To capture evidence of the efficacy and safety of pharmacological analgesia for uncomplicated acute sickle-cell pain in pediatric patients compared to placebo.
Sources of dataSearches for key evidence were performed from March 1 to 31, 2018, for randomized controlled trials of pharmacological analgesia compared to placebo for uncomplicated acute sickle-cell pain in a pediatric sample. The authors searched ten scientific databases including, among others, PubMed, MEDLINE, Embase, and Clinicaltrials.gov for this systematic review and meta-analysis.
Summary of the findingsFour trials (n=227) were selected by the inclusion criteria (intranasal fentanyl, intravenous magnesium, arginine, and inhaled nitric oxide). The quality of evidence ranged from low to moderate for each outcome. Meta-analysis of changes in the ladder of pain score (p=0.72), length-of-stay in hospital (p=0.65), and amount of narcotics used during the study (p=0.10) showed non-statistically significant differences and a lack of amelioration provided by pharmaceutical analgesics in treatment group. The adverse events reported that more participants in the intervention arm underwent pain, with statistically significant differences at the drug delivery site in studies using intranasal fentanyl and intravenous magnesium (p=0.03).
ConclusionsPharmacological analgesia appears to be uncertain in improving the intensity and providing relief of acute pain crisis in pediatric patients with sickle-cell anemia. With respect to clinical advantage, no decisive deduction about the clinical efficacy may be made regarding these medications in acute sickle-cell pain management in the pediatric age group.
Obter evidências da eficácia e segurança da analgesia farmacológica para dor falciforme aguda não complicada em pacientes pediátricos em comparação com placebo.
Fontes de dadosUma busca de evidências-chave foi feita de 1º a 31 de março de 2018 por ensaios clínicos randomizados controlados de analgesia farmacológica em comparação com placebo para dor aguda falciforme não complicada em uma amostra de pacientes pediátricos. Os autores pesquisaram dez bases de dados científicos que envolveram, entre outras, PubMed, Medline, Embase e Clinicaltrials.gov para esta revisão sistemática e metanálise.
Resumo dos dadosQuatro ensaios (n=227) foram considerados para critérios de inclusão (fentanil intranasal, magnésio intravenoso, arginina e óxido nítrico inalado). As evidências de qualidade variaram de baixas a moderadas para cada desfecho. A metanálise de alterações na escala de dor (p=0,72), o tempo de internação hospitalar (p=0,65) e a quantidade de analgésicos usados durante o estudo (p=0,10) mostraram diferença não estatisticamente significativa e a ausência de melhoria resultante do uso do fármaco analgésico no grupo de tratamento. Os eventos adversos relatados mostraram que no braço de intervenção mais participantes sofreram dor com diferença estatisticamente significativa no local de aplicação do fármaco com o uso de fentanil intranasal e magnésio intravenoso (p=0,03).
ConclusõesA analgesia farmacológica parece ter um efeito incerto na melhoria da intensidade e alívio da crise de dor aguda em pacientes pediátricos com doença falciforme. Em relação à vantagem clínica, ainda é incerta a eficácia clínica desses medicamentos no tratamento da dor aguda falciforme no grupo etário pediátrico.
Sickle-cell disease (SCD) is an inherited red cell disorder that encompasses a unit of disease that is the worldwide common molecular origin hemoglobinopathy.1–3 The most prevalent SCD genotypes quintessentially include the homozygous state (HbSS) and compound heterozygous conditions such as sickle trait, hemoglobin-C (HbSC) or beta (β) thalassemia (HbSβ+ and Hbβ0 thalassemia).4–7 Change to the polymer under a deoxygenated state of hemoglobin defines the hallmark of the condition that induces sickling formation; hence vaso-occlusive crisis (VOC), which is the label of SCD causing jammed microvascular beds, and leading to tissue ischemia.8–10 Acute pain crisis is a key feature of the external expression of VOC, varying from mild to burdensome and debilitating pain which may continue from hours to weeks. Most often this is the reason to seek medical care in the emergency department (ED), representing approximately 70% of children with SCD, and is the result of increased readmission rates in hospitals.11–17 The incidence of painful acute crisis tends to increment throughout childhood and reaches its highest in adolescence. The first years of life are, however, the critical phase of the onset of acute pain crisis.4,18 Despite the in-depth understanding of acute sickle-cell pain, relieving it remains a big challenge in children.
So far, objectifying therapy on the underlying mechanisms of VOC has shown no efficacy.19 Usually, symptomatic alleviation of uncomplicated sickle-cell acute pain crisis relies on the usage of non-steroidal anti-inflammatory drugs (NSAIDs), opioids, hydration, common analgesics, adjuvant medications, and with the recent tendency towards dissociative anesthetics. Generally speaking, the opiate derivatives have been believed to be the “gold standard” analgesic for managing sickle pain secondary to VOC.13,20,21 In case of uncontrolled pain after high-dose intravenous opiates, a dissociative anesthetic such as ketamine may be one of the alternatives.21 Dunlop’s intervention review, founded on nine randomized controlled trials (RCTs), concluded that the majority of studies have been unmet and that it was impossible to achieve relevant meta-analysis owing to insufficiency of data, small sample size, and inconsistencies in methods and reports. Hence, those authors judged that evidence for analgesic interventions in sickle-cell pain crises was lacking.22 In 2015, Archer et al. outlined the limits of opioids that they are rather in nociceptive frame regarding to the pathophysiology highlighting VOC and its inflammatory effects.23 Several approaches were referenced for the advancement of in-depth understanding of the physiopathogenesis underlying of the sickle-cell pain crisis.11,24,25 A potential novel therapy pathway is therefore believed necessary to manage the pain crisis.
Intranasal fentanyl is a suitable opioid choice compared to the parenteral opiate pathway in order to mitigate the pain promptly and efficiently, avoiding the use of intravenous or intramuscular opiate methods.18,20 Also, the nasal mucosa is an attractive and a broad area, with a rich vascular plexus, representing a rapid route for medication administration without going through gastrointestinal degradation or the hepatic metabolism.20 Based on breakthroughs in the pathophysiological comprehension of VOC, these three components, intravenous magnesium, arginine, and inhaled nitric oxide, have sparked the curiosity of scientific literature due to their critical role in endothelium and inflammatory syndrome regulation during the sickle-cell crisis.26–36
Thereby, the present authors chose to conduct a systematic review and meta-analysis of RCTs to examine the efficiency and safety of these pharmaceuticals with analgesic aims. This meta-analysis review was performed to gather an extensive available body of experimental trials evaluating the change in the ladder of pain score, length-of-stay in hospital, and amount of narcotics used in a pediatric sample with uncomplicated acute VOCs in SCD.
MethodsStudy research strategyThis systematic review and meta-analysis was initiated by manual search of the studies and references from two articles.23,37 Searches were performed irrespective of where the studies were conducted, without language restrictions, and were limited to humans. The study plan obeyed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for meta-analyses of interventional studies.38
The authors completed searches for the key evidence in ten scientific databases from March 1 to 31, 2018; trials were published between January 1, 2001, and December 31, 2017: MEDLINE (OvidSP), Embase, PubMed, CENTRAL, LILACS, ISRCTN Registry, CINAHL (EBSCOHost), Web of Science (CPCI-S) for RCTs, and for ongoing trials the authors searched Clinicaltrials.gov and WHO-ICTRP. For each database, a comprehensive search strategy was developed by using Boolean operators, and controlled through MeSH terms. Some of the search terms, as detailed in Table 1, included: "anemia, sickle cell," "pain," "acute pain," "randomized controlled trial," "placebo," "clinical trial," and "double blinded." Additionally, for further searches Google Scholar was used, and the authors manually searched the other bibliographies from included studies, which were considered for references supplied to other studies or were missed in the primary electronic search. Only the studies published under peer-review were considered in the search.
Electronic search strategy.
| Database/resource | Strategy |
|---|---|
| PubMed (2001 to 2017) search limited in Clinical Trial and "humans"[MeSH Terms] n=102 | #1 ("anemia, sickle cell" OR "sickle" OR "sickle cell" OR "sickle cell disease") |
| #2 ("pain" OR "acute pain" OR "painful") | |
| #3 ("randomized controlled trial" OR "controlled clinical trial" OR "randomized" OR "placebo" OR "double blinded") | |
| #4 #1 AND #2 AND #3 | |
| Clinicaltrials.gov (2001 to 2017) n=171 | Search terms: pain |
| Study type: interventional studies | |
| Conditions: anemia, sickle cell | |
| CENTRAL (Cochrane Central Register of Controlled Trials of the Cochrane Library) search limited in trials (2001 to 2017) n=110 | #1 MeSH descriptor: [Anemia, Sickle Cell] explode all threes |
| #2 ("sickle" or "sickle cell" or "sickle cell disease") | |
| #3 #1 or #2 | |
| #4 MeSH descriptor: [Pain] explode all trees | |
| #5 MeSH descriptor: [Acute Pain] this term only | |
| #6 "painful" | |
| #7 #4 or #5 or #6 | |
| #8 #3 and #7 | |
| LILACS n=12 | tw: (sickle) AND (instance:"regional") AND (db:("LILACS") AND |
| type_of_study:("clinical_trials")) | |
| WHO ICTRP (2001-2017) | [Title: pain |
| n=78 | Condition: sickle |
| Recruitment Status: ALL] | |
| MEDLINE (OvidSP) | 1. exp Anemia, Sickle Cell/ |
| Specific year range (2001-2017) n=66 | 2. (sickle or sickle cell or sickle cell disease).tw,kf. |
| 3. or/1-2 | |
| 4. exp Pain/ | |
| 5. Acute Pain/ | |
| 6. painful.tw. | |
| 7. or/4-6 | |
| 8. 3 and 7 | |
| 9. randomized controlled trial.pt. | |
| 10. controlled clinical trial.pt. | |
| 11. randomi*.tw. | |
| 12. placebo.ab. | |
| 13. clinical trial.sh. | |
| 14. randomly.ab. | |
| 15. groups.ab. | |
| 16. trial.tw. | |
| 17. or/9-16 | |
| 18. exp animals/ not humans/ | |
| 19. 17 not 18 | |
| 20. 8 and 19 | |
| ISRCTN Registry | Search 1: pain |
| n=11 | |
| n=6 | |
| Condition: sickle cell | |
| Search 2: pain | |
| Condition: sickle cell disease | |
| CINAHL (Cumulative Index to Nursing and Allied Health Literature) Complete (EBSCOHost): (2001-2017) n=88 | S1 (MH "anemia, sickle cell+") |
| S2 TX ("sickle" OR "sickle cell" OR "sickle cell disease") | |
| S3 S1 OR S2 | |
| S4 (MH "pain+") | |
| S5 TX ("acute pain") | |
| S6 TX ("painful") | |
| S7 S4 OR S5 OR S6 | |
| S8 S3 AND S7 | |
| S9 (MH "clinical trials+") | |
| S10 PT clinical trial | |
| S11 TI (clinic* N1 trial* OR controlled N1 trial*) | |
| S12 TI (doubl* N1 blind*) | |
| S13 TI (randomi* OR placebo*) | |
| S14 S9 OR S10 OR S11 OR S12 OR S13 | |
| S15 S8 AND S14 | |
| Embase (2001-2017) n=89 | #1 'sickle cell anemia'/exp |
| #2 'sickle cell anemia':ab,ti | |
| #3 'sickle cell':ab,ti | |
| #4 #1 OR #2 OR #3 | |
| #5 'pain'/exp | |
| #6 'pain':ab,ti | |
| #7 #5 OR #6 | |
| #8 #4 AND #7 | |
| #9 'randomized controlled trial':ab,ti | |
| #10 'controlled clinical trial':ab,ti | |
| #11 'placebo':ab,ti | |
| #12 'double blind procedure':ab,ti | |
| #13 #9 OR #10 OR #11 OR #12 | |
| #14 #8 AND #13 | |
| #15 'randomized controlled trial'/de | |
| #16 #14 AND #15 | |
| #17 [embase]/lim | |
| #18 #16 AND # 17 | |
| Web of Science CPCI-S (2001-2017) n=27 | TOPIC: (anemia, sickle cell OR sickle OR sickle cell OR sickle cell disease) AND TOPIC: (pain OR acute pain OR painful) AND TOPIC: (random OR randomly OR randomised OR randomized OR blind OR blinded OR control group OR placebo OR controlled study OR groups OR trial OR trials OR literature search OR medline OR pubmed OR cochrane OR embase) |
Two review authors (MIS and SS) independently searched the databases. Also, they independently identified the pertinence of the retrieved studies and examined the full-text papers to decide upon eligibility according the criteria. Any discrepancies about the inclusion of full-text studies were elucidated and resolved by consensus of all investigators.
Inclusion criteriaTo be considered, clinical trials needed to have at least ten participants in each study arm, in any clinical setting, and were required to be randomized, double-blinded, placebo-controlled studies, with parallel or single group and/or pilot study designs, and whose interventions had an analgesic purpose. The study population was pediatric patients that were aged between 3 and 21 years old, with three SCD genotypes – limited to HbSS, HbSC, and HbS-β thalassemia – experiencing an uncomplicated acute pain episode from VOCs. Non-RCTs and RCTs that reported the eligibility combination of both pediatric and adult participants, studies in animals, and studies that outlined outcomes in participants with complicated severe sickle-cell pain crisis (e.g., acute chest syndrome, acute splenic sequestration, and cerebrovascular accident) and chronic sickle-cell pain, studies published with only abstract, and studies with double-blinded active comparator trials of drugs were excluded. Therefore, seven studies were excluded: three non-RCTs,39–41 one study with a paucity of available participants,42 one including merely two genotypes,43 one with chronic pain,44 and one with an active comparator.45 The included studies had fully outlined two of these following outcome measures: assessing pain score, length-of-stay, cumulative amount of narcotic use, and adverse events (the definitions of outcome measures are reported in Table 2). Effectively, the studies that reported on disease-modifying therapy (e.g., hydroxyurea) for SCD were excluded, which is beneficial for VOCs. The studies addressed interventions particularly in regular blood transfusion, erythro-exchange regimens, colloids, fluid replacement, medications that drive to dwarf the less-deformable red blood cells, and anticoagulants which are thought to preclude the SCD-crisis and to minimize the frequency of pain by impeding the sickling process were also excluded. Others studies designed interventions featuring aggressive analgesic therapy with systemic treatment (oral, subcutaneous, epidural, and intramuscular administration) involving strong opioids, weak opioids (codeine and dextropropoxyphene), and non-opioids agents (paracetamol and NSAIDs) were not included in the review.
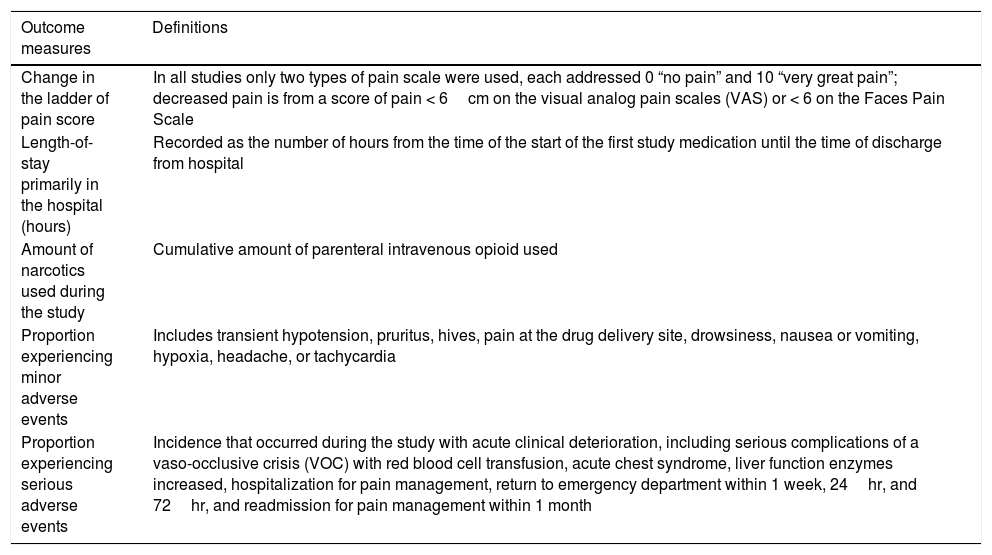
Definitions of outcome measures.
| Outcome measures | Definitions | |
|---|---|---|
| Change in the ladder of pain score | In all studies only two types of pain scale were used, each addressed 0 “no pain” and 10 “very great pain”; decreased pain is from a score of pain < 6cm on the visual analog pain scales (VAS) or < 6 on the Faces Pain Scale | |
| Length-of-stay primarily in the hospital (hours) | Recorded as the number of hours from the time of the start of the first study medication until the time of discharge from hospital | |
| Amount of narcotics used during the study | Cumulative amount of parenteral intravenous opioid used | |
| Proportion experiencing minor adverse events | Includes transient hypotension, pruritus, hives, pain at the drug delivery site, drowsiness, nausea or vomiting, hypoxia, headache, or tachycardia | |
| Proportion experiencing serious adverse events | Incidence that occurred during the study with acute clinical deterioration, including serious complications of a vaso-occlusive crisis (VOC) with red blood cell transfusion, acute chest syndrome, liver function enzymes increased, hospitalization for pain management, return to emergency department within 1 week, 24hr, and 72hr, and readmission for pain management within 1 month | |
One reviewer (MIS) performed the data extraction from the included trials, which was reviewed by all investigators (SS and DZ), and all discrepancies were also resolved with all authors by agreement. One investigator (MIS) extracted the general details from each study according to the Cochrane Handbook for Systematic Reviews of Interventions,46 which was subsequently crosschecked by all reviewers. The form included the first author and year of publication, study location, setting, design, characteristics of participants, description of interventions, and study results (pre-specified outcomes).
Quality assessment and risk of biasRisk of bias for methodological quality of the eligible trials was ascertained and assessed by one review author (MIS) and followed with a review by all investigators using each domain of relevant bias in accordance with the Cochrane risk assessment tools for randomized study designs.47 Each trial was judged as low, high, or unclear risk of selection bias (random sequence generation and allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective reporting), and other bias (Table 3, more details in Supplementary material). Any discrepancies were resolved by agreement of all investigators. As advocated in the Cochrane Collaboration, the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) Profile48 was used to rate the quality of the evidence for individual outcome by using the five leading domains, including the following: limitation of the study design or execution (risk of bias); inconsistency, indirectness, and imprecision of results; and, publication of bias. Hence, the quality of evidence was judged as very low, low, moderate, or high for each outcome.
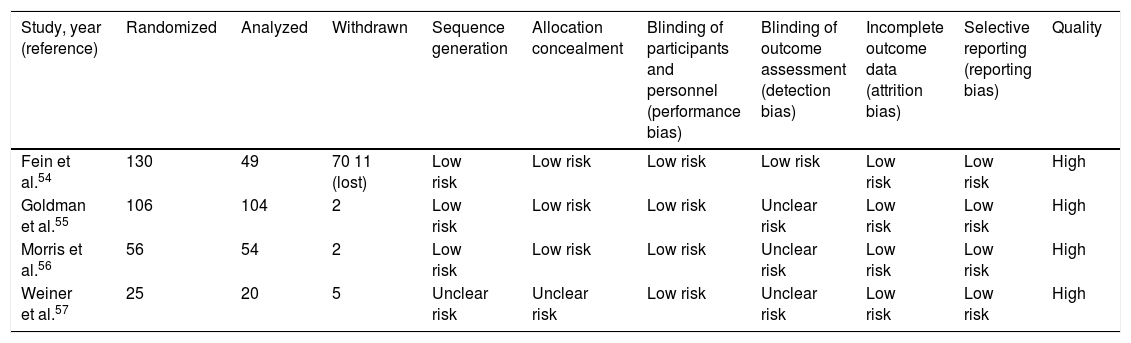
Quality judgment of the individual studies.
| Study, year (reference) | Randomized | Analyzed | Withdrawn | Sequence generation | Allocation concealment | Blinding of participants and personnel (performance bias) | Blinding of outcome assessment (detection bias) | Incomplete outcome data (attrition bias) | Selective reporting (reporting bias) | Quality |
|---|---|---|---|---|---|---|---|---|---|---|
| Fein et al.54 | 130 | 49 | 70 11 (lost) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | High |
| Goldman et al.55 | 106 | 104 | 2 | Low risk | Low risk | Low risk | Unclear risk | Low risk | Low risk | High |
| Morris et al.56 | 56 | 54 | 2 | Low risk | Low risk | Low risk | Unclear risk | Low risk | Low risk | High |
| Weiner et al.57 | 25 | 20 | 5 | Unclear risk | Unclear risk | Low risk | Unclear risk | Low risk | Low risk | High |
From each outcome measure, the authors retrieved and directly extracted the mean (standard deviation [SD]) or calculated it from the median and interquartile ranges (IQRs) by using the method of Hozo et al.49 Continuous outcomes were recorded as mean difference (MD) along with 95% confidence intervals (CIs) in outcomes measured using the same scale among studies (length-of-stay, primarily in-hospital), and as standardized mean difference (SMD) among studies that had used scales or units in different ways for measuring identical outcomes (change in ladder of pain score and amount of narcotics used during the study). Also, the number of events from each study in the two arms (split by the same investigator into minor and serious) and the summary statistic, presented by dichotomous outcomes as risk ratios (RR) and 95% CIs, were extracted. Contact was established with the principal authors of the identified reviews and trials to ask for any unpublished or missing data. The present authors derived pooled estimates of SMD, MD, and 95% CIs from the mean (SD) with inverse variance (IV)-weighted random-effects models where heterogeneity was moderated. In contrast, fixed-effects models were used where heterogeneity was considered as unimportant or nil. Overall summary estimates of RR and 95% CIs were computed with Mantel–Haenszel weighted fixed-effects models for events during the study. To determine the heterogeneity assessment between the effects of intervention, the authors used Cochran’s chi-squared (chi2) test along with p-value less than 0.10 to assess for subgroup analyses. The authors also used an alternative measurement of the statistical heterogeneity with the inconsistency (I2) method, which expresses a range from 0% to 100%, and it was calculated as unimportant (I2<50%), moderate (I2≥50%), or substantial (I2<80%).50,51
Analyses were performed to explore the impact of the pharmaceutical analgesic interventions versus control on the SMD of the change in the ladder of pain score for uncomplicated acute sickle-cell pain crisis, on the MD of hospital length-of-stay, and on the SMD of the amount of narcotics used during the study. Subgroup analyses were performed to consider the diverse adverse events that occurred in the two arms throughout each study.
Assessment of publication bias in order to generate funnel plots and sensitivity analyses could not be performed, since only a few studies were retrieved (less than 10).50,52 Furthermore, it would not be possible to achieve a meta-analysis review if the heterogeneity identified was 80% or more. All analyses were performed with Review Manager (Review Manager (RevMan) [Computer program]. version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).53
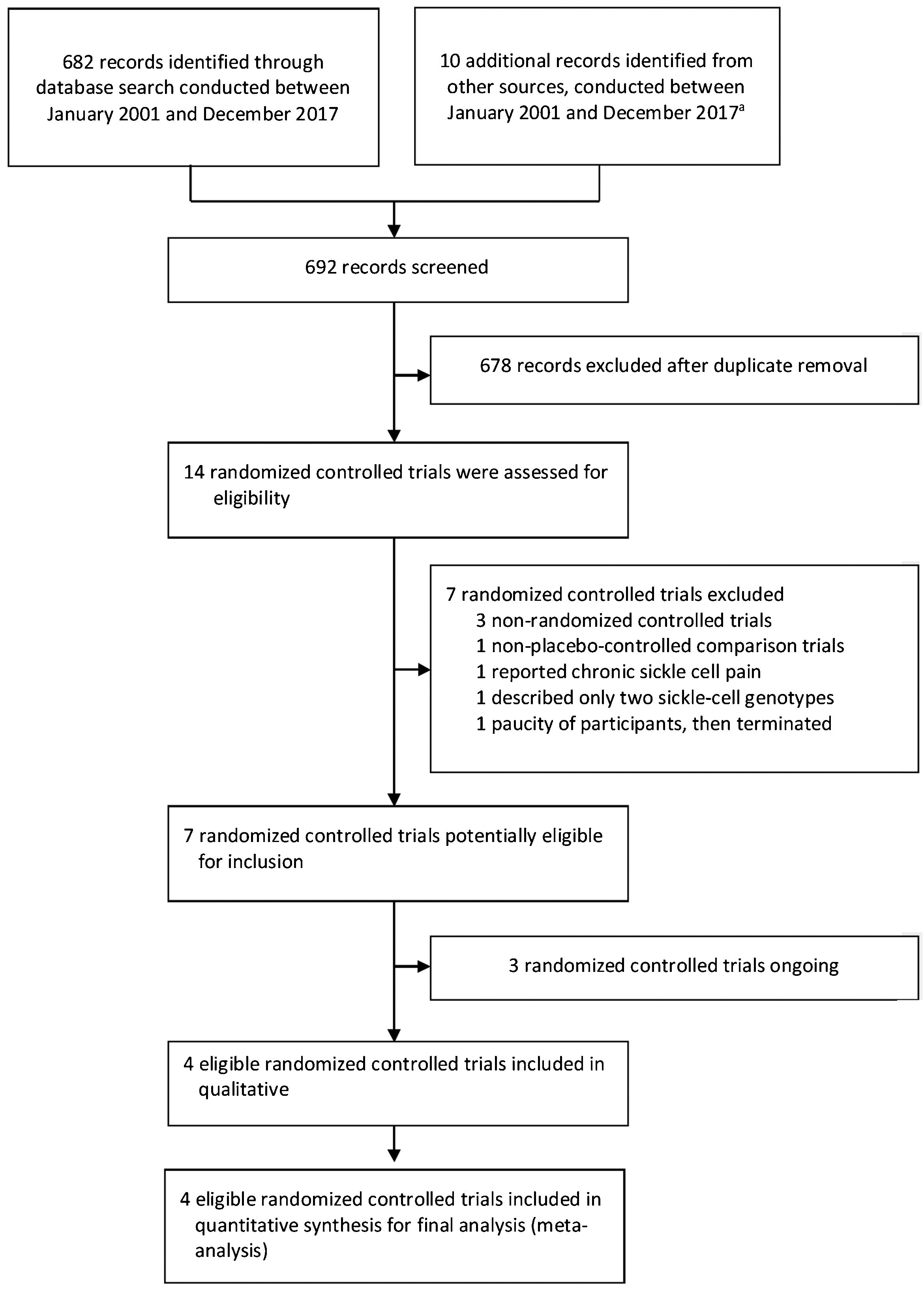
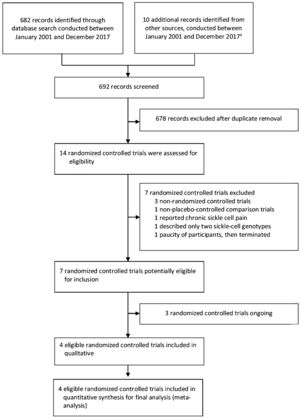
ResultsThe search for key evidence retrieved 692 studies, of which 14 potentially relevant studies were selected for full-text review. From these, four randomized controlled trials were considered that met the inclusion criteria (Fig. 1).
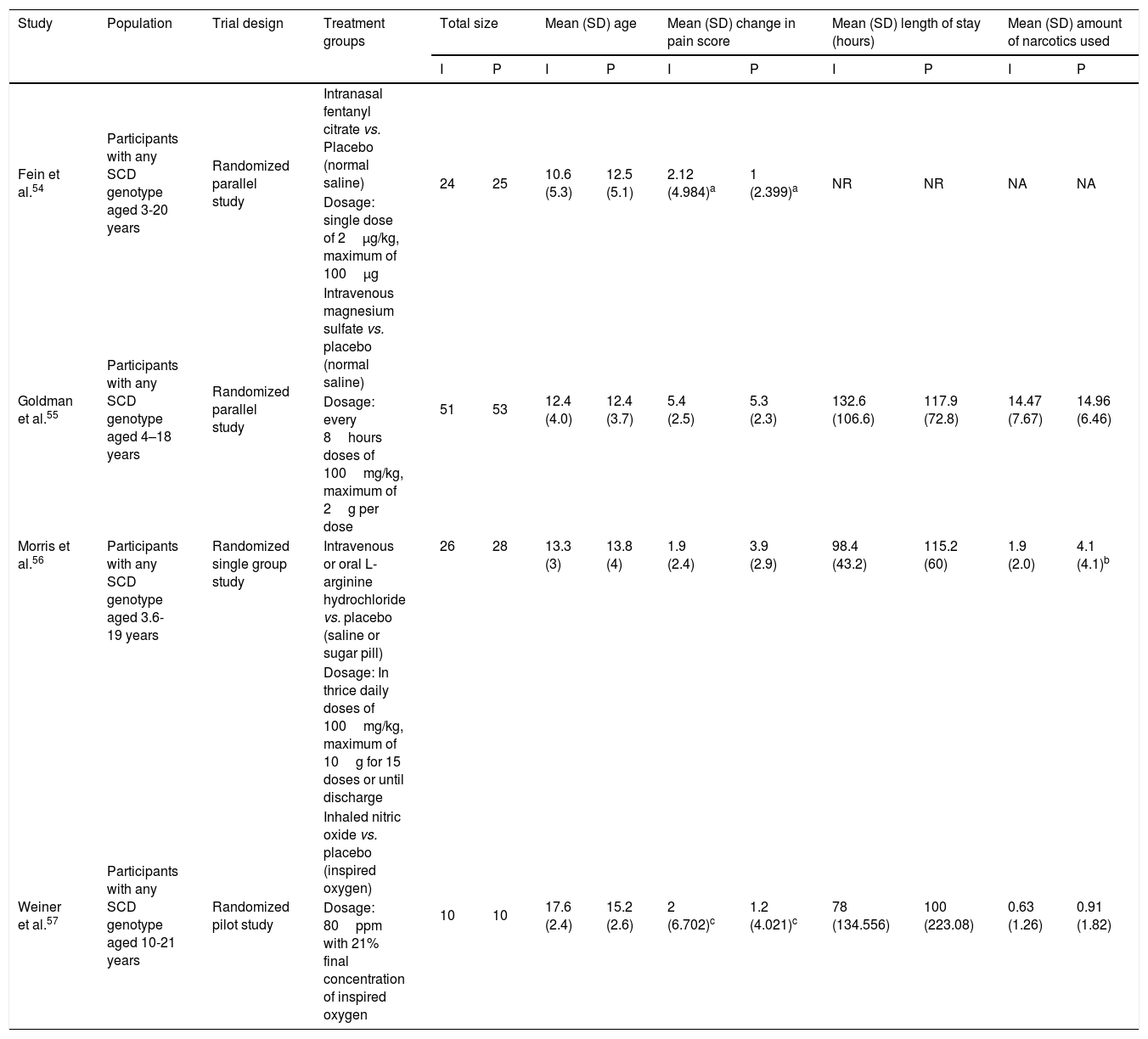
Study characteristicsThe summary of the study characteristics are described in Table 4 and in Supplementary Material. Moreover, these studies were published between the year 2003 and 2016. A total of four trials involved 227 participants.54–57 Among these, 111 patients were incorporated into intervention arms and 116 into placebo arms.
Main characteristics and summary of the studies outcomes included in the meta-analysis.
| Study | Population | Trial design | Treatment groups | Total size | Mean (SD) age | Mean (SD) change in pain score | Mean (SD) length of stay (hours) | Mean (SD) amount of narcotics used | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | P | I | P | I | P | I | P | I | P | ||||
| Fein et al.54 | Participants with any SCD genotype aged 3-20 years | Randomized parallel study | Intranasal fentanyl citrate vs. Placebo (normal saline) | 24 | 25 | 10.6 (5.3) | 12.5 (5.1) | 2.12 (4.984)a | 1 (2.399)a | NR | NR | NA | NA |
| Dosage: single dose of 2μg/kg, maximum of 100μg | |||||||||||||
| Goldman et al.55 | Participants with any SCD genotype aged 4–18 years | Randomized parallel study | Intravenous magnesium sulfate vs. placebo (normal saline) | 51 | 53 | 12.4 (4.0) | 12.4 (3.7) | 5.4 (2.5) | 5.3 (2.3) | 132.6 (106.6) | 117.9 (72.8) | 14.47 (7.67) | 14.96 (6.46) |
| Dosage: every 8hours doses of 100mg/kg, maximum of 2g per dose | |||||||||||||
| Morris et al.56 | Participants with any SCD genotype aged 3.6-19 years | Randomized single group study | Intravenous or oral L-arginine hydrochloride vs. placebo (saline or sugar pill) | 26 | 28 | 13.3 (3) | 13.8 (4) | 1.9 (2.4) | 3.9 (2.9) | 98.4 (43.2) | 115.2 (60) | 1.9 (2.0) | 4.1 (4.1)b |
| Dosage: In thrice daily doses of 100mg/kg, maximum of 10g for 15 doses or until discharge | |||||||||||||
| Weiner et al.57 | Participants with any SCD genotype aged 10-21 years | Randomized pilot study | Inhaled nitric oxide vs. placebo (inspired oxygen) | 10 | 10 | 17.6 (2.4) | 15.2 (2.6) | 2 (6.702)c | 1.2 (4.021)c | 78 (134.556) | 100 (223.08) | 0.63 (1.26) | 0.91 (1.82) |
| Dosage: 80ppm with 21% final concentration of inspired oxygen | |||||||||||||
NR, not reported; NA, not applicable; I, intervention; P, placebo; vs., versus.
In the studies selected for inclusion in meta-analysis, three RCTs were found with interventional drugs that have crucial interventional role in the pathological mechanism of VOCs, including intravenous magnesium, arginine, and nitric oxide, and one RCT of intranasal fentanyl, which is an opioid derivative. All of the RCTs were compared with a non-treatment arm as placebo control, and those drugs were subsequently investigated. There were two studies with parallel design54,55; one single group design56 and one pilot design.57 All trials included were of patients admitted to the emergency department (ED) for an acute episode of pain crisis caused by VOC that used scale measurement of pain; and that were of shorter duration of treatment, and were achieved in a normal period. Three of the included studies were done in United States54,56,57 and one was conducted in Canada.55
The total number of participants in the included trials was not different regarding the intervention (111) and placebo (116) arms, and total number of participants within each trial was between 20 and 104. All studies focused on unpredictable and uncomplicated acute episodes of sickle-cell pain crisis with phenotypes identified as HbSS, HbSC, and HbSβ-thalassemia. Of the 227 participants involved, 149 (65.6%) had HbSS, 61 (26.9%) had HbSC, and only 17 (7.5%) had HbSβ-thalassemia; 115 (50.66%) were male and 112 (49.34%) were female. Forty-nine participants were eligible and were included for analysis in the intranasal fentanyl trial, 104 in the intravenous magnesium trial, 54 in the arginine trial, and only 20 in the nitric oxide trial. Across two groups (treatment and control) in all studies, the lowest mean (SD) age of participants ranged from 10.6 (5.3) to the highest mean (SD) of 17.6 (2.4) years. The splits of participants in each arm (intervention and placebo) through all trials were almost equal (24–25) in the intranasal fentanyl trial, 51–53 in the intravenous magnesium trial, 26–28 in the arginine trial; and ten-ten in the nitric oxide trial.54–57
Risk of biasThe Cochrane risk of bias assessment tool for individual trials is shown in Table 3 and in Supplementary Material. Additionally, the quality of the evidence is shown for each outcome, which in accordance with the GRADE approach was classified from “low” to “moderate” (Table 5). The three randomized controlled trials were judged to be at low risk of selection bias given that randomization method was reported by using the tables in block, and the sequence was engendered through the pharmacy research.54–56 One trial was judged to have an unclear risk of selection bias, as the study did not suitably describe the method of randomization and had insufficient information regarding allocation concealment (Supplementary material).57 All four studies were graded to be at low risk of performance, attrition, and selective reporting bias because all reported that their trials were double-blinded and that the placebo treatments were not discernible from the study intervention. All analyzed participants had been included in the final data. Reports of all intended pre-specified outcome measures were cited in the online trial registry.54–57 Three trials had an unclear risk of detection bias because in the trial registry there were insufficient clear details to judge, and no supplementary statements of information were given for the outcome assessors that were incorporated into the blinding.55–57 One trial was considered as having a low risk of detection bias because the blinding incorporated outcome assessors that were stated in the trial registry.54 Furthermore, all studies had a high risk of other potential sources of bias; the reason was that the target sample size was not attained (Supplementary material).
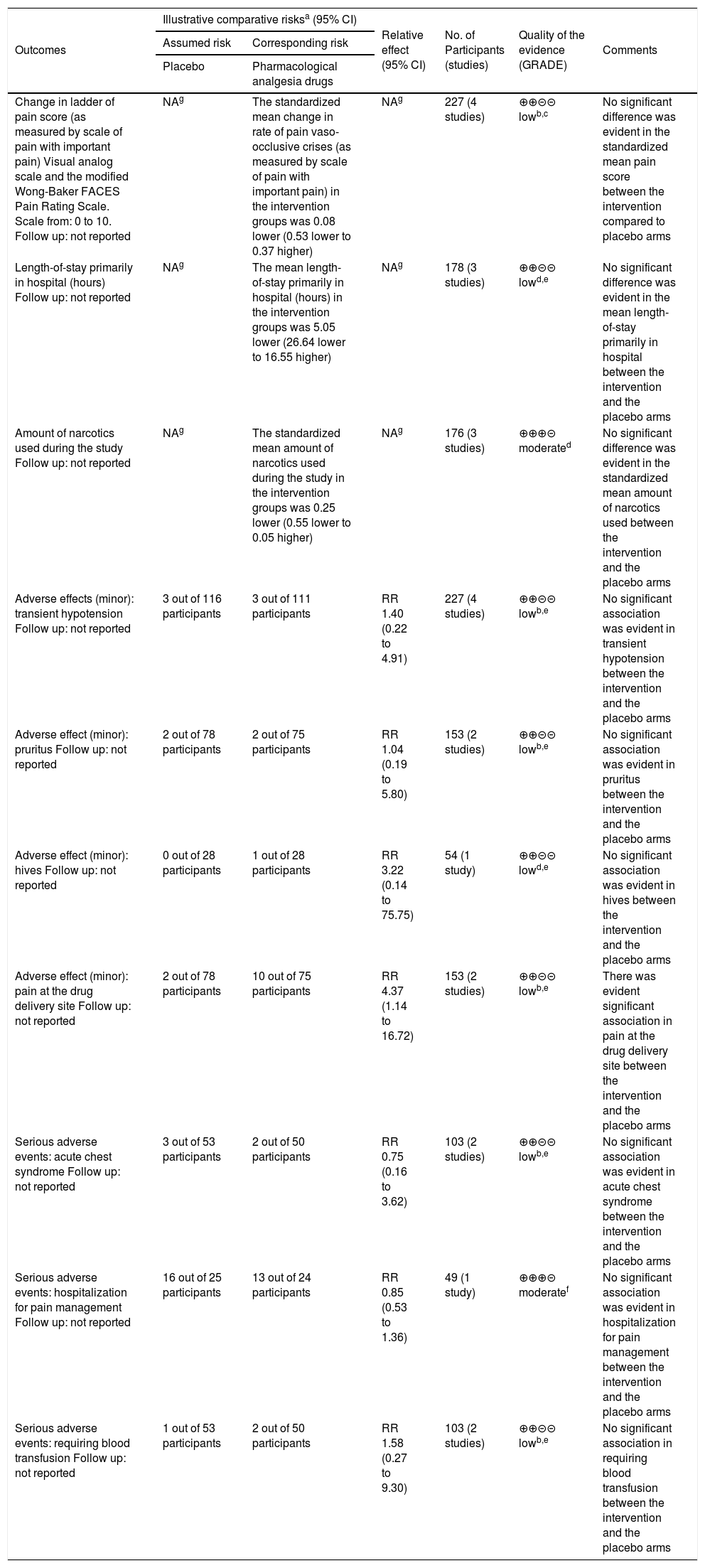
Pharmacological analgesic drugs compared to placebo for pediatric patients with acute painful crisis in sickle-cell disease (SCD). Summary of findings.
| Outcomes | Illustrative comparative risksa (95% CI) | Relative effect (95% CI) | No. of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Placebo | Pharmacological analgesia drugs | |||||
| Change in ladder of pain score (as measured by scale of pain with important pain) Visual analog scale and the modified Wong-Baker FACES Pain Rating Scale. Scale from: 0 to 10. Follow up: not reported | NAg | The standardized mean change in rate of pain vaso-occlusive crises (as measured by scale of pain with important pain) in the intervention groups was 0.08 lower (0.53 lower to 0.37 higher) | NAg | 227 (4 studies) | ⊕⊕⊝⊝ lowb,c | No significant difference was evident in the standardized mean pain score between the intervention compared to placebo arms |
| Length-of-stay primarily in hospital (hours) Follow up: not reported | NAg | The mean length-of-stay primarily in hospital (hours) in the intervention groups was 5.05 lower (26.64 lower to 16.55 higher) | NAg | 178 (3 studies) | ⊕⊕⊝⊝ lowd,e | No significant difference was evident in the mean length-of-stay primarily in hospital between the intervention and the placebo arms |
| Amount of narcotics used during the study Follow up: not reported | NAg | The standardized mean amount of narcotics used during the study in the intervention groups was 0.25 lower (0.55 lower to 0.05 higher) | NAg | 176 (3 studies) | ⊕⊕⊕⊝ moderated | No significant difference was evident in the standardized mean amount of narcotics used between the intervention and the placebo arms |
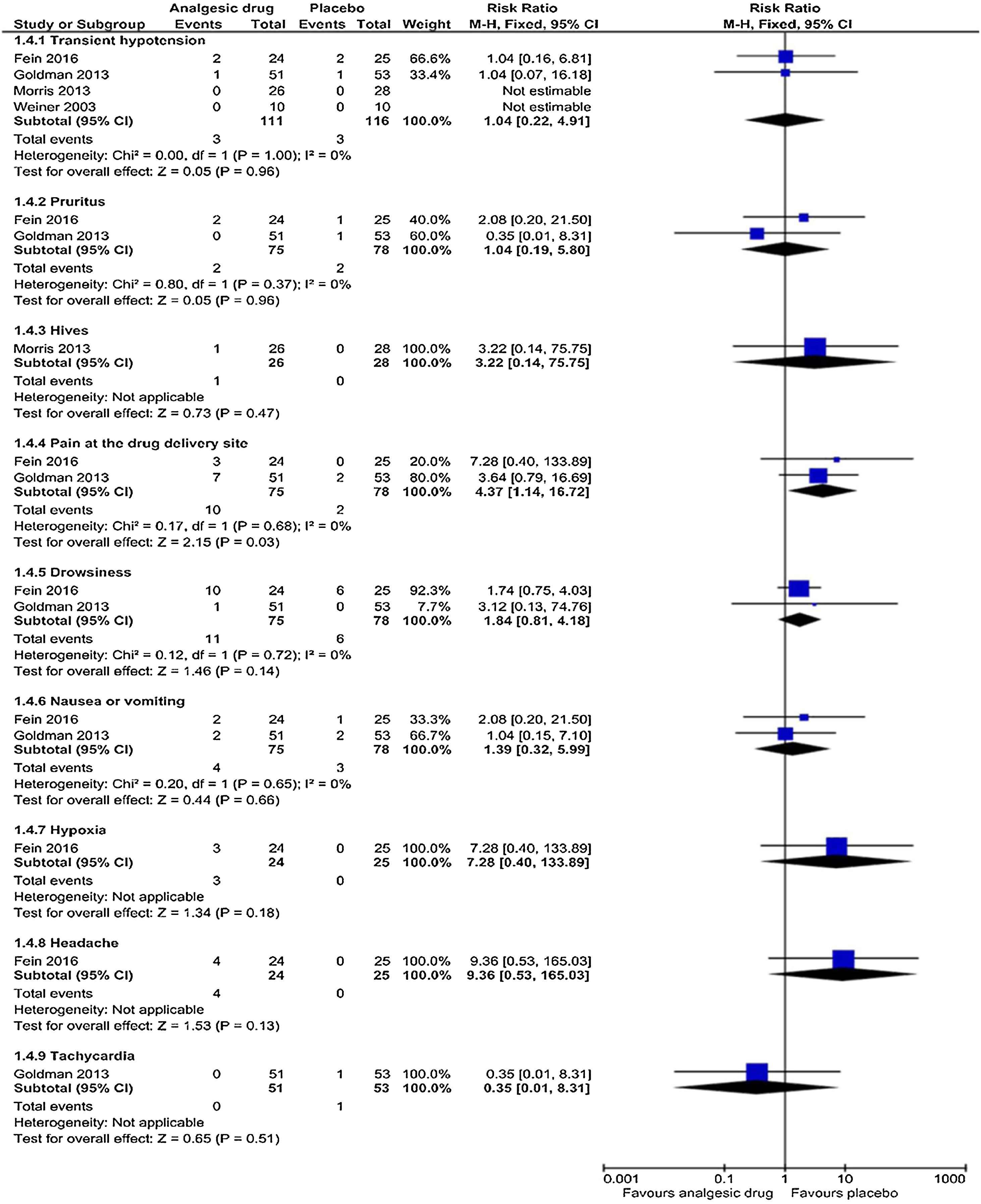
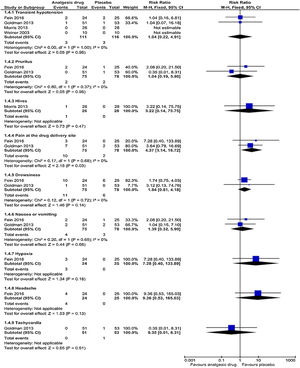
| Adverse effects (minor): transient hypotension Follow up: not reported | 3 out of 116 participants | 3 out of 111 participants | RR 1.40 (0.22 to 4.91) | 227 (4 studies) | ⊕⊕⊝⊝ lowb,e | No significant association was evident in transient hypotension between the intervention and the placebo arms |
| Adverse effect (minor): pruritus Follow up: not reported | 2 out of 78 participants | 2 out of 75 participants | RR 1.04 (0.19 to 5.80) | 153 (2 studies) | ⊕⊕⊝⊝ lowb,e | No significant association was evident in pruritus between the intervention and the placebo arms |
| Adverse effect (minor): hives Follow up: not reported | 0 out of 28 participants | 1 out of 28 participants | RR 3.22 (0.14 to 75.75) | 54 (1 study) | ⊕⊕⊝⊝ lowd,e | No significant association was evident in hives between the intervention and the placebo arms |
| Adverse effect (minor): pain at the drug delivery site Follow up: not reported | 2 out of 78 participants | 10 out of 75 participants | RR 4.37 (1.14 to 16.72) | 153 (2 studies) | ⊕⊕⊝⊝ lowb,e | There was evident significant association in pain at the drug delivery site between the intervention and the placebo arms |
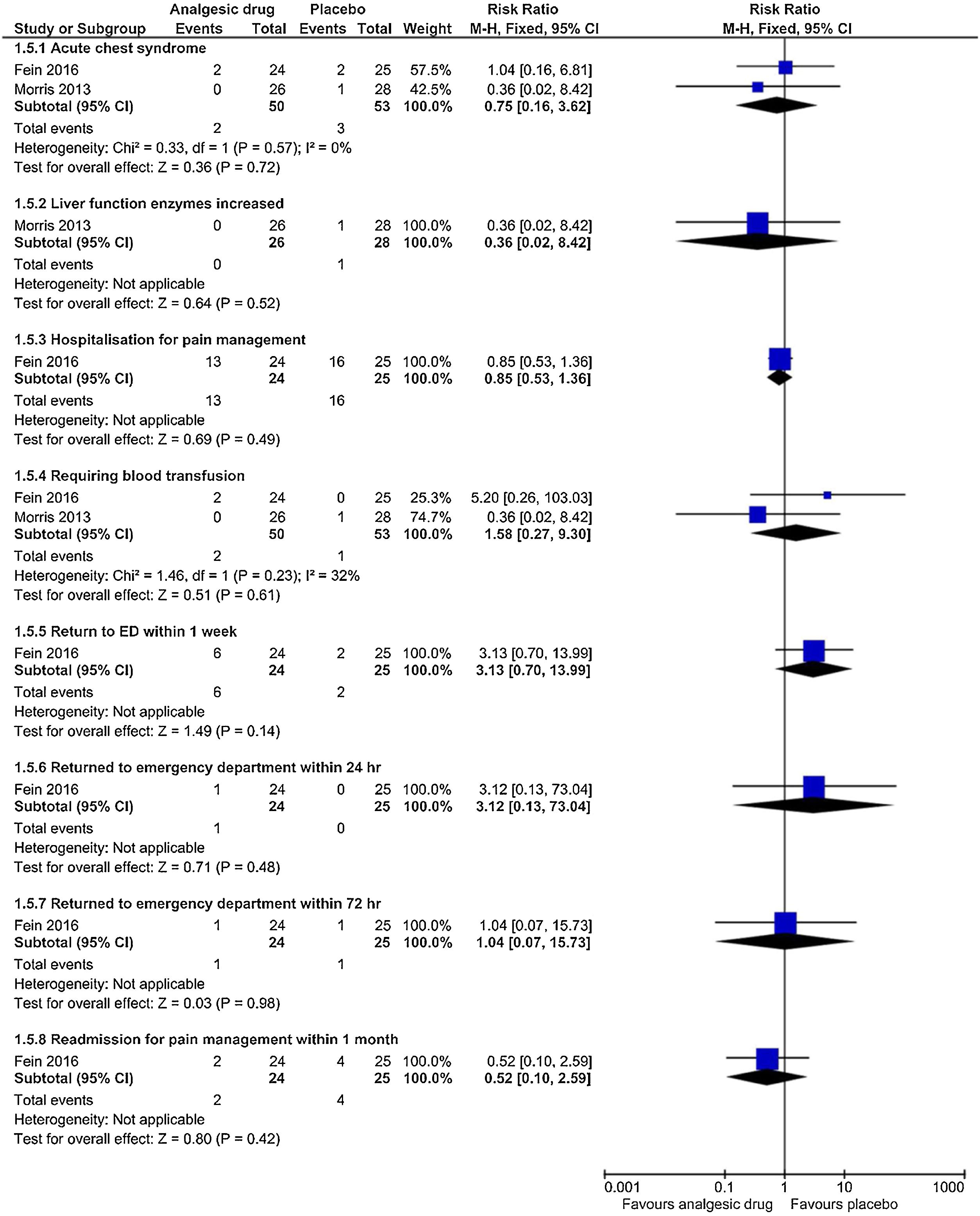
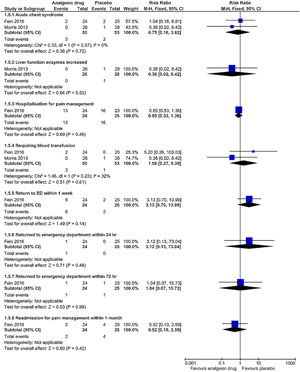
| Serious adverse events: acute chest syndrome Follow up: not reported | 3 out of 53 participants | 2 out of 50 participants | RR 0.75 (0.16 to 3.62) | 103 (2 studies) | ⊕⊕⊝⊝ lowb,e | No significant association was evident in acute chest syndrome between the intervention and the placebo arms |
| Serious adverse events: hospitalization for pain management Follow up: not reported | 16 out of 25 participants | 13 out of 24 participants | RR 0.85 (0.53 to 1.36) | 49 (1 study) | ⊕⊕⊕⊝ moderatef | No significant association was evident in hospitalization for pain management between the intervention and the placebo arms |
| Serious adverse events: requiring blood transfusion Follow up: not reported | 1 out of 53 participants | 2 out of 50 participants | RR 1.58 (0.27 to 9.30) | 103 (2 studies) | ⊕⊕⊝⊝ lowb,e | No significant association in requiring blood transfusion between the intervention and the placebo arms |
Patient or population: pediatric patients with SCD. Settings: large urban quaternary children’s hospital (pediatric emergency department [ED], Montefiore, New York, United States); ED (Toronto, Canada), children’s hospital research center (Oakland, California, United States); urban, tertiary care academic children’s hospital (United States). Intervention: pharmacological analgesic drugs. Comparison: placebo CI, confidence interval; NA, not applicable; RR, risk ratio. GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on confidence in the estimate of effect and is likely to change the estimate. Very low quality: The authors are very uncertain about the estimate.
The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
Evidence downgraded by one due to serious limitation of risk of bias: blinding of outcome assessment was not clearly described in some study and the aimed sample size intended for studies was not attained.
Evidence downgrade due to moderate inconsistency: studies analysis represent a moderate heterogeneity. Heterogeneity was explained by differences in outcomes (diminishing treatment effect with time). The quality of evidence was downgraded by one level from high to moderate.
The authors downgraded the quality of evidence by one due to serious risk of bias. Method of blinding of outcome assessment was not clearly reported; thus, it was downgraded by one level from high.
Evidence downgraded by one due to imprecision: wide 95% confidence intervals of the effect of outcomes of the intervention.
Owing to the poor number of included trials into the meta-analysis review (less than ten), implementation of a funnel plot to explore the risk of publication bias and sensitivity analyses were not feasible.
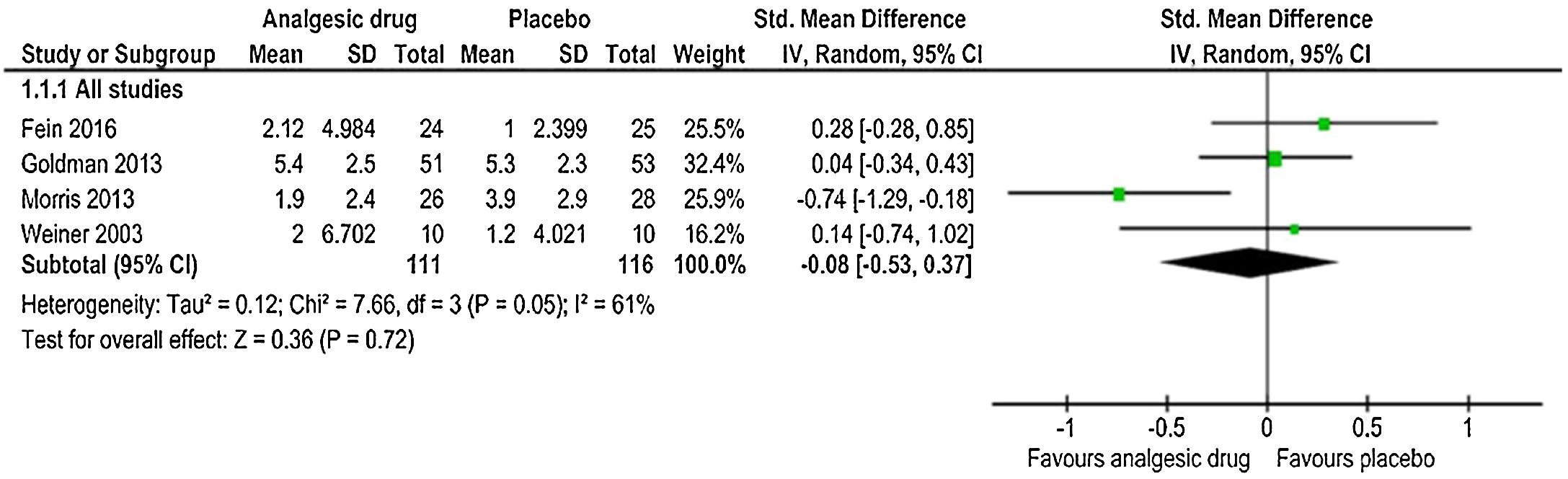
Change in the ladder of pain scoreIn the analysis of the change in the ladder of pain score, pooling the four studies (pain intensity rating was assessed either by visual-analogue scale (VAS) or other tools with age-appropriateness, i.e., the Faces Pain Scale) in an IV-weighted random-effects meta-analysis (Fig. 2) indicated that the SMD estimate did not show a significant difference between analgesic drug and control arms (SMD: −0.08, 95% CI: −0.53, 0.37); p=0.72. There was moderate heterogeneity (I2>50%) among studies that estimated the outcome of change in pain rating (chi2=7.66, p=0.05, I2=61%). The quality of evidence was low (Table 5).
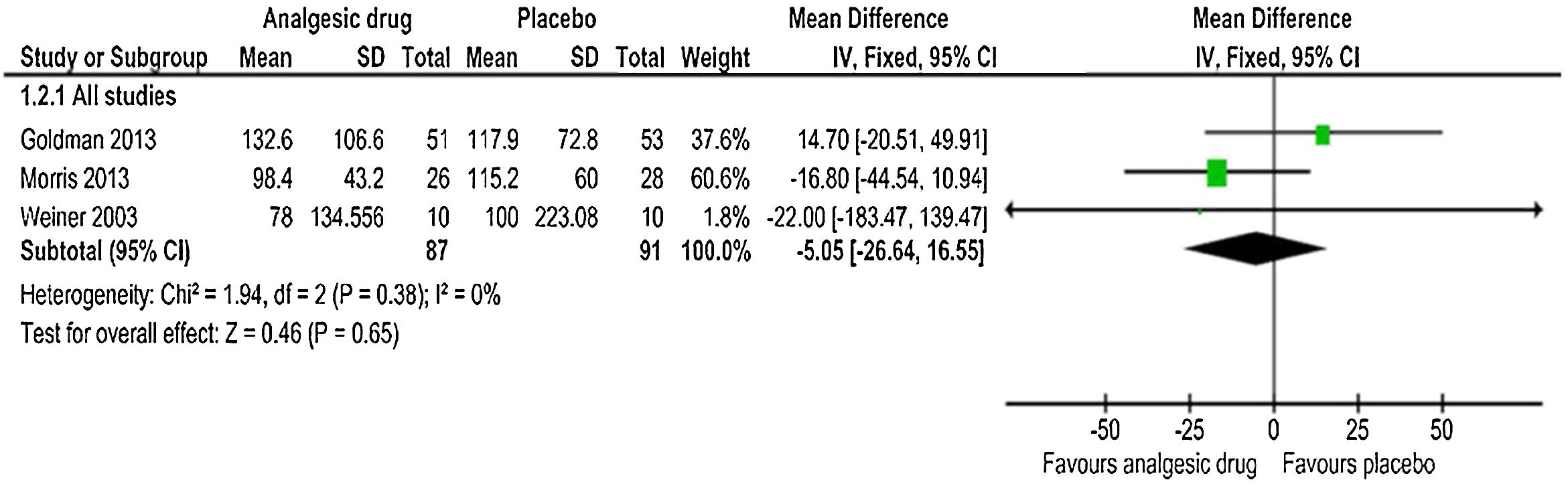
Length-of-stay in hospitalFor the three trials that reported hospital length-of-stay for uncomplicated acute episodes of sickle-cell pain crisis,55–57 the pooled mean difference estimate calculated with IV-weighted fixed-effects meta-analysis (Fig. 3) indicated no statistically significant difference between the analgesic drug and control groups (MD: −5.05h, 95% CI: −26.64, 16.55); p=0.65. The quality of evidence was low (Table 5), with no evidence of heterogeneity for the hospital length-of-stay outcome among those three trials (χ2=1.94, p=0.38, I2=0%).
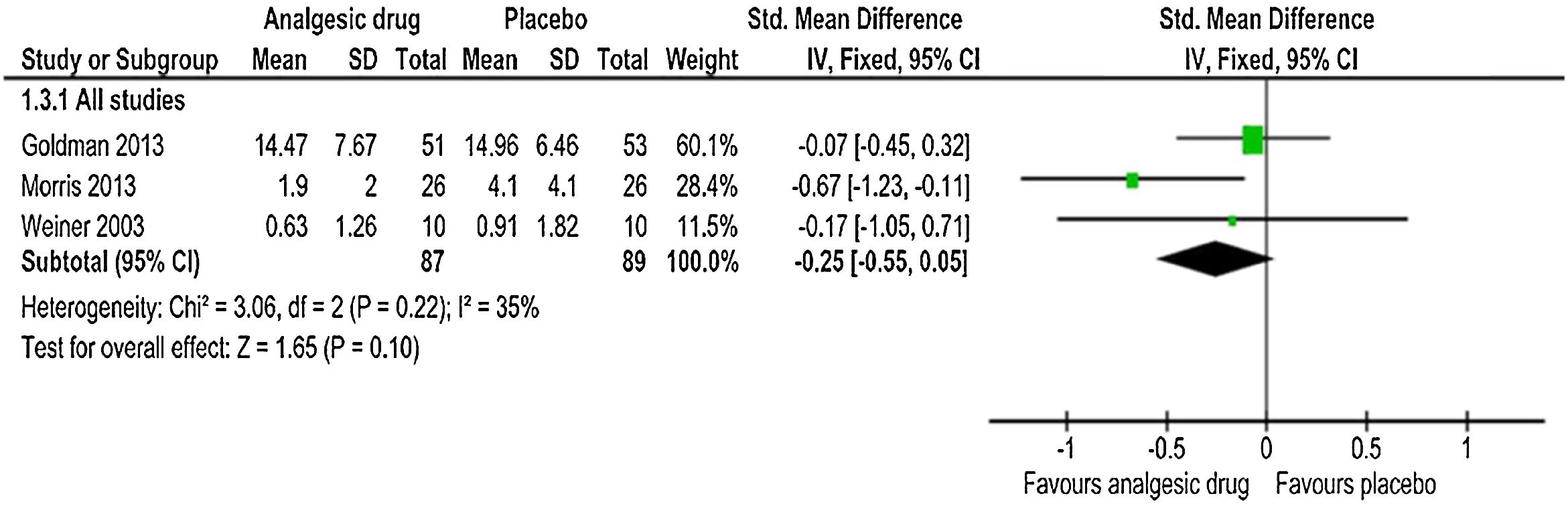
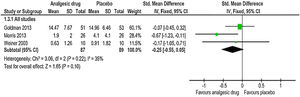
Amount of narcotics used during the studyThe same three trials55–57 reported the estimated pooled standardized mean difference, calculated with IV-weighted fixed-effects meta-analysis (SMD: −0.25, 95% CI: −0.55, 0.05); p=0.10 (Fig. 4). A non-statistically significant difference between the analgesic drug and control arms for the amount of narcotics used throughout the study was found, along with no important heterogeneity (χ2=3.06, p=0.22, I2=35%). The quality of evidence was moderate (Table 5). This outcome analysis was reported by one study (intranasal fentanyl) as an event.54 Five (20%) participants in placebo group received parenteral morphine compared to one (4%) participant in intervention group. In another study (arginine),56 two participants in placebo arms were ruled out from the analysis of the amount of opioid consumption on account of the narcotic records being incomplete. Thus, the number of participants in placebo arm for the total opioid use outcome in this study was decreased from 28 to 26. However, these participants were involved in all other outcomes analyses.
Adverse eventsThe subgroup analyses results are shown in Figs. 5 and 6. Each trial had their own manner to describe and tabulate the different numbers of adverse events. Therefore, the present authors planned to exhibit adverse events data in proportion to the minor (transient hypotension, pruritus, hives, pain at the drug delivery site, drowsiness, nausea or vomiting, hypoxia, headache, and tachycardia) and serious adverse events (acute chest syndrome; liver function enzymes increased; hospitalization for pain management; requiring blood transfusion; return to ED within 24h, 72h, or one week; and readmission for pain management within one month) during the study. For the proportion experiencing minor adverse events presented by pain at the drug delivery site, there was a statistically significant difference (p=0.03) in the combination of data from two studies (intranasal fentanyl citrate and intravenous magnesium)54,55 along with no important heterogeneity (χ2=0.17, p=0.68, I2=0%). The quality of evidence was low (Table 5).
DiscussionThe results of this systematic review and meta-analysis show that most of the studies that reported on pain score ratings demonstrate a non-significant improvement to the changes in the ladder of pain scores for acute sickle-cell pain crisis. Only three studies reported on hospital length-of-stay and amount of narcotics used, finding a non-significant reduction in these outcome measures. In the adverse event outcome of those experiencing minor side-effects, significant evidence of association of pain at the drug delivery site was demonstrated only in the studies that used intravenous magnesium and intranasal fentanyl citrate. These pharmacological analgesics have inadequate treatment impacts on managing uncomplicated and isolated acute sickle-cell pain crisis in pediatric departments.
Since opioid derivatives have been assigned as the “gold standard” to manage sickle-cell pain crisis, their actions are limited only to a nociceptive, but they do not show relevant action upon the tremendous cascade that gives rise to VOC associated with inflammatory syndrome. Thus, there has been an increase in the ongoing search for new treatment options.20,23 Given these breakthroughs, the pathophysiological comprehension of VOC has sparked curiosity in the scientific literature. These three components (intravenous magnesium, arginine, and inhaled nitric oxide) have therefore revolutionized this in-depth understanding, as well as promoting new therapeutic approaches,9,10,25,43,58 but they require more evidence.
To the authors’ knowledge, the present study is the most recent investigation exhibiting the gaps of pharmacological drug effects in the pediatric age group with acute sickle cell pain crisis. Moreover, three of the included trials (intravenous magnesium, arginine, and inhaled nitric oxide) used interventions with non-analgesic properties but having an analgesic purpose, which is to limit the sickle-cell pathophysiological mechanisms from VOC. The findings of this meta-analysis are consistent with the previous meta-analysis conducted by Dunlop and Bennett22 there were gaps of cross advantage of treatment trials in dwarfed of the acute episode sickle-cell pain crisis as well as in opioid consumption, and hospital length-of-stay. However, their meta-analysis involved nine RCTs (six with placebo control and three inter-treatment trials), and included children and adults without combining them. Their findings did show the complete alleviation of acute sickle-cell pain crisis in all participants, but distinctly proved that none of the intervention trials were able to entirely manage this crisis state, and additionally the evidence bases were inadequate.
The heterogeneity test of the included studies across all outcomes in the efficacy of these treatments in pediatric patients with acute sickle-cell pain crisis was not substantial (I2>80%). There was moderate risk of statistical heterogeneity across the meta-analysis of the change in the ladder of pain score. This heterogeneity could be explained by the different tools used and the methods of pain assessment, such as the VAS or the Faces Pain Scale, and the small sample sizes of the included studies. The non-risk of statistical heterogeneity in meta-analysis of hospital length-of-stay is accounted for by the use across studies of the same or similar methods to estimate the length-of-stay in hospital; beside to it minimizes the inconstantly of this outcome. Also, the non-important risk of statistical heterogeneity in the amount of narcotics used is partially consistent with the moderate level of the quality of evidence.
The meta-analysis excluded studies that recruited and combined different age groups, and those that compared between-treatments, which was done in order decrease the risk of inclusion bias. In four small-scale studies of uncomplicated acute sickle-cell pain crisis treatment, contributing data from 227 registered patients, the present study did not have enough evidence emphasizing the adequate management of the pediatric age group with sickle-cell pain crisis. Furthermore, a multicenter sickle-cell randomized placebo-controlled trial with intravenous magnesium reported only sickle-cell cases including the HbSS and HbSβ0 phenotypes, with inadequate and trivial results regarding length-of-stay and opioid derivatives used.43 Besides, there were the immense barriers during the literature search, as studies appraising acute sickle-cell painful episode management have ended in an untimely manner because of the lack of available participants in trials.42,58,59 The adverse events were split as the proportion experiencing minor and serious events for this meta-analysis, data were overly diverse and were difficult to investigate, hindering distinct pooled examination. In contrast, it was possible to pool two studies showing that intravenous magnesium and intranasal fentanyl citrate caused pain at the drug delivery site. The intravenous magnesium event was consistent with another study that showed warmth at the delivery site in the treatment arm.43
The pertinent strengths of this systematic review and meta-analysis include that the study provided the latest evaluation of the efficiency and safety of uncomplicated acute sickle-cell pain crisis treatment in the pediatric age group. The authors also found three ongoing studies,60–62 focusing only on participants aged 3–22. One of them defines the ideal level of intranasal ketamine that has shown a salutary effect for analgesia in subsequent trials of pediatric patients in prehospital medicine, emergency medicine, and oncology.62,63 Prior studies confirm that ketamine also provides clinically substantial analgesia for sickle-cell pain, as well as reducing opiate consumption.21,64,65 In one study that compared the analgesic benefits of intranasal fentanyl to intranasal ketamine, it was found that both provide equivalent analgesia.66 All confidence intervals in the present study were revealed to be wide, thus highlighting the significance and magnitude of continued research in this realm to fully manage the crisis and to recognize the additional reason for these important differences. The meta-analysis of other outcomes apart from the change in the ladder of pain score showed the non-importance of statistical heterogeneity, although these outcomes had insufficient statistical power. Notwithstanding, the GRADE analysis of the quality for outcomes almost evinces a low level of strength of evidence owing to the presence of bias, inconsistency, and imprecision; except for the amount of narcotics used, for which the evidence had a moderate level of quality.
This meta-analysis review has some limitations that can be reduced in prospective research. The majority of the trials involved in this review originated in areas with a low burden of SCD, despite the authors’ extensive scientific research strategy, which identified numerous pertinent trials. Thereby, the greater part of the eligible studies had inadequate sample size, which may have promoted the confounders of reduction of the precision in the effect size estimate, the lessening of the significance of findings, and the under-powered design of the studies. Some included studies were conducted in a single center, which may lead to confounding factors in the insufficient sample size. Most of the RCTs exploring sickle-cell pain crisis recruited and combined three different age groups (children, young adults, adults and seniors), and did not provide unique data for each participants’ age group, the use of which would have involved many trials in the review. The pain scale assessments and methods – whether regarding its intensity or relief – used in the studies included in the meta-analysis review were not standardized; for instance, two trials used two types of pain scales.55,56 Thus, the comparison between trials and the pooling of data were challenging. Nevertheless, these circumstances might affect the analysis outcomes. Owing to the poor number of eligible trials into the meta-analysis review, implementation of a funnel plot to explore the risk of publication bias and sensitivity analyses was not feasible.
ConclusionIn this study, pharmacological analgesic treatments appeared dubious in the improvement of pain intensity and relief for acute pain crisis in pediatric patients with SCD. Therefore, no decisive deduction about the clinical efficacy of these pharmacological analgesics was made. More RCTs are required and may attest the strengths evidence of each study. This may provide further information regarding these analgesic drugs in order to improve reduction in the length of stay, primarily in-hospital, and in reducing the amount of narcotics used. Well-designed studies along with plenty of participants are needed for demonstrating significant differences. Studies should ensure the inclusion of a large sample size and a balance of each SCD phenotype in trials, to avert overestimation of results in the upcoming research. Single-center trials should be joined to multi-center trials in order to have enough participants and to have sufficient study power. Researchers should consider expanding their studies in settings with a high burden of SCD.
FundingThis work was supported by grants from the Chinese National Natural Science Fund, No. 81170005 and No. 81670007.
Conflicts of interestThe authors declare no conflicts of interest.
This work was supported by grants from the Chinese National Natural Science Fund, No. 81170005 and No. 81670007. The authors would like to recognize the contributions, encouragements, and endorsements from Professor Yi Guo, and Mrs. Loraine S. Castellano. Finally, they would like to express their gratitude to Dr. Salama A. Ali and Dr. Elsam Koshy for their immense support in reviewing the article.
Please cite this article as: Saramba MI, Shakya S, Zhao D. Analgesic management of uncomplicated acute sickle-cell pain crisis in pediatrics: a systematic review and meta-analysis. J Pediatr (Rio J). 2020;96:142–58.