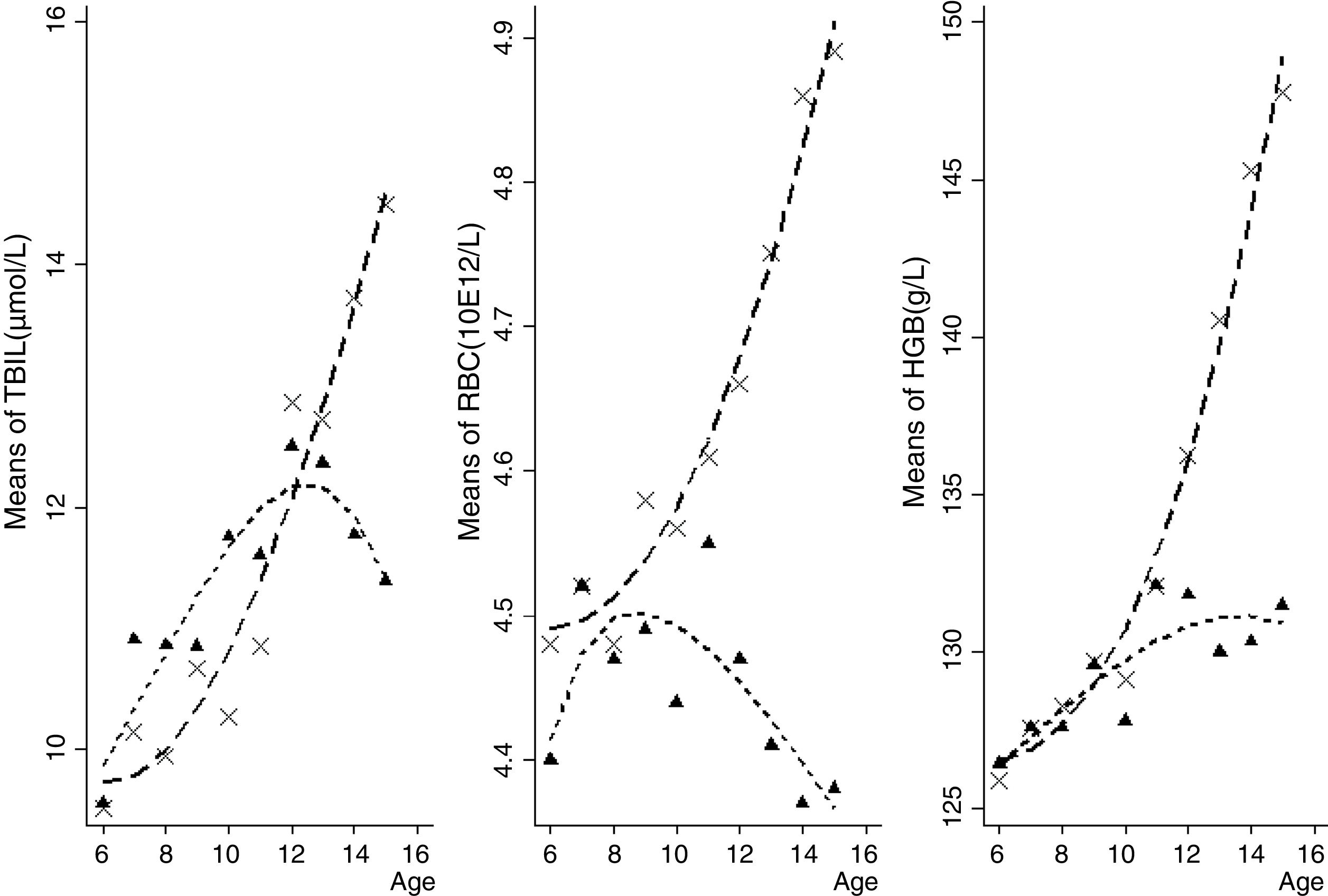
Total bilirubin is beneficial for protecting cardiovascular diseases in adults. The authors aimed to investigate the association of total bilirubin, red blood cell, and hemoglobin levels with the prevalence of high blood pressure in children and adolescents.
MethodsA total of 3776 students (aged from 6 to 16 years old) were examined using cluster sampling. Pre-high blood pressure and high blood pressure were respectively defined as the point of 90th and 95th percentiles based on the Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. Both systolic and diastolic blood pressure were standardized into z-scores.
ResultsPeripheral total bilirubin, red blood cell and hemoglobin levels were significantly correlated with age, and also varied with gender. Peripheral total bilirubin was negatively correlated with systolic blood pressure in 6- and 9-year-old boys, whilst positively correlated with diastolic blood pressure in the 12-year-old boys and 13- to 15-year-old girls (p<0.05). Higher levels of red blood cell and hemoglobin were observed in pre-high blood pressure and high blood pressure students when compared with their normotensive peers (p<0.01). The increases in red blood cell and hemoglobin were significantly associated with high blood pressure after adjusting for confounding factors. The ORs (95% CI) of each of the increases were 2.44 (1.52–3.92) and 1.04 (1.03–1.06), respectively. No statistical association between total bilirubin and high blood pressure was observed (p>0.05).
ConclusionTotal bilirubin could be weakly correlated with both systolic and diastolic blood pressure, as correlations varied with age and gender in children and adolescents; in turn, the increased levels of red blood cell and hemoglobin are proposed to be positively associated with the prevalence of high blood pressure.
A bilirrubina total é benéfica para proteger contra doenças cardiovasculares em adultos. Nosso objetivo foi investigar a associação dos níveis de bilirrubina total, glóbulos vermelhos e hemoglobina com a prevalência de pressão arterial elevada em crianças e adolescentes.
MétodosUm total de 3.776 estudantes (com idade entre 6-16 anos) foram examinados utilizando uma amostra em blocos. A pressão arterial elevada anterior e a pressão arterial elevada foram definidas como o 90° e 95° percentil, respectivamente, com base nos critérios do Quarto Relatório sobre Diagnóstico, Avaliação e Tratamento da Pressão Arterial elevada em Crianças e Adolescentes. A pressão arterial sistólica e pressão arterial diastólica foram padronizadas no escore z.
ResultadosOs níveis periféricos de bilirrubina total, glóbulos vermelhos e hemoglobina foram significativamente correlacionados à idade, que também variou de acordo com o sexo. A bilirrubina total periférica apresentou uma correlação negativa com a pressão arterial sistólica em meninos com 6 e 9 anos, ao passo que apresentou uma correlação positiva com a pressão arterial diastólica em meninos de 12 anos e meninas de 13 a 15 anos (p < 0,05). Foram observados níveis mais elevados de glóbulos vermelhos e hemoglobina em estudantes com pressão arterial elevada anterior e pressão arterial elevada em comparação a indivíduos normotensos (p < 0,01). Os aumentos de glóbulos vermelhos e hemoglobina tiveram uma associação significativa com a pressão arterial elevada após ajuste dos fatores de confusão. As RC (IC de 95%) de cada um dos aumentos foram 2,44 (1,52-3,92) e 1,04 (1,03-1,06) respectivamente. Não foi observada nenhuma associação estatística entre o nível de bilirrubina total e a pressão arterial elevada (p > 0,05).
ConclusãoA bilirrubina total pode ter correlações fracas com a pressão arterial sistólica e a pressão arterial diastólica, variando de acordo com a idade e o sexo em crianças e adolescentes, enquanto isso, propõe-se que o aumento dos níveis de glóbulos vermelhos e hemoglobina está positivamente associado à prevalência de pressão arterial elevada.
High blood pressure (HBP) is widely recognized as one of the most important challenges of the global public health among adults in both developed and developing countries.1 Being a leading risk factor of cardiovascular diseases (such as stroke, ischemic heart disease, and hypertensive heart disease), HBP is one of the main causes of morbidity and mortality worldwide.2 It has been demonstrated that BP levels in childhood and adolescence greatly impact the onset of hypertension in adulthood.3 A school-based survey in China with individuals aged from 6 to 13 years revealed that the overall prevalence of HBP was 18.4% (boys: 20.2%; girls: 16.3%),4 indicating that, in this population, HBP is likely to be a serious public health issue. Therefore, elevated BP in children should be of significant concern, calling for early detection and intervention to prevent future cardiovascular sequelae of pediatric HBP.
Bilirubin (BIL), a member of the super family of tetrapyrrolic compounds, is a metabolic end-product of heme degradation in the systemic circulation.5 BIL deriving from the degradation of hemoglobin (HGB) in anile red blood cells (RBC)6 has been widely used as a marker of cholestasis in the clinical assessment of liver function.7 Recently, several prospective population-based cohort studies have reported an inverse association between total BIL (TBIL) and cardiovascular diseases.8,9 A moderate elevation of peripheral BIL level has been shown to lower the risk of the onset and progression of coronary artery disease and atherosclerosis.10 It was suggested that BIL could play a role in the cell defense machinery in response to oxidative stress, a primary stimulus to the pathogenesis of hypertension.11–13 However, the relationship between peripheral TBIL and HBP in children and adolescents is barely understood. Thus, the authors aimed to explore the relationship between peripheral TBIL, RBC, and HGB with the prevalence of HBP, as well as to provide novel insights into HBP in children and adolescents.
MethodsA total of 5595 students, aged 6–16 years, from four primary schools and three junior high schools participated in the local medical examination program in September, 2014 (Guanlin town, Yixing city, Jiangsu province, Southern China). Parents/guardians of 4482 students received information about the study and signed an informed consent form. The demographic data was collected by the teachers through interviewer-administered questionnaires. Students aged under 6 years (n=14) or over 16 years (n=23), those with an incomplete physical examination record (n=197), and those who refused to draw blood (n=482) were excluded. Due to their small number, students aged 16 years were merged into the 15-year group. The study protocols were approved by the Research Ethics Committee of Nanjing Medical University.
BP measurements were performed twice in all participants, according to a standard protocol. After at least 5min of rest, the electronic sphygmomanometers (OMROM, HEM-7207, Dalian, China), a kind of oscillometric device, were used for BP measurement with an appropriate cuff size, depending on the circumference of the right upper arm of the participant. If the difference between the two measurements of either systolic blood pressure (SBP) or diastolic blood pressure (DBP) was over 8mmHg, then, an additional BP measurement was performed after 30s. In turn, in students who had a low DBP (<40mmHg) outlier reading from Korotkoff phases V measurement, a mercury sphygmomanometer was used instead to measure the BP.14 Likewise, an additional BP measurement was needed if the difference between the two measurements of SBP or DBP was over 8mmHg. Finally, the mean values of SBP and DBP were calculated for analysis.
According to the reference values of ‘The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents’,15 which considered age, sex, and height, normal BP was defined as both the SBP and DBP lower than the 90th percentile. Individuals with mean SBP and/or DBP above 90th but lower than 95th, and/or those with SBP≥120 or DBP≥80mmHg regardless of age, gender, and height, were classified into the category of pre-HBP (high-normal BP). HBP was defined as mean SBP and/or DBP persistently above the 95th percentile, and/or SBP≥140 or DBP≥90mmHg regardless of age, gender, and height. As age, gender, and height were recognized as key covariates associated with BP,16 the BP value was transformed into a BP z-score relatively to age, gender, and height in this study.
Physical examination and non-fasting blood collection for each student were conducted by uniformly trained and qualified staffs. Height was measured using a standard column stadiometer, and body weight was measured using an electronic scale after students removed their heavy clothes and shoes. Both height and body weight were measured twice and rounded to the closest decimal. Body mass index (BMI) was calculated as weight (kg)/height squared (m2). All measurements were taken using the same type of apparatus and followed the same procedures.
Samples of 3mL of venous blood were collected by the local physicians before 10:00am. Peripheral TBIL, high-density lipoprotein (HDL), low-density lipoprotein (LDL), total cholesterol (TC), and triglyceride (TG) concentrations were measured by an automated biochemical analyzer (Mindray BS-800, Shenzhen, China). HGB and RBC count were detected by an automated blood cell analyzer (Mindray BC5800®, Shenzhen, China). All the aforementioned biochemical indices were obtained in the clinical laboratory of Guanlin Hospital.
Continuous variables were presented as mean±standard deviation (SD) and the extreme values (over mean±3 SD) were translated into mean±3SD. Comparisons of the continuous variables among the normotensive, pre-HBP, and HBP groups were performed by applying the univariate analysis of variance, while the category parameters were compared by the chi-squared tests. The relationship of peripheral TBIL, RBC, and HGB with BP was examined by Spearman's partial correlation in different age and gender groups. The comparison of peripheral TBIL, RBC, and HGB levels among three BP groups were determined by the general linear models, after adjusting for covariates. The distribution of peripheral TBIL, RBC, and HGB levels, stratified by age and gender, was fitted by a fractional polynomial regression model. Multinomial logistic regression and ordinal logistic regression models were used to investigate the association of peripheral TBIL, RBC, and HGB with pre-HBP and HBP. Log transformation was performed for variables with significant deviation from normal distribution before further analyses. Two-tailed p-values <0.05 were considered to be statistically significant. Statistical analyses were performed using SPSS (SPSS for Windows, version 13.0. Chicago, USA).
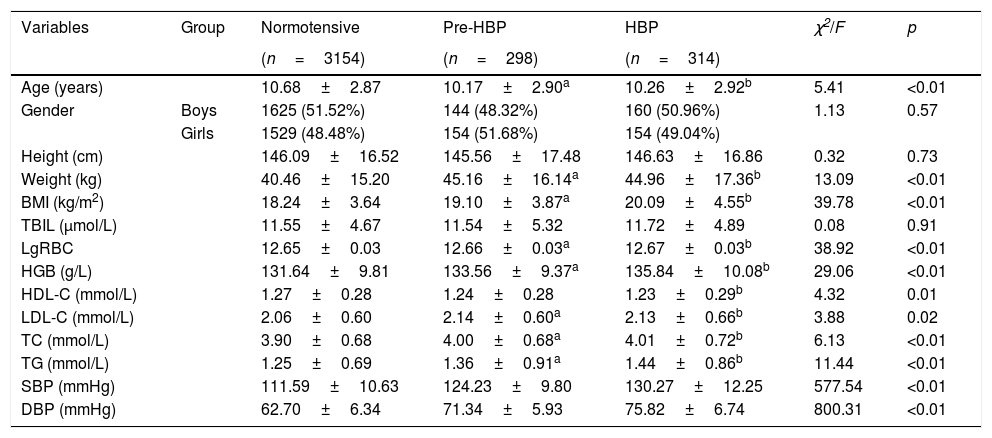
ResultsThe demographic characteristics of participants are listed in Table 1. A total of 3766 students (1929 boys and 1837 girls) were included in the current study. Of these, 3154 students (1625 boys and 1529 girls) were classified as normotensive, 298 (144 boys and 154 girls) as pre-HBP, and 314 (160 boys and 154 girls) as HBP.
Demographic characteristics of normotensive, pre-HBP, and HBP students.
| Variables | Group | Normotensive | Pre-HBP | HBP | χ2/F | p |
|---|---|---|---|---|---|---|
| (n=3154) | (n=298) | (n=314) | ||||
| Age (years) | 10.68±2.87 | 10.17±2.90a | 10.26±2.92b | 5.41 | <0.01 | |
| Gender | Boys | 1625 (51.52%) | 144 (48.32%) | 160 (50.96%) | 1.13 | 0.57 |
| Girls | 1529 (48.48%) | 154 (51.68%) | 154 (49.04%) | |||
| Height (cm) | 146.09±16.52 | 145.56±17.48 | 146.63±16.86 | 0.32 | 0.73 | |
| Weight (kg) | 40.46±15.20 | 45.16±16.14a | 44.96±17.36b | 13.09 | <0.01 | |
| BMI (kg/m2) | 18.24±3.64 | 19.10±3.87a | 20.09±4.55b | 39.78 | <0.01 | |
| TBIL (μmol/L) | 11.55±4.67 | 11.54±5.32 | 11.72±4.89 | 0.08 | 0.91 | |
| LgRBC | 12.65±0.03 | 12.66±0.03a | 12.67±0.03b | 38.92 | <0.01 | |
| HGB (g/L) | 131.64±9.81 | 133.56±9.37a | 135.84±10.08b | 29.06 | <0.01 | |
| HDL-C (mmol/L) | 1.27±0.28 | 1.24±0.28 | 1.23±0.29b | 4.32 | 0.01 | |
| LDL-C (mmol/L) | 2.06±0.60 | 2.14±0.60a | 2.13±0.66b | 3.88 | 0.02 | |
| TC (mmol/L) | 3.90±0.68 | 4.00±0.68a | 4.01±0.72b | 6.13 | <0.01 | |
| TG (mmol/L) | 1.25±0.69 | 1.36±0.91a | 1.44±0.86b | 11.44 | <0.01 | |
| SBP (mmHg) | 111.59±10.63 | 124.23±9.80 | 130.27±12.25 | 577.54 | <0.01 | |
| DBP (mmHg) | 62.70±6.34 | 71.34±5.93 | 75.82±6.74 | 800.31 | <0.01 |
Continuous variables were presented as mean±SD; p-values were calculated by univariate analysis of variance or chi-squared test.
BMI, body mass index; HBP, high blood pressure; HGB, hemoglobin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; RBC, red blood cell count; TBIL, total bilirubin; TC, total cholesterol; TG, triglyceride; lgRBC, logarithmically transformed red blood cell count.
a and b refers to the significant difference compared with normotensive by post hoc multiple test.
Gender, height, and TBIL did not differ among three BP groups (p>0.05). Post hoc multiple comparisons showed normotensive students were slightly older than those in the pre-HBP and HBP group, and that the HDL level in normotensive group was higher than in the HBP group. However, weight, BMI, LDL, TC, TG, RBC, and HGB in the normotensive group were lower than those in pre-HBP and HBP groups (p<0.05). Overall, these characteristics were adjusted as confounding factors for further association analysis.
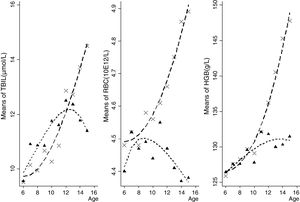
Boys presented higher TBIL, RBC, and HGB than girls (all p<0.05, data not shown in tables). Furthermore, the consecutive tendency of peripheral TBIL, RBC, and HGB levels by age and gender groups is depicted in Fig. 1. Peripheral TBIL concentration was positively correlated with age both in boys (r=0.14, p<0.01) and girls (r=0.15, p<0.01), after adjusting for HDL, LDL, TC, TG, RBC, and HGB. RBC counts and HGB were positively correlated with age in boys (r=0.35, p<0.01; and r=0.59, p<0.01, respectively) after adjusting for lipids. In girls, HGB had a positive (r=0.19, p<0.01) whilst RBC had a negative (r=−0.05, p<0.05) correlation with the age.
Both RBC and HGB were positively correlated with SBP (Supplements, Fig. S1) as well as with DBP (Supplements, Fig. S2), whereas no positive relationship was observed between TBIL and BP. Specifically, further stratification analysis by age and gender was conducted to assess the correlation of TBIL, RBC, and HGB with BP (Supplements, Fig. S3–5). Negative correlations between TBIL and SBP were observed in 6- and 9-year-old boys (r=−0.20, p<0.01; r=−0.22, p<0.01) while a positive correlation of peripheral TBIL and DBP was observed in 12-year-old boys (r=0.16, p<0.01). Moreover, positive correlations of TBIL and DBP were observed in 13-, 14- and 15-year-old girls (r=0.15, p<0.01; r=0.16, p<0.01; r=0.27, p<0.01, respectively).
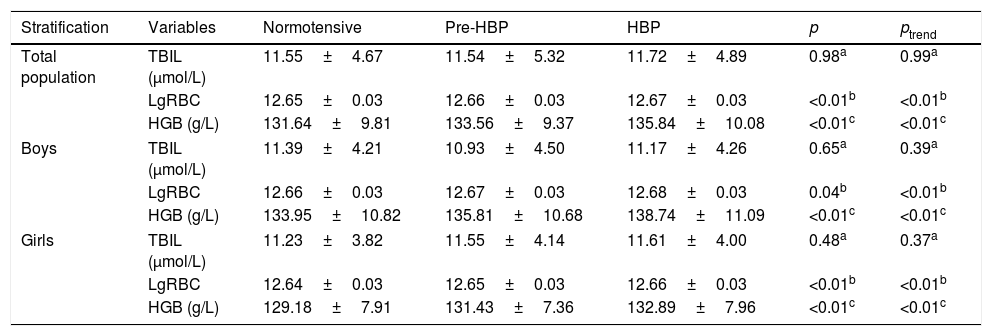
No significant differences in peripheral TBIL were observed among the normotensive, pre-HBP, and HBP groups in the entire population (p>0.05). When stratified by gender, similar results were observed even after adjusting for age, HDL, LDL, TC, TG, RBC, and HGB (all with p>0.05). Students in the pre-HBP and HBP groups presented a higher level of lgRBC and HGB when compared with their normotensive peers (Table 2). In addition, the general linear model analysis demonstrated significant ascending trends of lgRBC and HGB level across the three BP groups (both ptrend<0.01).
Comparison of TBIL, lgRBC, and HGB among normotensive, pre-HBP, and HBP students.
| Stratification | Variables | Normotensive | Pre-HBP | HBP | p | ptrend |
|---|---|---|---|---|---|---|
| Total population | TBIL (μmol/L) | 11.55±4.67 | 11.54±5.32 | 11.72±4.89 | 0.98a | 0.99a |
| LgRBC | 12.65±0.03 | 12.66±0.03 | 12.67±0.03 | <0.01b | <0.01b | |
| HGB (g/L) | 131.64±9.81 | 133.56±9.37 | 135.84±10.08 | <0.01c | <0.01c | |
| Boys | TBIL (μmol/L) | 11.39±4.21 | 10.93±4.50 | 11.17±4.26 | 0.65a | 0.39a |
| LgRBC | 12.66±0.03 | 12.67±0.03 | 12.68±0.03 | 0.04b | <0.01b | |
| HGB (g/L) | 133.95±10.82 | 135.81±10.68 | 138.74±11.09 | <0.01c | <0.01c | |
| Girls | TBIL (μmol/L) | 11.23±3.82 | 11.55±4.14 | 11.61±4.00 | 0.48a | 0.37a |
| LgRBC | 12.64±0.03 | 12.65±0.03 | 12.66±0.03 | <0.01b | <0.01b | |
| HGB (g/L) | 129.18±7.91 | 131.43±7.36 | 132.89±7.96 | <0.01c | <0.01c | |
Variables were presented as mean±SD; p-values were calculated by univariate analysis of variance; ptrend indicates the linear trend test among normotensive, pre-HBP and HBP groups.
HBP, high blood pressure; HGB, hemoglobin; TBIL, total bilirubin; lgRBC, logarithmically transformed red blood cell count; HGB, hemoglobin; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; RBC, red blood cell count; TC, total cholesterol; TG, triglyceride.
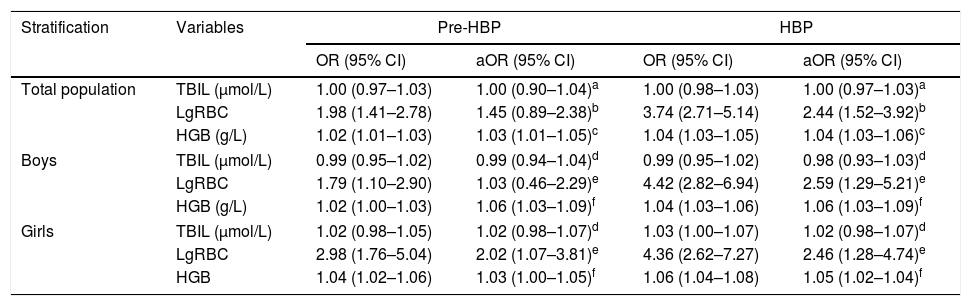
Logistic regression analysis showed that, when compared with the normotensive, there was no significant association of peripheral TBIL with pre-HBP and/or HBP, neither in the whole population nor in the sub-population stratified by gender (Table 3). However, increased RBC and HGB were significantly associated with the risk of pre-HBP, with ORs (95% CIs) of 1.98 (1.41–2.78) and 1.02 (1.01–1.03), respectively (p<0.01). RBC and HGB were significantly associated with HBP, and the adjusted ORs (95% CIs) were 2.44 (1.52–3.92) and 1.05 (1.03–1.07), respectively. Significant associations of RBC and HGB with HBP were also observed in boys and girls. Furthermore, ordinal logistic regression analysis showed that the increases of RBC and HGB were significantly associated with the cumulative risk of pre-HBP and of HBP; the adjusted ORs (95% CIs) were 1.95 (1.36–2.79) and 1.04 (1.03–1.05), respectively.
Logistic regression analysis of TBIL, RBC, and HGB with pre-HBP and HBP.
| Stratification | Variables | Pre-HBP | HBP | ||
|---|---|---|---|---|---|
| OR (95% CI) | aOR (95% CI) | OR (95% CI) | aOR (95% CI) | ||
| Total population | TBIL (μmol/L) | 1.00 (0.97–1.03) | 1.00 (0.90–1.04)a | 1.00 (0.98–1.03) | 1.00 (0.97–1.03)a |
| LgRBC | 1.98 (1.41–2.78) | 1.45 (0.89–2.38)b | 3.74 (2.71–5.14) | 2.44 (1.52–3.92)b | |
| HGB (g/L) | 1.02 (1.01–1.03) | 1.03 (1.01–1.05)c | 1.04 (1.03–1.05) | 1.04 (1.03–1.06)c | |
| Boys | TBIL (μmol/L) | 0.99 (0.95–1.02) | 0.99 (0.94–1.04)d | 0.99 (0.95–1.02) | 0.98 (0.93–1.03)d |
| LgRBC | 1.79 (1.10–2.90) | 1.03 (0.46–2.29)e | 4.42 (2.82–6.94) | 2.59 (1.29–5.21)e | |
| HGB (g/L) | 1.02 (1.00–1.03) | 1.06 (1.03–1.09)f | 1.04 (1.03–1.06) | 1.06 (1.03–1.09)f | |
| Girls | TBIL (μmol/L) | 1.02 (0.98–1.05) | 1.02 (0.98–1.07)d | 1.03 (1.00–1.07) | 1.02 (0.98–1.07)d |
| LgRBC | 2.98 (1.76–5.04) | 2.02 (1.07–3.81)e | 4.36 (2.62–7.27) | 2.46 (1.28–4.74)e | |
| HGB | 1.04 (1.02–1.06) | 1.03 (1.00–1.05)f | 1.06 (1.04–1.08) | 1.05 (1.02–1.04)f | |
aOR, adjusted odds ratio; CI, confidence interval; HBP, high blood pressure; HGB, hemoglobin; TBIL, total bilirubin; lgRBC, logarithmically transformed red blood cell count; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; RBC, red blood cell count; TC, total cholesterol; TG, triglyceride.
The current study firstly evaluated the relationship between peripheral TBIL and BP variation in children and adolescents aged 6–15 years. Almost 80% of endogenous TBIL in plasma derived from the degradation of HGB in the anile RBC.6 Thus, the interplay of RBC and HGB were also considered in this study.
Peripheral RBC and HGB were significantly elevated in children and adolescents as age progressed,17 whereas the divergent trend in gender was not well elucidated. It was observed that peripheral TBIL, RBC, and HGB levels increased significantly with age in boys, while a downward trend was observed in girls, appearing approximately at the 13-, 9- and 14-year groups, respectively. This indicates that the fluctuations of peripheral TBIL, RBC, and HGB levels with aging in puberty may be significantly different between boys and girls. The potential mechanism might be that adolescent girls are at high risk of developing iron deficiency because of increased iron demands during puberty, menstrual losses, and limited dietary iron intake.18
BP is strongly related to pubertal status at increasing ages, and increases at an accelerated rate during puberty in adolescents.19 Besides, the growth hormone and gonadal steroids could play critical roles in changes of peripheral TBIL, RBC, and HGB and then induce BP alteration.20,21 Hitherto, whether peripheral TBIL level are associated with BP in children and adolescents is poorly understood. Herein, stratification analysis by age and gender was used to explore the relationship of TBIL, RBC, and HGB with BP in youth.
Overall, a negative correlation of peripheral TBIL and SBP was observed in 6- and 9-year-old boys, while a positive correlation between TBIL and DBP was shown for 12-year-old boys. Slightly positive correlations of TBIL and DBP were observed in girls aged 13, 14, and 15 years. These results indicated that TBIL could have a weak correlation with SBP and DBP where the correlations varied with age and gender. These findings are helpful to reveal differential correlation of TBIL and BP varied with age and gender. Further research would be warranted to clarify the trajectory effect of the RBC and HGB hormonal changes in physical development on BP regulation.
In the current study, RBC and HGB were linearly elevated across the normotensive, pre-HBP, and HBP groups, but not TBIL. The increased levels of RBC and HGB were associated with an increased risk for HBP, which was firstly observed in children and adolescents, being consistent with several studies in adults.22,23 The RBC and HGB elevation might raise the blood viscosity and circulation volume, and consequently increase the BP level.24,25 Moreover, it has been reported that HGB is strongly related to arterial stiffness.26 HGB is a scavenger of nitric oxide (NO) produced by the endothelial cells.27 NO relaxes the muscle cells surrounding the vessel and inhibits several atherosclerosis processes,28 which is considered as an critical protective role against the onset and progression of cardiovascular disease. Increased HGB may bind to NO, leading to vascular constriction and, consequently, an elevation of BP.
Nevertheless, the present study presented limitations. First, the BP reference in the Fourth Report was measured by mercury sphygmomanometers, while electronic sphygmomanometers were used in the present study. Second, the subjects of this study were sampled in clusters; the selection bias may have occurred and further replication in the other populations would be warranted. Third, hereditary factors and food intake habits have been reported to be correlated with TBIL, but neither of these factors were examined in this study.29,30 Finally, peripheral TBIL, RBC, and HGB were examined at the same time of pre-HBP and HBP classification, and further study are required to verify the causal inference.
In conclusion, the current study indicated that age and gender had a significant effect on the peripheral TBIL, RBC, and HGB concentrations in the children and adolescents. A weak correlation was observed between peripheral TBIL and BP, varying with age and gender, whereas no significant correlations between TBIL and pre-HBP and/or HBP were observed. Significant correlations of RBC and HGB with BP were observed; in turn, the increases of RBC and HGB were significantly associated with pre-HBP and HBP. This merits further studies to elucidate the mechanisms underlying these results and providing room for further mechanistic research in this field.
FundingThis work was supported by grants from National Natural Science Foundation of China (No. 81273165, No. 81573232), and the Priority Academic Program for the Development of Jiangsu Higher Education Institutions (Public Health and Preventive Medicine).
Conflicts of interestThe authors declare no conflicts of interest.
This work was supported by grants from National Natural Science Foundation of China, and the Priority Academic Program for the Development of Jiangsu Higher Education Institutions (Public Health and Preventive Medicine). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Please cite this article as: Chen X-t, Yang S, Yang Y-m, Zhao H-l, Chen Y-c, Zhao X-h, et al. Exploring the relationship of peripheral total bilirubin, red blood cell, and hemoglobin with blood pressure during childhood and adolescence. J Pediatr (Rio J). 2018;94:532–8.