To identify risk factors for chronic kidney disease progression in Brazilian children and to evaluate the interactions between factors.
MethodsThis was a multicenter prospective cohort in São Paulo, involving 209 children with CKD stages 3–4. The study outcome included: (a) death, (b) start of kidney replacement therapy, (c) eGFR decrease >50% during the followup. Thirteen risk factors were tested using univariate regression models, followed by multivariable Cox regression models. The terms of interaction between the variables showing significant association with the outcome were then introduced to the model.
ResultsAfter a median follow-up of 2.5 years (IQR=1.4–3.0), the outcome occurred in 44 cases (21%): 22 started dialysis, 12 had >50% eGFR decrease, seven underwent transplantation, and three died. Advanced CKD stage at onset (HR=2.16, CI=1.14–4.09), nephrotic proteinuria (HR=2.89, CI=1.49–5.62), age (HR=1.10, CI=1.01–1.17), systolic blood pressure Z score (HR=1.36, CI=1.08–1.70), and anemia (HR=2.60, CI=1.41–4.77) were associated with the outcome. An interaction between anemia and nephrotic proteinuria at V1 (HR=0.25, CI=0.06–1.00) was detected.
ConclusionsAs the first CKD cohort in the southern hemisphere, this study supports the main factors reported in developed countries with regards to CKD progression, affirming the potential role of treatments to slow CKD evolution. The detected interaction suggests that anemia may be more deleterious for CKD progression in patients without proteinuria and should be further studied.
Identificar os fatores de risco para progressão da DRC em crianças do Brasil e avaliar as interações entre os fatores.
MétodosCoorte prospectiva multicêntrica em São Paulo, envolvendo 209 crianças com DRC em estágios 3-4. O desfecho do estudo incluiu: a) óbito, b) início da terapia de substituição renal, c) redução de > 50% na taxa estimada de filtração glomerular (eGFR) durante o acompanhamento. Foram testados 13 fatores de risco com o modelo de regressão univariada seguido do modelo de regressão multivariado de Cox. Os termos de interação entre as variáveis mostraram associação significativa e foram introduzidos ao modelo.
ResultadosApós média de acompanhamento de 2,5 anos (IIQ=1,4 a 3,0), 44 casos (21%) apresentaram desfecho: 22 iniciaram diálise, 12 apresentaram redução de > 50% na eGFR, sete foram submetidos a transplante e três morreram. Estágio avançado de DRC no acometimento (RR=2,16, IC=1,14-4,09), proteinúria nefrótica (RR = 2,89, IC=1,49-5,62), idade (RR – 1,10, IC=1,01-1,17), escore Z da pressão arterial sistólica (RR=1,36, IC=1,08-1,70) e anemia (RR=2,60, IC – 1,41-4,77) foram associados ao resultado. Foi detectada interação entre anemia e proteinúria nefrótica na primeira visita (V1) (RR=0,25, IC=0,06-1,00).
ConclusõesComo a primeira coorte de DRC no hemisfério sul, este estudo é concordante com os principais fatores relatados em países desenvolvidos com relação à progressão da DRC, afirmando o possível papel dos tratamentos para mostrar a evolução da DRC. A interação detectada sugere que a anemia pode ser mais nociva na progressão da DRC em pacientes sem proteinúria e deve ser ainda mais estudada.
Chronic kidney disease (CKD) has devastating consequences in children and adolescents. It is a progressive disease ranging from anatomical changes and mild functional urinary tract disorders to the complete loss of kidney functions. CKD is defined as a glomerular filtration rate (GFR) lower than 90mL/min/1.73m2 for more than three months, and is classified in five stages based on the severity of GFR decrease.1
Several types of treatment can be used to slow disease progression or to minimize associated comorbidities.2 The treatment is different for each stage of CKD, and the disease evolution rate is not uniform. Thus, knowledge of the risk factors for CKD progression has important practical implications, because it allows preparation for and optimization of therapy.
Previous studies have suggested that a higher CKD stage is associated with a greater likelihood of disease advance.3 Furthermore, nephrotic proteinuria, hypoalbuminemia, high blood pressure (BP), dyslipidemia, male gender, and anemia could accelerate GFR decrease.4,5 Puberty can also increase the rate of CKD progression; it is believed that sex hormones can promote faster deterioration of kidney function.6
The aforementioned studies were conducted in economically developed countries in the northern hemisphere. However, the factors associated with faster CKD progression found in these studies may not be entirely applicable to other geographic regions or to less economically developed countries, which limits the generalizability of the results. Therefore, it is important to determine the factors involved in CKD progression in other geographic regions because of the genetic, social, and cultural differences. Moreover, differences in the infrastructure of health services among the continents can result in distinct patterns of disease progression, because CKD treatment is expensive and involves an elaborate organization of specialized health services. Thus, it is possible that macroeconomic factors interfere with the provision of therapy, which may affect the progression of CKD in children.
In addition, the risk factors for CKD progression may interact in such a way that a particular risk factor may change the effect of another risk factor in the development of CKD, and these potential interactions have seldom been assessed in children.
The aim of this study was to identify the main risk factors for the progression of CKD in a sample of Brazilian children and adolescents and to evaluate potential interactions among these factors.
Materials and methodsThis study evaluated data from a multicenter prospective cohort study involving children and adolescents who underwent medical follow-up for CKD stages 3 or 4 in seven different centers in the state of São Paulo, Brazil (SP-CKDkid). The authors stipulated in advance that it would be feasible to recruit a convenience sample including approximately 200 patients; their first visit would be at the onset of the study and a follow-up period of approximately 2 years, with visits every six months.
The inclusion criteria were (a) age from 1 to 17 years at the beginning of the study, (b) estimated GFR lower than 60mL/min/1.73m2 and higher than 15mL/min/1.73m2 for at least three months, (c) signing of the informed consent form by parents or legal guardians, and of the term of assent by children older than 12 years, and (d) previous follow-up with proper adherence to follow-up for at least three months.
Patients who received any transplant, those with malignancies treated in the past 24 months, those with human immunodeficiency virus (HIV), and those who planned to move to another city subsequently to the day of invitation to participate in the cohort were excluded from the study.
The combined outcome of the study included the occurrence of at least one of the following events during follow up: (a) death, (b) start of kidney replacement therapy (dialysis or transplantation), or (c) a decrease by more than 50% of the estimated GFR.
GFR was estimated from measurements of height and serum creatinine levels made at each medical center and at each visit using the Schwartz formula.7
The potential risk factors considered were: (a) patient age at the first visit (V1), (b) sex, (c) etiology of CKD as a binary variable defined as congenital anomalies of the kidney and urinary tract (CAKUT) or other causes combined, (d) race, on the basis of self-definition by the patient and/or legal guardian and classified as a binary variable of white or non-white (for this variable, data from 179 patients were available), (e) socioeconomic status of the patient as established by the criteria of the Brazilian Association of Research Companies (Associação Brasileira de Empresas de Pesquisa [ABEP]) in 2011, in which A is the wealthiest class, (f) occurrence of puberty during the follow-up defined as Tanner≥2 for any characteristic; (g) CKD stage at V1, defined as a binary variable identified by GFR≥30 and <60 or GFR<30, (h) nephrotic proteinuria at V1, defined as a binary variable by the presence of a protein/creatinine ratio greater than 2g/g of creatinine in the isolated urine samples, (i) BP at V1, defined as the Z-score of the mean of three consecutive BP measurements,8 (j) anemia at V1, defined as a hemoglobin level lower than the fifth percentile for sex and age,9 (k) serum cholesterol at V1, defined as a continuous quantitative variable in mg/dL, (l) serum bicarbonate at V1, defined as a continuous variable in mEquiv./L, and (m) serum parathyroid hormone (PTH) at V1, defined as a continuous quantitative variable in pg/mL.
All clinical variables and laboratory analyses were collected and processed in the participating medical centers, and a central laboratory was not used in this study.
Median and interquartile range (IQR) were used to describe quantitative variables, and the qualitative variables were described using frequency tables. The effect of each potential risk factor on the combined outcome was evaluated by a Cox regression analysis for each variable (univariate analysis). The variables with p-values smaller than 0.2 in the univariate analysis were then included in a multivariable Cox model. In this model, all preselected variables were included; subsequently, the variables that did not attain a statistically significant association with the outcome were removed (backward selection). Furthermore, terms of interaction between the variables that showed a statistically significant association with the combined outcome were introduced to the Cox model to assess the occurrence of interactions between the variables and the combined outcome of the study.
A 5% level of significance (α<0.05) was adopted in all tests to refute the null hypothesis, and the software Stata (Stata Statistical Software: Release 14. College Station, TX, USA), was used for all statistical calculations.
The Research Ethics Committees of the seven participating centers approved the study protocol, and all study procedures were conducted after the parents/legal guardians signed the informed consent form and patients older than 12 years signed the term of assent.
ResultsFrom August 2013 to August 2016, 245 patients were preselected for the study; however, 36 cases (15%) were excluded from the analysis for the following reasons: (a) individuals with CKD stage 5 (15 cases) or stage 2 (four cases) at V1, or (b) missing data (17 cases). Compared with the children included in the study, the participants excluded for the aforementioned reasons showed no significant differences in age (9.3 [IQR=5.4–13.2] years vs. 9.9 [IQR=5.3–13.5] years, respectively, p=0.752), sex distribution (male=59% and 69%, respectively, p=0.251), and percentage of CAKUT as the etiology of CKD (73% vs. 86%, respectively, p=0.088).
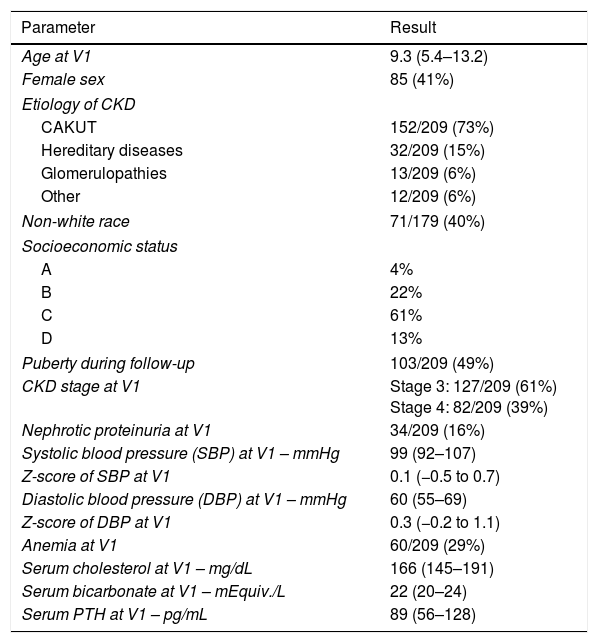
After exclusion, the analysis was based on a sample of 209 children with CKD. The median follow-up period was 2.5 years (IQR=1.4–3.0) with 88% of patients retained for a second visit, 70% for a third visit, and 67% for a fourth visit. Sample demographics and potential risk factors are shown in Table 1.
Demographics and potential risk factors in the sample.
| Parameter | Result |
|---|---|
| Age at V1 | 9.3 (5.4–13.2) |
| Female sex | 85 (41%) |
| Etiology of CKD | |
| CAKUT | 152/209 (73%) |
| Hereditary diseases | 32/209 (15%) |
| Glomerulopathies | 13/209 (6%) |
| Other | 12/209 (6%) |
| Non-white race | 71/179 (40%) |
| Socioeconomic status | |
| A | 4% |
| B | 22% |
| C | 61% |
| D | 13% |
| Puberty during follow-up | 103/209 (49%) |
| CKD stage at V1 | Stage 3: 127/209 (61%) Stage 4: 82/209 (39%) |
| Nephrotic proteinuria at V1 | 34/209 (16%) |
| Systolic blood pressure (SBP) at V1 – mmHg | 99 (92–107) |
| Z-score of SBP at V1 | 0.1 (−0.5 to 0.7) |
| Diastolic blood pressure (DBP) at V1 – mmHg | 60 (55–69) |
| Z-score of DBP at V1 | 0.3 (−0.2 to 1.1) |
| Anemia at V1 | 60/209 (29%) |
| Serum cholesterol at V1 – mg/dL | 166 (145–191) |
| Serum bicarbonate at V1 – mEquiv./L | 22 (20–24) |
| Serum PTH at V1 – pg/mL | 89 (56–128) |
Quantitative variables are expressed as the median (interquartile range).
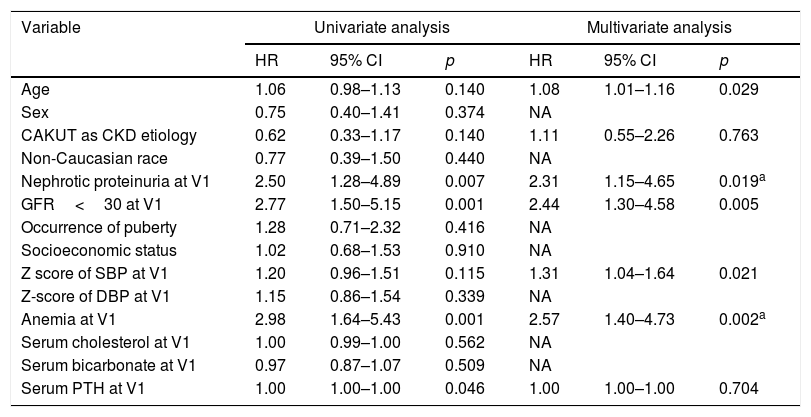
The baseline GFR was 33.1mL/min/1.73m2 (IQR=24.7–40.9) and during follow-up this rate decreased by −1.6mL/min/1.73m2 (IQR=−6.3 to 1.6). At the end of follow-up, the combined outcome occurred in 44 cases (21%). Of these patients, 22 started dialysis, 12 lost >50% of the GFR, seven received kidney transplants, and three died. The results of the Cox regression analysis are shown in Table 2.
Analysis of potential risk factors in the progression of CKD.
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Age | 1.06 | 0.98–1.13 | 0.140 | 1.08 | 1.01–1.16 | 0.029 |
| Sex | 0.75 | 0.40–1.41 | 0.374 | NA | ||
| CAKUT as CKD etiology | 0.62 | 0.33–1.17 | 0.140 | 1.11 | 0.55–2.26 | 0.763 |
| Non-Caucasian race | 0.77 | 0.39–1.50 | 0.440 | NA | ||
| Nephrotic proteinuria at V1 | 2.50 | 1.28–4.89 | 0.007 | 2.31 | 1.15–4.65 | 0.019a |
| GFR<30 at V1 | 2.77 | 1.50–5.15 | 0.001 | 2.44 | 1.30–4.58 | 0.005 |
| Occurrence of puberty | 1.28 | 0.71–2.32 | 0.416 | NA | ||
| Socioeconomic status | 1.02 | 0.68–1.53 | 0.910 | NA | ||
| Z score of SBP at V1 | 1.20 | 0.96–1.51 | 0.115 | 1.31 | 1.04–1.64 | 0.021 |
| Z-score of DBP at V1 | 1.15 | 0.86–1.54 | 0.339 | NA | ||
| Anemia at V1 | 2.98 | 1.64–5.43 | 0.001 | 2.57 | 1.40–4.73 | 0.002a |
| Serum cholesterol at V1 | 1.00 | 0.99–1.00 | 0.562 | NA | ||
| Serum bicarbonate at V1 | 0.97 | 0.87–1.07 | 0.509 | NA | ||
| Serum PTH at V1 | 1.00 | 1.00–1.00 | 0.046 | 1.00 | 1.00–1.00 | 0.704 |
SBP, systolic blood pressure; DBP, diastolic blood pressure.
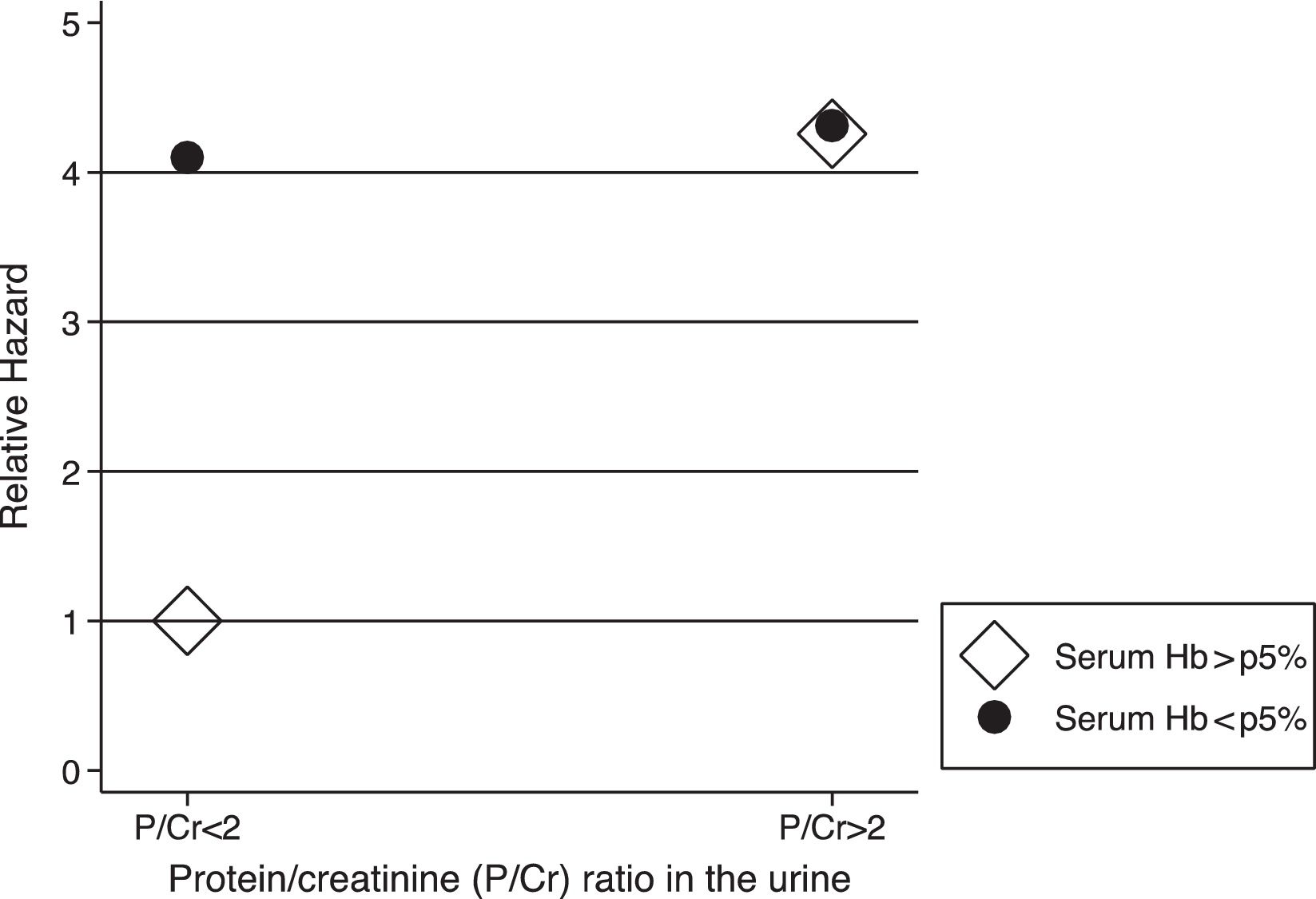
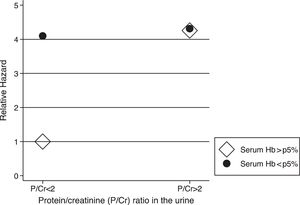
The analysis of the interactions between risk factors revealed an association between anemia at V1 and nephrotic proteinuria (hazard ratio (HR) for the interaction=0.25; 95% CI=0.06–1.00; p=0.05), indicating that hemoglobin levels greater than fifth percentile were associated with a lower pace of CKD progression only in children who did not present with nephrotic proteinuria, and that the effect was not evident in cases involving nephrotic proteinuria (Fig. 1).
DiscussionThe main finding of this study was the corroboration of the factors previously known to be associated with CKD progression, including advanced CKD stage, nephrotic proteinuria, increased age, high BP, and anemia. Furthermore, we demonstrated the presence of interactions between risk factors for the development of CKD, in which the effect of a particular factor could be modified by another factor, suggesting a possible modulation of the effects of two or more factors in determining the rate of loss of kidney function. In this sample, the deleterious effects of anemia seemed to be more important in children without proteinuria, and this could have implications for clinical practice.
CKD stage is a well-known factor that is associated with disease progression, and a plausible explanation is a faster impairment of kidney function as the disease progresses.3,5,10
Proteinuria, another recognized marker of poor CKD prognosis, was also associated with the study's outcome. In the present series, the combined outcome was observed in 40% of the children with nephrotic proteinuria, versus 16% of those without nephrotic proteinuria. These findings support the association between nephrotic proteinuria and faster CKD progression.10,11 This association is so clear that proteinuria was recently postulated to be not only a biomarker of progression, but also a causal factor of kidney injury. Experimental data indicate that albuminuria has toxic effects on kidney tissue and leads to a progressive loss of kidney function. The mechanisms by which albuminuria accelerates kidney injury include the stimulation of pro-inflammatory and fibrosis-inducing factors, culminating in damage to the interstitial tubules.12 Whether proteinuria is a marker or an agent of kidney injury in CKD is unknown at present.13–15
The increase in BP in the present model was associated with worse progression of CKD, and this result is in agreement with those of previous studies conducted in several regions of the northern hemisphere, both in the west5,16 and the east.17 In an American study, children with elevated BP had a 38% abbreviated time to attain the composite outcome, which is in agreement with the 31% increase in the risk of reaching the composite outcome of the present sample.5 This association appears to be robust, since an European study also showed that the hazard for progression to the end point was 0.65 (95% CI, 0.44–0.94) with intensified BP control, implying that an optimal BP control can slow the progression of CKD.18
In the present cohort, we chose to express BP as the Z-score instead of using BP values in mmHg because this sample comprised different age groups, including children aged 1–17 years. According to the model obtained in this analysis, an increase of 1unit in the Z-score of systolic BP at V1 was associated with a 1.31-fold higher probability of reaching the combined outcome of the study. A possible explanation for the association between BP and increased risk of CKD progression could be a selection bias, due to the possibility of higher BP in children with worse kidney function. In this case, the effect would be explained by the poor kidney function and not by BP. This result was not observed in this study, and the difference in the Z-score of systolic BP among children in stages 3 and 4 at V1 was only 0.002 SD, which suggests that BP has an independent effect on the progression of CKD. The underlying mechanism of the effect of BP on the progression of CKD is similar to that hypothesized to explain the effect of BP on the cardiovascular system, with a prominent role of high BP causing vascular stiffness, which secondarily affects renal microcirculation.19,20 Whatever the explanation for the effect of BP on the progression of CKD, it appears to be very pervasive, since similar results are reported in studies involving different racial and genetic backgrounds.
The effect of age on the progression of CKD may be explained by three hypotheses: (a) an increase in the loss of kidney function associated with the development of puberty, (b) a higher proportion of children in more advanced stages of CKD, and (c) a higher rate of glomerular diseases that evolve more rapidly in older children. In the present sample, the effect of age may possibly be explained by the first hypothesis, because the predominant diagnostic profile of this sample was CAKUT, with little representation of glomerular diseases in all age groups. This lower frequency of glomerular diseases may be due to the delay in the diagnosis and referral of patients with these diseases in Brazil. However, the present results did not allow the confirmation of this hypothesis. Finally, the second hypothesis cannot be accepted for this sample, because the percentage of children in stages 3 and 4 was similar among the different age groups.
The presence of anemia at study onset was also associated with poor prognosis of CKD. This result is corroborated by a report of a seven-center cohort study on CKD progression in 108 adolescents from the United States, which demonstrated that the annualized decline in GFR was greater among patients with anemia with an accelerated decline of 8.2mL/min/1.73m2 (95% CI: 4.1–12),21 as well as the data from the collaborative NAPRTCS study, which evaluated 4166 children and revealed that patients with anemia at the beginning of follow-up had a 52% increase in the risk of disease progression compared with that of patients without anemia.22 The mechanism proposed to explain the effect of anemia on CKD progression is that kidney tissue hypoxia stimulates the production of cytokines that induce fibrosis and trials in adults have suggested that correction of anemia with erythropoietin may decrease the risk of CKD progression.9,23
Altogether, the present results are in agreement with those of previous studies. However, some factors regarded as important in other studies, such as baseline diagnosis, male gender, puberty, and dyslipidemia, were not associated with a poor outcome of CKD in the present study. Considering the baseline diagnosis, we suppose that this factor was not identified probably because of the evident predominance of CAKUT cases in the present cohort, which limited the present study's ability to establish differences with other baseline diagnoses. Moreover, although no significant association was observed between the occurrence of puberty and CKD progression, age was associated with worse outcomes and may be an indirect indication of the effect of puberty. For other factors (male sex and dyslipidemia), there may be real differences between the populations. However, the possibility that the power of the present study was insufficient to demonstrate the presence of these associations cannot be ruled out because of the number of participants in this cohort and the relatively short follow-up.
The study that was most similar to the present was conducted in Brazil aiming to identify a model for predicting CKD progression; it included 147 children, who were retrospectively evaluated for approximately 4.5 years.24 The most accurate model included the estimated GFR and proteinuria at admission and primary kidney disease, with glomerulopathy representing a risk factor for faster disease progression. Except for the etiologic diagnosis of CKD, the findings of that study were similar to the present results.
A limitation of the present study was that the biochemical analyses were conducted in individual laboratories at each center, which reduced the methodological uniformity of these measurements. GFR estimates were based on creatinine measurements made at each center, and this source of variation is a potential limitation of the present findings, because the combined outcome of the study included a decrease by more than 50% in the estimated GFR at any point of the follow-up. However, this potential risk bias must be low, because the present’ statistical model was repeated considering only the clinical outcomes observed among the cohort members: (a) death or (b) initiation of kidney replacement therapy (dialysis or transplantation). In this post hoc analysis, the variables associated with the outcome were similar to those described in the primary analysis: start of follow-up in CKD stage 4, nephrotic proteinuria, and anemia at V1. However, the effects of age and BP at V1 were not confirmed.
Another factor that should be considered as a possible source of bias in the present study is that the GFR was defined using the Schwartz formula, and proteinuria was defined as the protein/creatinine ratio in urine, which may not represent the most precise measurements of these variables.
Despite its potential limitations, this cohort was based on the everyday clinical practice of seven pediatric nephrology centers in a populous region of a developing country located in the southern hemisphere, with a predominance of patients in an underprivileged economic situation. Most children and adolescents worldwide live in an analogous situation, and we believe that this study potentially adds to the understanding of risk factors for CKD progression in children in geographical regions with similar conditions.
In conclusion, this study demonstrated the importance of CKD stage at baseline, older age, massive proteinuria, anemia, and systolic BP as factors associated with faster CKD progression. Of these factors, at least three (hypertension, proteinuria, and anemia) may be addressed by specific therapies, which has significant practical implications. Unfortunately, the diagnosis of CKD in Brazil frequently is established lately in the course of the disease, when renal replacement therapies are indicated.25 This fact limits the potential usefulness of therapies to delay CKD progression, which reinforces the need for an early diagnosis of the disease. Finally, an interaction was observed between serum hemoglobin and proteinuria over the progression of CKD, suggesting that the effect of hemoglobin may be more important in children without proteinuria; due to its potential practical implications, this hypothesis should be further tested.
FundingThis study was funded by the Brazilian Ministry of Health through the Program for Institutional Development of the Unified Health System (Programa de Apoio ao Desenvolvimento Institucional do Sistema Único de Saúde [PROADI-SUS]) under Protocol No. 25000.180613/2011-11
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank the following researchers, who contributed to this study:
Débora Helena Silveira Dias, Marta Liliane Maia, Anelise Del Vechio Gessullo, Luciana de Santis Feltran, Anna Cristina Gervásio Lutaif, Sumara Zuanazzi Pinto Rigatto, Helen Sasaki Takagi, Erika Arai Furusawa, Maria Cristina Andrade, Maria Luiza Dautro Moreira do Val, Enzo Ricardo Russo, Elaine Mara Lourenço.
Please cite this article as: Belangero VM, Prates LC, Watanabe A, Schvartsman BS, Nussenzveig P, Cruz NA, et al. Prospective cohort analyzing risk factors for chronic kidney disease progression in children. J Pediatr (Rio J). 2018;94:525–31.
Study conducted at Hospital Samaritano de São Paulo, São Paulo, SP, Brazil.