The aim of this study was to systematically review the literature and answer the following central question: “What are the risk factors associated with worse clinical outcomes of pediatric bacterial meningitis patients?”
MethodsThe articles were obtained through literary search using electronic bibliographic databases: Web of Science, Scopus, MEDLINE, and LILACS; they were selected using the international guideline outlined by the Preferred Reporting Items for Systematic Reviews and Meta-analysis Protocols.
ResultsThe literature search identified 1,244 articles. After methodological screening, 17 studies were eligible for this systematic review. A total of 9,581 patients aged between 0 days and 18 years were evaluated in the included studies, and several plausible and important prognostic factors are proposed for prediction of poor outcomes after bacterial meningitis in childhood. Late diagnosis reduces the chances for a better evolution and reinforces the importance of a high diagnostic suspicion of meningitis, especially in febrile pictures with nonspecific symptomatology. S. pneumoniae as a causative pathogen was demonstrated to be related to clinical severity.
ConclusionsEarly prediction of an adverse outcome may help determine which children require more intensive or longer follow-up and may provide the physician with rationale for parental counseling about their child's prognosis in an early phase of the disease.
O objetivo deste estudo é revisar sistematicamente a literatura e responder à seguinte questão central: “Quais são os fatores de risco associados a piores desfechos clínicos de pacientes pediátricos com meningite bacteriana?”.
MétodosOs artigos foram obtidos através de pesquisa bibliográfica, nas bases de dados eletrônicas Web of Science, Scopus, Medline e Lilacs, e selecionados com diretriz internacional delineada pela abordagem Prisma (Preferred Reporting Items for Systematic Reviews and Meta-Analysis).
ResultadosA pesquisa bibliográfica identificou 1.244 artigos. Após a triagem metodológica, 17 estudos foram considerados elegíveis para esta revisão sistemática. Foram avaliados 9.581 pacientes até 18 anos nos estudos incluídos e vários fatores prognósticos plausíveis e importantes são propostos para a previsão de desfechos piores após meningite bacteriana na infância. O diagnóstico tardio reduz as chances de uma melhor evolução e reforça a importância de uma alta suspeita diagnóstica de meningite, especialmente em quadros febris com sintomatologia inespecífica. S. pneumoniae como patógeno causador demonstrou estar relacionado à gravidade clínica.
ConclusõesA previsão precoce de um desfecho clínico desfavorável pode ajudar a determinar quais crianças necessitam de uma abordagem mais invasiva ou seguimento mais prolongado e pode fornecer ao médico a justificativa para o aconselhamento dos pais sobre o prognóstico de seu filho em uma fase inicial da doença.
Acute bacterial meningitis is considered a medical emergency, because it is a life-threatening infection that requires immediate treatment. Diagnosing this disease is not always easy, since the symptoms may be nonspecific, especially in infants, and cerebrospinal fluid (CSF) analysis is not always available due to clinical conditions and the absence of time for analysis of specific outcomes.1,2 In addition, although signs and symptoms of fever, irritability, lethargy, headache, vomiting, and nuchal rigidity are strongly associated with bacterial meningitis, other comorbidities or conditions should be considered in the differential diagnosis, such as viral, fungal, and mycobacterial infections of the central nervous system, rickettsial diseases, arboviruses, leptospirosis, and neck or retropharyngeal abscesses. Noninfectious conditions such as autoinflammatory vasculitis, Kawasaki disease, brain tumors, and drug reactions should also be considered.
Early diagnosis, identification of the pathogen, and time to initiation of adequate antibiotic therapy are important variables that can improve the clinical outcomes of bacterial meningitis in children. However, even with an early approach and adequate treatment with effective antibiotics, death and neurological sequelae may occur as outcomes of this infection, especially in younger patients.1–6
Mortality rates remain extremely high, with a range from 5% to 30% of cases, and approximately 50% of survivors evolve with neurological sequelae.5 In children who survive an episode of bacterial meningitis, the most frequent sequelae include hearing loss, developmental delays, and poor school performance.2,6 The time of diagnosis of those sequelae may vary according to the type or intensity of the neurological injury.
The main objective of this study was to systematically review the literature, answering the following main question: “What are the risk factors associated with worse clinical outcomes of pediatric bacterial meningitis patients?” For this purpose, the search was conducted according to the acronym PECOS: population, exposition, comparison, outcome, and study type. PECOS was defined as follows: P, infants with bacterial meningitis; E, risk factors; C, without risk factors; O, death or sequelae; and S, original studies.
MethodsArticles were selected according to the recommendations of Preferred Reporting Items for Systematic Reviews and Meta-analysis Protocols (PRISMA-P),7,8 responsible for coordinating the process of performing meta-analyses and systematic reviews. The review protocol (PROSPERO) was registered at York University under the following identification number: CRD CRD42018089133.
Population, exposure, and outcomesThis systematic review included studies with pediatric patients, who were defined as children aged <18 years, with a diagnosis of bacterial meningitis that evaluated the presence of risk factors that might influence the clinical outcomes, which ranged from death to different levels of sequelae.
The articles were selected using a structure search strategy in Web of Science, Scopus, MEDLINE, and LILACS. The following Medical Subject Heading (MeSH) terms were used: “risk factors,” “mortality,” “complications,” “child,” “pediatrics,” “hospitalized child,” and “bacterial meningitis”. These terms were adapted for use with other bibliographic databases in combination with specific database filters for controlled trials. Searches were performed in February 2019 without language restrictions.
Selection criteriaThe inclusion criteria reflect those noted above for the participants/populations, as well as the following: cross-sectional, case-control, cohort design, and diagnostic studies performed with both males and females younger than 18 years of age and studies that evaluated worsening clinical evolution.
The exclusion criteria were as follows: duplicate publications, studies conducted with adults and elderly individuals, studies that evaluated risk factors for bacterial meningitis but did not associate these factors with a worsening clinical status, studies that evaluated meningitis caused by non-bacterial agents, review studies, experimental studies with animals, and research whose theme was not in accordance with the objective of this review. The types of studies most suitable for this etiological review were mainly observational (analytical) studies that compared groups and produced odds ratios, predictive values, or likelihood ratios (case-control and cohort studies, including both retrospective and prospective studies).
Data analysis and synthesisSummaries of all articles found were independently screened by two reviewers, in order to identify and remove possible duplicates or studies that did not answer the central question of this review. Those potentially eligible for inclusion underwent comprehensive reading of the full text, and subsequently a decision regarding final selection was made during a consensus meeting. Data were extracted regarding year of publication, country and design of the studies, population characteristics, age of infection, causative pathogen, duration of follow-up, outcomes, and statistically significant prognostic factors.
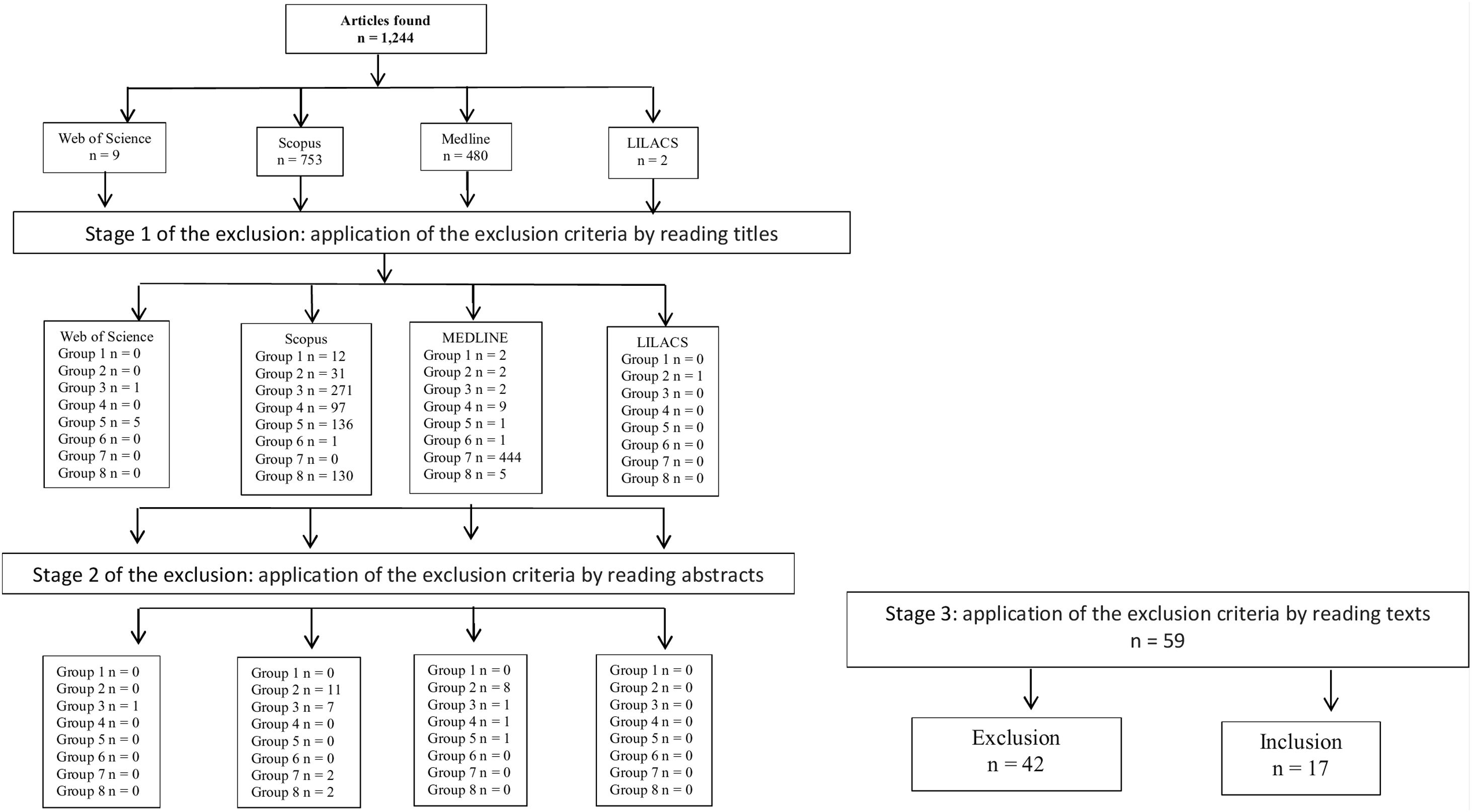
The analysis was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) approach. The number of studies assessed at each screening level were evidenced in the included PRISMA diagram (Fig. 1). The excluded articles were classified as follows: group 1, duplicates; group 2, age group not consistent with the revision proposal (adults or elderly); group 3, articles that did not report the association of the event with the outcome or only cited one of these two observations; group 4, non-bacterial meningitis; group 5, narrative, systematic reviews or meta-analysis articles; group 6, experimental studies; group 7, theme not suitable for the purpose of this review; and finally group 8, studies performed with other bacterial infections.
Studies were assessed for the evaluation of the quality of evidence and to provide a summary of the findings of the included studies by The Grading of Recommendations, Assessment, Development, and Evaluation (GRADE),9,10 as recommended by The Cochrane Collaboration. In this approach, observational studies are considered low-quality evidence and randomized trials are considered high-quality evidence. Methodological limitations, inconsistencies, inaccuracies, indirect evidence, and publication bias can reduce the GRADE score, while magnitude of effect, dose response, and control of all plausible confounding factors can increase quality of evidence.9,10
ResultsThe literature search identified 1244 articles. After methodological screening, 17 studies were eligible for this systematic review. Fig. 1 describes the manuscript inclusion and exclusion steps.
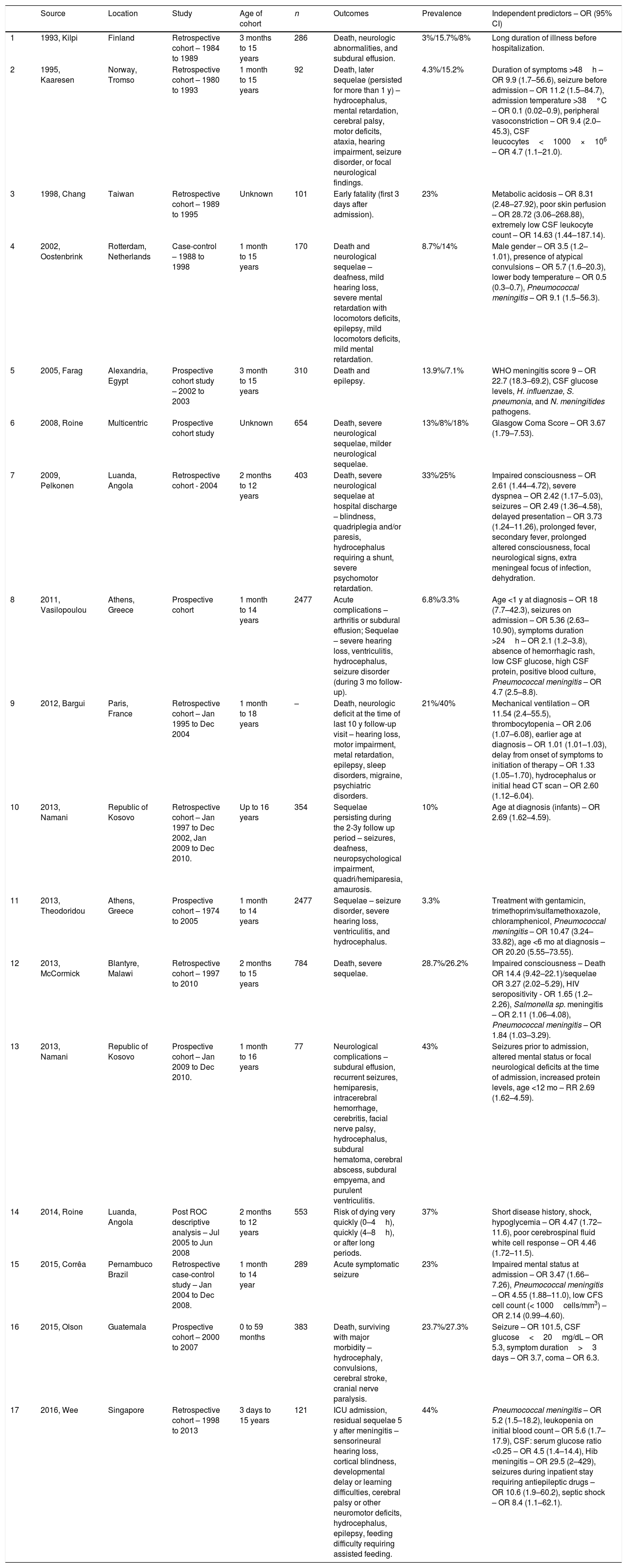
Among the 17 evaluated studies, 14 were retrospective or prospective cohorts, two were case-control studies, and one was a cross-sectional study. No sample calculation information was available in any of the articles, and only one study was conducted in Brazil (Pernambuco). Table 1 summarizes the study characteristics of all included publications.
Characteristics of included studies on risk factors associated with the outcomes of pediatric bacterial meningitis.
| Source | Location | Study | Age of cohort | n | Outcomes | Prevalence | Independent predictors – OR (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| 1 | 1993, Kilpi | Finland | Retrospective cohort – 1984 to 1989 | 3 months to 15 years | 286 | Death, neurologic abnormalities, and subdural effusion. | 3%/15.7%/8% | Long duration of illness before hospitalization. |
| 2 | 1995, Kaaresen | Norway, Tromso | Retrospective cohort – 1980 to 1993 | 1 month to 15 years | 92 | Death, later sequelae (persisted for more than 1 y) – hydrocephalus, mental retardation, cerebral palsy, motor deficits, ataxia, hearing impairment, seizure disorder, or focal neurological findings. | 4.3%/15.2% | Duration of symptoms >48h – OR 9.9 (1.7–56.6), seizure before admission – OR 11.2 (1.5–84.7), admission temperature >38°C – OR 0.1 (0.02–0.9), peripheral vasoconstriction – OR 9.4 (2.0–45.3), CSF leucocytes<1000×106 – OR 4.7 (1.1–21.0). |
| 3 | 1998, Chang | Taiwan | Retrospective cohort – 1989 to 1995 | Unknown | 101 | Early fatality (first 3 days after admission). | 23% | Metabolic acidosis – OR 8.31 (2.48–27.92), poor skin perfusion – OR 28.72 (3.06–268.88), extremely low CSF leukocyte count – OR 14.63 (1.44–187.14). |
| 4 | 2002, Oostenbrink | Rotterdam, Netherlands | Case-control – 1988 to 1998 | 1 month to 15 years | 170 | Death and neurological sequelae – deafness, mild hearing loss, severe mental retardation with locomotors deficits, epilepsy, mild locomotors deficits, mild mental retardation. | 8.7%/14% | Male gender – OR 3.5 (1.2–1.01), presence of atypical convulsions – OR 5.7 (1.6–20.3), lower body temperature – OR 0.5 (0.3–0.7), Pneumococcal meningitis – OR 9.1 (1.5–56.3). |
| 5 | 2005, Farag | Alexandria, Egypt | Prospective cohort study – 2002 to 2003 | 3 month to 15 years | 310 | Death and epilepsy. | 13.9%/7.1% | WHO meningitis score 9 – OR 22.7 (18.3–69.2), CSF glucose levels, H. influenzae, S. pneumonia, and N. meningitides pathogens. |
| 6 | 2008, Roine | Multicentric | Prospective cohort study | Unknown | 654 | Death, severe neurological sequelae, milder neurological sequelae. | 13%/8%/18% | Glasgow Coma Score – OR 3.67 (1.79–7.53). |
| 7 | 2009, Pelkonen | Luanda, Angola | Retrospective cohort - 2004 | 2 months to 12 years | 403 | Death, severe neurological sequelae at hospital discharge – blindness, quadriplegia and/or paresis, hydrocephalus requiring a shunt, severe psychomotor retardation. | 33%/25% | Impaired consciousness – OR 2.61 (1.44–4.72), severe dyspnea – OR 2.42 (1.17–5.03), seizures – OR 2.49 (1.36–4.58), delayed presentation – OR 3.73 (1.24–11.26), prolonged fever, secondary fever, prolonged altered consciousness, focal neurological signs, extra meningeal focus of infection, dehydration. |
| 8 | 2011, Vasilopoulou | Athens, Greece | Prospective cohort | 1 month to 14 years | 2477 | Acute complications – arthritis or subdural effusion; Sequelae – severe hearing loss, ventriculitis, hydrocephalus, seizure disorder (during 3 mo follow-up). | 6.8%/3.3% | Age <1 y at diagnosis – OR 18 (7.7–42.3), seizures on admission – OR 5.36 (2.63–10.90), symptoms duration >24h – OR 2.1 (1.2–3.8), absence of hemorrhagic rash, low CSF glucose, high CSF protein, positive blood culture, Pneumococcal meningitis – OR 4.7 (2.5–8.8). |
| 9 | 2012, Bargui | Paris, France | Retrospective cohort – Jan 1995 to Dec 2004 | 1 month to 18 years | – | Death, neurologic deficit at the time of last 10 y follow-up visit – hearing loss, motor impairment, metal retardation, epilepsy, sleep disorders, migraine, psychiatric disorders. | 21%/40% | Mechanical ventilation – OR 11.54 (2.4–55.5), thrombocytopenia – OR 2.06 (1.07–6.08), earlier age at diagnosis – OR 1.01 (1.01–1.03), delay from onset of symptoms to initiation of therapy – OR 1.33 (1.05–1.70), hydrocephalus or initial head CT scan – OR 2.60 (1.12–6.04). |
| 10 | 2013, Namani | Republic of Kosovo | Retrospective cohort – Jan 1997 to Dec 2002, Jan 2009 to Dec 2010. | Up to 16 years | 354 | Sequelae persisting during the 2-3y follow up period – seizures, deafness, neuropsychological impairment, quadri/hemiparesia, amaurosis. | 10% | Age at diagnosis (infants) – OR 2.69 (1.62–4.59). |
| 11 | 2013, Theodoridou | Athens, Greece | Prospective cohort – 1974 to 2005 | 1 month to 14 years | 2477 | Sequelae – seizure disorder, severe hearing loss, ventriculitis, and hydrocephalus. | 3.3% | Treatment with gentamicin, trimethoprim/sulfamethoxazole, chloramphenicol, Pneumococcal meningitis – OR 10.47 (3.24–33.82), age <6 mo at diagnosis – OR 20.20 (5.55–73.55). |
| 12 | 2013, McCormick | Blantyre, Malawi | Retrospective cohort – 1997 to 2010 | 2 months to 15 years | 784 | Death, severe sequelae. | 28.7%/26.2% | Impaired consciousness – Death OR 14.4 (9.42–22.1)/sequelae OR 3.27 (2.02–5.29), HIV seropositivity - OR 1.65 (1.2–2.26), Salmonella sp. meningitis – OR 2.11 (1.06–4.08), Pneumococcal meningitis – OR 1.84 (1.03–3.29). |
| 13 | 2013, Namani | Republic of Kosovo | Prospective cohort – Jan 2009 to Dec 2010. | 1 month to 16 years | 77 | Neurological complications – subdural effusion, recurrent seizures, hemiparesis, intracerebral hemorrhage, cerebritis, facial nerve palsy, hydrocephalus, subdural hematoma, cerebral abscess, subdural empyema, and purulent ventriculitis. | 43% | Seizures prior to admission, altered mental status or focal neurological deficits at the time of admission, increased protein levels, age <12 mo – RR 2.69 (1.62–4.59). |
| 14 | 2014, Roine | Luanda, Angola | Post ROC descriptive analysis – Jul 2005 to Jun 2008 | 2 months to 12 years | 553 | Risk of dying very quickly (0–4h), quickly (4–8h), or after long periods. | 37% | Short disease history, shock, hypoglycemia – OR 4.47 (1.72–11.6), poor cerebrospinal fluid white cell response – OR 4.46 (1.72–11.5). |
| 15 | 2015, Corrêa | Pernambuco Brazil | Retrospective case-control study – Jan 2004 to Dec 2008. | 1 month to 14 year | 289 | Acute symptomatic seizure | 23% | Impaired mental status at admission – OR 3.47 (1.66–7.26), Pneumococcal meningitis – OR 4.55 (1.88–11.0), low CFS cell count (< 1000cells/mm3) – OR 2.14 (0.99–4.60). |
| 16 | 2015, Olson | Guatemala | Prospective cohort – 2000 to 2007 | 0 to 59 months | 383 | Death, surviving with major morbidity – hydrocephaly, convulsions, cerebral stroke, cranial nerve paralysis. | 23.7%/27.3% | Seizure – OR 101.5, CSF glucose<20mg/dL – OR 5.3, symptom duration>3 days – OR 3.7, coma – OR 6.3. |
| 17 | 2016, Wee | Singapore | Retrospective cohort – 1998 to 2013 | 3 days to 15 years | 121 | ICU admission, residual sequelae 5 y after meningitis – sensorineural hearing loss, cortical blindness, developmental delay or learning difficulties, cerebral palsy or other neuromotor deficits, hydrocephalus, epilepsy, feeding difficulty requiring assisted feeding. | 44% | Pneumococcal meningitis – OR 5.2 (1.5–18.2), leukopenia on initial blood count – OR 5.6 (1.7–17.9), CSF: serum glucose ratio <0.25 – OR 4.5 (1.4–14.4), Hib meningitis – OR 29.5 (2–429), seizures during inpatient stay requiring antiepileptic drugs – OR 10.6 (1.9–60.2), septic shock – OR 8.4 (1.1–62.1). |
CT, computed tomography; CSF; cerebral spinal fluid; ICU; intensive care unit.
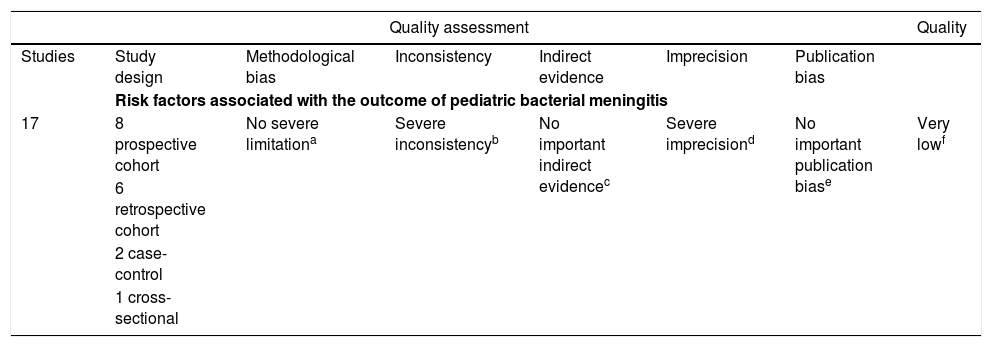
A total of 9581 patients aged between 0 days and 18 years were evaluated in the included studies, and a large number of potentially important prognostic factors were presented. The prevalence of unfavorable clinical outcomes, such as sequelae or death, varied between 3% and 44%, with a calculated arithmetic mean of 17.6%. Prevalence estimates of unfavorable outcomes included data from all studies; however, Chang et al.11 and Roine et al.12 did not mention the ages of the children in their cohorts, and Bargui et al.13 did not mention the sample size and thus were excluded from this analysis. The results of the quality assessment are presented in Table 2.
Summary of studies considering risk factors associated with the outcome of pediatric bacterial meningitis.
| Quality assessment | Quality | ||||||
|---|---|---|---|---|---|---|---|
| Studies | Study design | Methodological bias | Inconsistency | Indirect evidence | Imprecision | Publication bias | |
| Risk factors associated with the outcome of pediatric bacterial meningitis | |||||||
| 17 | 8 prospective cohort | No severe limitationa | Severe inconsistencyb | No important indirect evidencec | Severe imprecisiond | No important publication biase | Very lowf |
| 6 retrospective cohort | |||||||
| 2 case-control | |||||||
| 1 cross-sectional | |||||||
Although the studies presented methodological differences between themselves and based on the population, exposition, comparison, outcome, and study type (PECOS) analysis of the review, no important indirect evidence was observed.
The absolute effect (difference between the exposed and non-exposed groups) was not calculated because the studies did not present effect measures.
Early prediction of an adverse outcome may help determine which children require more intensive or longer follow-up and may provide the physician with rationale for parental counseling about their child's prognosis in an early phase of the disease. Age, duration of symptoms at the time of diagnosis, and symptoms/signs suggestive of the clinical severity at admission, such as tachypnea, hemodynamic instability, and metabolic acidosis, were shown to be independent prognostic factors in the vast majority of studies. Age younger than 12 months at the time of diagnosis was considered a risk factor for early neurologic complications, such as hydrocephalus, ventriculitis, subdural effusion, and arthritis, and for long-term sequelae, such as seizure disorder and hearing loss, by Vasilopoulou et al.2 and Namani et al.,5 who conducted cohort studies with very significant samples. The patient age at diagnosis was not associated with higher mortality, as would be expected if the immature immune status resulted in more severe infections.5,8
Peripheral vasoconstriction was found to be an independent predictor of a poor outcome in the studies by Chang et al. and Kaanesen et al.11,14 Because this condition is considered an earlier sign of cardiovascular compromise than blood pressure in children, it should be considered a valuable predictor of poor outcomes. Approximately two-thirds of early fatalities in pediatric bacterial meningitis cases are a result of septic shock.11 This finding reinforces the idea that the presence of suggestive signs or symptoms of clinical severity at the time of hospital admission can define a poor outcome regardless of the treatment established. The main signs of severity assessed at the time of initial care that were subsequently associated with sequelae or death in the analyzed studies were metabolic acidosis (odds ratio [OR] 8.31; 95% confidence interval [95% CI] 2.48–27.92) and poor skin perfusion (OR 28.72; 95% CI 3.06–268.88) in a retrospective cohort study with 101 patients conducted by Chang et al.,11 impaired consciousness in four other studies (OR 3.67 [95% CI 1.79–7.53],12 OR 2.61 [95% CI 1.44–4.72],15 OR 14.4 [95% CI 9.42–22.1],16 and OR 3.47 [95% CI 1.66–7.26]17), and severe dyspnea (OR 2.42 [95% CI 1.17–5.03]) in a retrospective cohort with 403 patients conducted by Pelkonen et al.15
The delay from symptom onset to initiation of therapy, which varied from 24 to 72h, was independently associated with death, subdural effusion, and sequelae, such as hydrocephalus, cerebral palsy, hearing impairment, and seizure disorder, in six studies.2,3,13–15,18 These were cohort studies developed by Kilpi et al.,18 Kaaresen et al.,14 Pelkonen et al.,15 Vasilopoulou et al.,2 Bargui et al.,13 and Olson et al.,3 with a total sample of 3641 patients. In the opinion of the present authors, this finding can be directly associated with the clinical presentation and the signs and symptoms of severity, since delay of treatment allows increased bacterial multiplication and a stronger outflow of bacterial compounds, leading to brain damage or septic shock.
Pelkonen et al. performed a large cohort study in Angola with 403 patients and reported that secondary and prolonged fevers were independent risk factors for severe neurological sequelae at hospital discharge, including blindness, quadriplegia or paresis, hydrocephalus requiring a shunt, and severe psychomotor retardation.15 However, secondary and prolonged fevers were not defined and could be considered a source of bias. Kaaresen et al. reported in a smaller retrospective cohort study conducted in Norway over a 13-year period that 42% of 92 patients developed a secondary fever, which was defined as recurrence of fever after an afebrile period of 24h or more during the hospital stay; however, in that study, the secondary fever was not associated with death or later sequelae (OR 0.6 [95% CI 0.2–1.9]).14 Moreover, the authors did not mention whether this secondary or prolonged fever determined a change in the antibiotic treatment (i.e., drug or time) or was associated with suppurative complications, such as subdural effusion, purulent ventriculitis, cerebral abscess, or arthritis, which would explain the difference between the results.
Seizures were found to be independent risk factors for a poor outcome, such as death, subdural effusion, hydrocephalus, and seizure disorder, in seven of the studies. However, this potential risk factor was approached in different ways. For instance, Kaaresen et al., Vasilopoulou et al., Namani et al., and Olson et al. specified that the risk for a poor outcome was associated with early seizure that was present prior to or at the time of hospital admission.2,3,14,19 For Wee et al., intensive care unit (ICU) admission or residual sequelae five years after meningitis was associated with development of seizure during the inpatient stay and required antiepileptic drugs (OR 10.6 [95% CI 1.9–60.2]).20 Pelkonen et al. associated seizures at any time point – before, during, or after hospital admission – as an independent predictor of severe neurological sequelae (OR 9.34 [95% CI 3.49–25.00]).15 Oostenbrik et al. found an association between death, deafness, and mental retardation with atypical convulsions (OR 5.7 [95% CI 1.6–20.3]).21 Importantly, in that study, the authors excluded all meningitis cases caused by Haemophilus influenzae type B, which represented nearly 31% of the initially selected patients. This exclusion strategy would interfere not only with the prevalence of sequelae and lethality, but also with the identified risk factors, especially when compared with studies developed before the introduction of routine immunization of infants against this pathogen.
Analysis of the risk associated with the causative organism of bacterial meningitis was difficult, because most studies included patients with laboratory and clinical findings suggestive of meningitis, but lacked isolation of the etiologic agent using either microbiological or molecular tests.2,5,14,21–23 In addition, older studies evaluated independent predictors of the prognosis during a period before introduction of vaccines, which modified the epidemiology of agents involved in the disease.24 However, Pneumococcal meningitis was identified as an independent risk factor for acute symptomatic seizure; ICU admission; acute complications, such as subdural effusion, ventriculitis, and hydrocephalus; and severe sequelae, such as hearing loss and mental retardation or death, in seven of the studies.16,17,19–22,24 Wee et al. conducted a retrospective study of children under 18 years of age who were admitted with acute bacterial meningitis with positive identification of the causative organism from the cerebrospinal fluid. The multivariate analysis showed that risk factors associated with ICU admission, which were used as an indicator of the disease severity, included pneumococcus as the causative organism (OR 5.2 [95% CI 1.5–18.2]).20 The only Brazilian study included in this review, which was conducted by Correa et al., evaluated only patients with bacterial meningitis with a causative organism identified from the cerebrospinal fluid or a positive blood culture. That study found that patients with pneumococcal meningitis were approximately four times more likely to develop acute seizures (OR 4.55 [95% CI 1.88–11.00]).12
Oostenbrink et al. developed a case control study with 170 patients aged from 1 month to 15 years and found an incidence of Streptococcus pneumonia meningitis of 17%. After a stepwise multivariate analysis, the pathogen S. pneumonia was considered an independent predictor for neurological sequelae, with an OR of 9.1 (95% CI 1.5–56.3). However, that study had two important limitations: the inclusion of patients with meningeal irritations with an increased CSF leukocyte count and negative bacterial culture attributed to the use of antimicrobials before the lumbar puncture, and the exclusion of meningitis cases caused by H. influenzae.21 Theodoridou et al. conducted a prospective cohort study with the largest sample, which included 2477 patients aged from 1 month to 14 years who were admitted to Agia Sofia Children's Hospital in Athens from 1974 to 2005. Pneumococcal meningitis was an independent risk factor for development of seizure disorder (OR 10.47 [95% CI 3.24–33.82]), although Oostenbrink et al. included data from patients with laboratory findings consistent with acute bacterial meningitis without isolating the pathogen (29.6%). The long duration of this cohort also contributes bias because of differences in the prevalence of pathogens after introduction of the conjugate H. influenzae type b vaccine.22
The main concern about interpretation of the present findings is that they are limited by the age quality and heterogeneity of the literature. Although all of these studies have evaluation of prognostic factors associated with meningitis in pediatric patients as their main objective, the evaluated outcomes are very different, ranging from mild hearing loss to severe neurological complications, suppurative complications, or death. The timing of these outcomes also varies greatly. Some studies evaluated complications only during hospitalization or until hospital discharge,11,12,15,21,24 whereas others were able to guarantee follow-up of patients for long periods; for instance, Bargui et al. presented an average follow-up of ten years after hospital discharge.5,13,14,19,20 This difference in the follow-up and outcome evaluation also has an impact on the differences in the prevalence of complications found in the different studies. Most studies adequately evaluated the independent values of predictors using multivariate analysis to minimize bias. Only Kilpi et al., Namani et al., and Roine et al. did not cite multivariate adjustments.12,18,19
Another reason for potential differences between studies is the different populations studied. Although all studies were performed on the characteristics of children with bacterial meningitis, the cases were selected using different age, pathogen type, or disease severity criteria, and different definitions of bacterial meningitis were applied. Finally, the small number of patients with sequelae or death included in these studies would introduce an important bias, which implies a need for other studies to focus on these aspects.
ConclusionSeveral plausible and important prognostic factors are proposed for prediction of poor outcomes after bacterial meningitis in childhood. Unfavorable evolution and late diagnosis reduce the chances for a better evolution of the patient and reinforce the importance of a high diagnostic suspicion of meningitis, especially in febrile pictures with nonspecific symptomatology when a clear infectious focus cannot identified. Early suspicion and antimicrobial treatment improve the chance for a cure without sequelae. The disease still presents high lethality and a high prevalence of associated sequelae in survivors, which range from mild neurological sequelae, such as partial hearing loss and mild cognitive deficits, to severe neurological sequelae, such as epilepsy and mental retardation or death, even in the post-vaccination era.
Another prognostic factor that was demonstrated to be related to severity was S. pneumoniae as a causative pathogen, which had more pathogenic action than the other bacterial species. Mild neurological complications had a variable prevalence and were not evaluated in all studies, since they were associated with difficulties with the diagnostic criteria, which required specialized outpatient follow-up for a prolonged period after discharge. However, these complications should not be neglected, since they account for a large portion of the children affected by the disease.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Teixeira DC, Diniz LM, Guimarães NS, Moreira HM, Teixeira CC, Romanelli RM. Risk factors associated with the outcomes of pediatric bacterial meningitis: a systematic review. J Pediatr (Rio J). 2020;96:159–67.