningitis were made. In blood samples collected at the same time, serum leukocyte count, serum glucose, and blood culture were measured. The exclusion criteria comprised children with any of the following: critical illness, recent neurosurgical intervention, trauma, any non-meningitic focus of infection, and those who had received antibiotics prior to hospitalization.
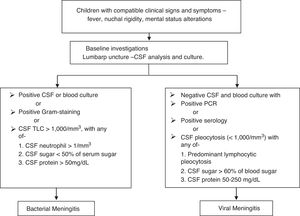
Fig. 1 presents the definitions of meningitis. In the absence of proven viral etiology, meningitis was considered to be viral if a cure was achieved without any antibiotic treatment, apart from antiviral therapy.
The following data were recorded: demographics (age, gender, weight), total number of patients admitted, number of children excluded and the reasons, medical history, clinical findings, and the results of the tests performed. The study was approved by the hospital's ethical committee. A written informed consent was obtained from all parents/guardians.
SPSS (SPSS Statistics for Windows, Version 20.0, NY, USA) and XLSTAT 2016 (Microsoft® Excel/XLSTAT© 2016, Addinsoft, Inc., Brooklyn, NY, USA) were used for statistical analysis. The results were expressed as means and 95% CI. Mean values were compared using Mann–Whitney's nonparametric test, and the threshold of statistical significance was set at p<0.05. Diagnostic efficiency of different CSF lactate was expressed as sensitivity, specificity, positive predictive value, and negative predictive value. The discriminative power of the various parameters studied was determined using receiver operating characteristic (ROC) curves.12
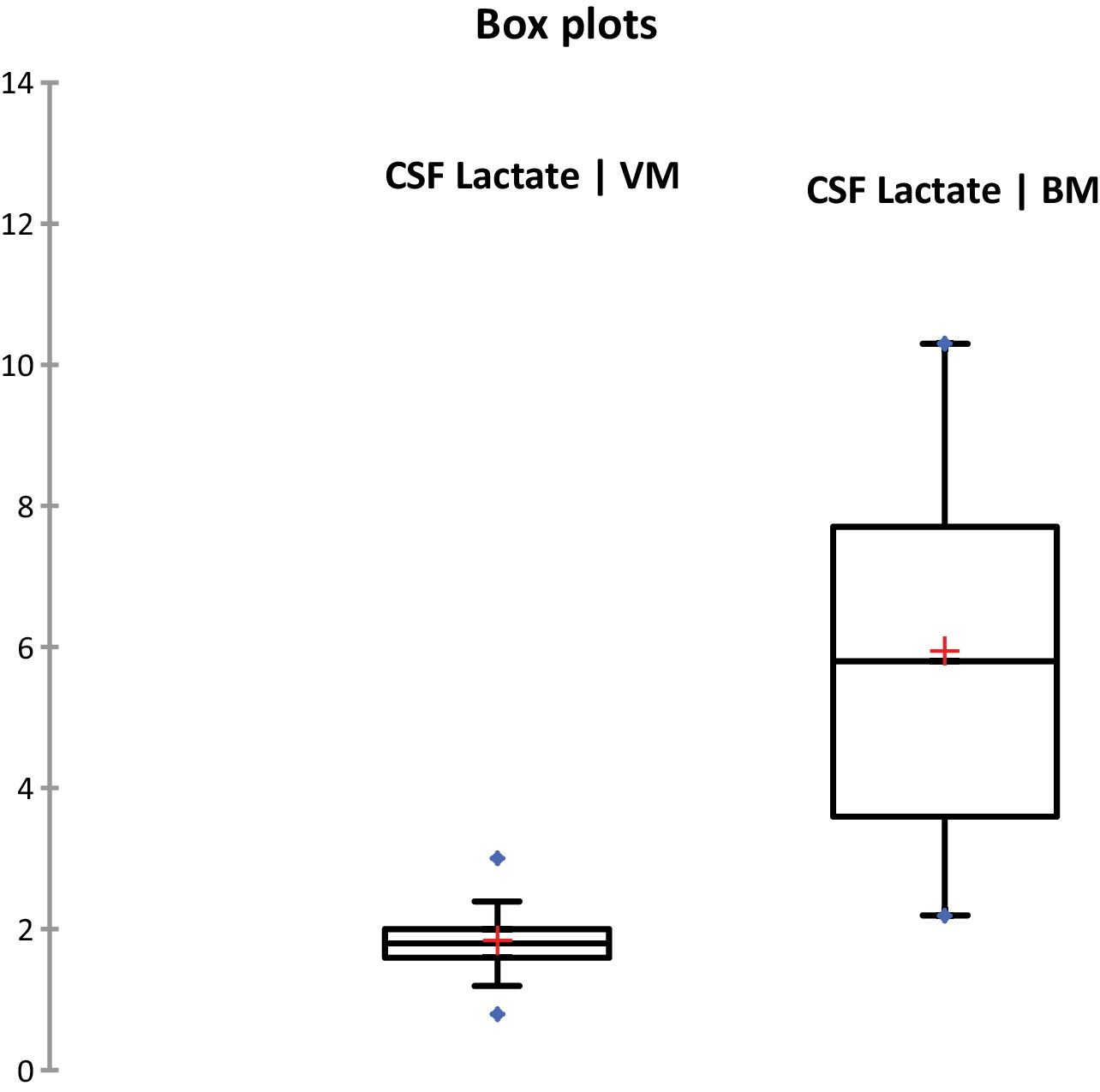
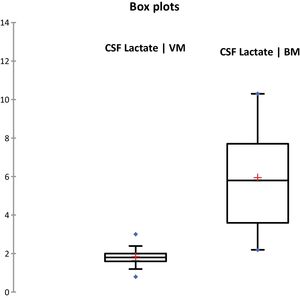
ResultsDuring the study period, 378 patients qualified for a lumbar puncture. BM was diagnosed in 60 (15.8%) patients, and 156 (41.3%) patients met the criteria for viral meningitis. The group of 162 (42.8%) patients who did not fit in either of the groups was excluded as not having meningitis. Both the groups were comparable regarding baseline characteristics (Table 1). CSF culture was positive in 19 (31.7%) patients and blood culture yielded positive results in nine (15%) patients. The following bacteria were identified in CSF cultures: Streptococcus pneumoniae (12), Haemophilus influenza (1), Klebsiella pneumoniae (4), and Escherichia coli (2). The following bacteria were identified in blood culture: S. pneumoniae (5), K. pneumoniae (3), and E. coli (1). Gram-staining was positive in 16 (26.7%) patients with BM. Among the 156 patients with VM, herpes meningitis was identified in six patients through PCR. The CSF levels of different parameters are summarized in Table 1. Fig. 2 shows the diagnostic efficiency of CSF lactate, and Fig. 3 shows the box-plot comparison of CSF lactate in BM and VM. At a cut-off value of 3mmol/L, CSF lactate had a sensitivity of 0.90, specificity of 1.0, positive predictive value of 1.0, and negative predictive value of 0.963, with an accuracy of 0.972. In the comparison between BM and VM, the area under the curve (AUC) for CSF lactate was 0.979. The mean (95% CI) CSF lactate in patients with positive and negative blood and/or CSF culture were 6.22 (5.05–7.39) and 5.84 (4.90–6.79)mmol/L, respectively (p=0.24). No statistically significant difference was observed in CSF lactate concentrations between patients with Gram-positive and Gram-negative BM (6.91[5.22–8.61] vs. 5.05 [3.65–6.45]mmol/L; p=0.116).
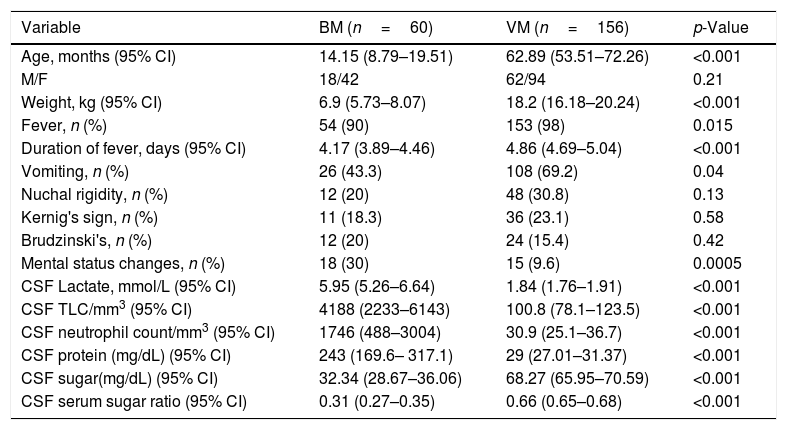
Demographic, clinical and CSF characteristics of the patients.
| Variable | BM (n=60) | VM (n=156) | p-Value |
|---|---|---|---|
| Age, months (95% CI) | 14.15 (8.79–19.51) | 62.89 (53.51–72.26) | <0.001 |
| M/F | 18/42 | 62/94 | 0.21 |
| Weight, kg (95% CI) | 6.9 (5.73–8.07) | 18.2 (16.18–20.24) | <0.001 |
| Fever, n (%) | 54 (90) | 153 (98) | 0.015 |
| Duration of fever, days (95% CI) | 4.17 (3.89–4.46) | 4.86 (4.69–5.04) | <0.001 |
| Vomiting, n (%) | 26 (43.3) | 108 (69.2) | 0.04 |
| Nuchal rigidity, n (%) | 12 (20) | 48 (30.8) | 0.13 |
| Kernig's sign, n (%) | 11 (18.3) | 36 (23.1) | 0.58 |
| Brudzinski's, n (%) | 12 (20) | 24 (15.4) | 0.42 |
| Mental status changes, n (%) | 18 (30) | 15 (9.6) | 0.0005 |
| CSF Lactate, mmol/L (95% CI) | 5.95 (5.26–6.64) | 1.84 (1.76–1.91) | <0.001 |
| CSF TLC/mm3 (95% CI) | 4188 (2233–6143) | 100.8 (78.1–123.5) | <0.001 |
| CSF neutrophil count/mm3 (95% CI) | 1746 (488–3004) | 30.9 (25.1–36.7) | <0.001 |
| CSF protein (mg/dL) (95% CI) | 243 (169.6– 317.1) | 29 (27.01–31.37) | <0.001 |
| CSF sugar(mg/dL) (95% CI) | 32.34 (28.67–36.06) | 68.27 (65.95–70.59) | <0.001 |
| CSF serum sugar ratio (95% CI) | 0.31 (0.27–0.35) | 0.66 (0.65–0.68) | <0.001 |
BM, bacterial meningitis; VM, viral meningitis; CSF, cerebrospinal fluid; TLC, total leukocyte count; CI, confidence interval.
The neurological outcomes of BM are often poor, making the early diagnosis and treatment important.13 Fortunately, BM is less common than aseptic meningitis; in one series of 3295 children with pleocytosis, only 4% had BM.14 The incidence of BM in the present study was 15.8%. The mean age of patients in BM group was significantly lower than those in VM group. In other words, BM in the present study was more prevalent in lower age ranges. This observation is in agreement with a surveillance study conducted in Louisville, Kentucky,15 which concluded that patients most affected with pneumococcal BM are those younger than 2 years; pneumococci were also the most common etiological organisms in the present study.
Nuchal rigidity, fever, and altered mental state are among the most commonly reported signs and symptoms in adults with BM,8 although one or more of these signs and symptoms is commonly absent.16,17 The authors reported fever (90%) as the most commonly presenting symptom, followed by recurrent vomiting (43.3%) and mental status alterations (30%). Nuchal rigidity (20%) and Brudzinski's signs (20%) were the most common presenting signs. In the present study, the classical triad was observed only in 20% of patients with BM. van de Beek et al. reported that all the three features were present in only 44% of 696 adults with proven BM,18 but the absence of all three excluded the diagnosis, with a sensitivity of 99%. Berkley et al. observed that 50% to 90% of patients with BM reported neck stiffness.19 Thomas et al. further concluded that the poor diagnostic value of neck stiffness is not improved by the presence of Kernig's or Brudzinski's signs, because neither has a sensitivity of more than 10%.16
The present results showed that, overall, clinical history and examination have a low diagnostic accuracy when used alone. This observation is in agreement with the findings of earlier studies in children and adults.20,21 Therefore, the onus of final diagnosis lies on CSF examination and bacterial isolation through cultures, in a clinically compatible case. Nigrovic et al. reported that the combined assessment of history, CSF microscopy, and CSF biochemistry had a sensitivity of 100% and a specificity of 66% in differentiating between BM and VM in children.14 However, the atypical manifestation of CSF examination, including culture negative and negative Gram-staining, can result in a missed diagnosis of BM. Studies in adults have indicated that adding CSF lactate to routine CSF examination is better in estimating the chance of BM in a very short time.21,22
The mechanism of the increase in concentration of lactate in the CSF of patients with meningitis is not clear, but it has been linked with anaerobic glycolysis of brain tissue due to a decrease cerebral blood flow and oxygen uptake.23 In the present study, a statistically significant increase in CSF lactate was observed in patients with BM, when compared to those with VM. At a cut-off value of 3mmol/L, CSF lactate had a high sensitivity, specificity, and accuracy in distinguishing BM from VM. The cut-off values studied for CSF lactate concentration ranged from 2.1 to 4.44mmol/L, in different studies in adults and children.22,24 Although the epidemiology of BM differs by age,25 the diagnostic value of CSF lactate is similar between children and adults.21 Huy et al., in a systemic review on assessment of CSF lactate concentration to distinguish BM from aseptic meningitis, reported a sensitivity ranging from 0.86 to 1.00 (mean: 0.96; 95% CI: 0.95–0.98), and a specificity that varied widely from 0.43 to 1.00 (mean: 0.94; 95% CI: 0.93–0.96). The mean positive likelihood ratio (LR+) was calculated at 14.53 (95% CI; 8.07–26.19), and the mean negative likelihood ratio (LR−), at 0.07 (95% CI: 0.05–0.09).22
In the present study, the AUC of CSF lactate concentration was 0.979, indicating an excellent26 level of overall accuracy. This observation was in good agreement with previous literature, with observed AUCs ranging from 0.977 to 0.988.22,27 The positive and negative likelihood ratios were 23.6 and 0.1, respectively. Sakushima et al., in their systematic review, found that CSF lactate had LR+ of 22.9 (95% CI: 12.6–41.9), LR− of 0.07 (95% CI: 0.05–0.12), and diagnostic odds ratio of 313 (95% CI: 141–698). They concluded that the very low LR− indicated that lack of CSF lactate is particularly good for discarding BM.21
In the present study, no significant differences were observed in CSF lactate in patients with Gram-positive or Gram-negative BM. Since the CSF lactate concentration is neither specific for BM nor for any specific bacteria in patients with BM, the results should always be interpreted in line with clinical findings and the results of conventional assays, including CSF concentrations of protein, cells, and glucose, as well as a microbiological CSF.28 Furthermore, CSF lactate cannot be used for antibiotic selection, which must be based on the results of microscopic smear examination and/or culture for bacteria.
The present study had a number of limitations. Only a single measurement of lactate was made, upon hospital admission; repeat assessments to monitor treatment and response were not performed. Furthered, the results were not compared with conventional serum markers and CSF biomarkers (such as CRP).
It can be concluded that CSF lactate has high sensitivity and specificity in differentiating BM from VM. While at a cut-off value of 3mmol/L, CSF lactate has high diagnostic accuracy for BM, mean levels in VM remain essentially below 2mmol/L. Further research including cost-effectiveness studies should be performed to investigate the efficiency of CSF lactate as a diagnostic marker of BM and to evaluate the economic impact of using this technique as a routine assay in hospital settings to distinguish BM from VM.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Nazir M, Wani WA, Malik MA, Mir MR, Ashraf Y, Kawoosa K, et al. Cerebrospinal fluid lactate: a differential biomarker for bacterial and viral meningitis in children. J Pediatr (Rio J). 2018;94:88–92.