The aim of this study was to identify the causes of congenital microcephaly in Rio Grande do Sul, a state in southern Brazil, where no ZIKV outbreak was detected, from December 2015 to December 2016, which was the period when ZIKV infection was at its peak in northeast Brazil.
MethodsThis was a cross-sectional study where all notifications of congenital microcephaly in the state of Rio Grande do Sul were included for analysis. Evaluation of cases followed the guidelines of the Brazilian Ministry of Health. Dysmorphological and neurological evaluations were performed by a specialized team, and genetic tests and neuroimaging were performed when clinically indicated. STORCH infections were diagnosed using standard tests. ZIKV infection was diagnosed through maternal serum RT-PCR and/or neuroimaging associated with clinical/epidemiological criteria.
ResultsFrom 153744 registered live births in the study period, 148 cases were notified, but 90 (60.8%) of those were later excluded as “non-confirmed” microcephaly. In the 58 confirmed cases of microcephaly (prevalence = 3.8/10000 live births), congenital infections (syphilis, toxoplasmosis, cytomegalovirus, and ZIKV) constituted the predominant etiology (50.0%), followed by isolated CNS (15.5%), and genetic syndromes (10.3%). Congenital ZIKV syndrome (CZS) with typical phenotype was diagnosed in three cases (5.2% of all confirmed microcephaly cases or 10.4% of all congenital infections).
ConclusionIn Rio Grande do Sul, where no outbreak of ZIKV infection was recorded, congenital infections were the leading cause of congenital microcephaly, and the attributable risk for CZS in the etiology of microcephaly was 5.2%.
Identificar as causas da microcefalia congênita no Rio Grande do Sul, Região Sul do Brasil, onde não foi detectado surto de ZIKV, de dezembro de 2015 a dezembro de 2016. Esse foi o período em que a infecção por ZIKV estava em seu auge no Nordeste do Brasil.
MétodosEste é um estudo transversal no qual todas as notificações de microcefalia congênita no estado do Rio Grande do Sul foram incluídas para análise. A avaliação dos casos seguiu as orientações do Ministério da Saúde. A avaliação dismorfológica e neurológica foi feita por uma equipe especializada e os testes genéticos e as neuroimagens foram feitos quando indicado clinicamente. As infecções STORCH (Sífilis, Toxoplasmose, Rubéola, Citomegalovírus e Herpes simples) foram diagnosticadas utilizando testes padrão. A infecção por ZIKV foi diagnosticada por meio da transcriptase reversa seguida de reação em cadeia da polimerase (RT-PCR) no soro materno e/ou neuroimagem associada a critérios clínicos/epidemiológicos.
ResultadosDe 153.744 nascidos vivos registrados no período do estudo, 148 bebês foram casos notificados, porém 90 (60,8%) casos foram excluídos posteriormente como microcefalia “não confirmada”. Nos 58 casos confirmados de microcefalia (prevalência = 3,8/10.000 nascidos vivos), as infecções congênitas (sífilis, toxoplasmose, citomegalovírus e ZIKV) constituíram a etiologia predominante (50,0%), seguidas de doenças ligadas ao SNC isolado (15,5%) e síndromes genéticas (10,3%). A síndrome congênita do ZIKV (SCZ) com fenótipo típico foi diagnosticada em três casos (5,2% de todos os casos confirmados de microcefalia ou 10,4% de todas as infecções congênitas).
ConclusãoNo Rio Grande do Sul, Brasil, onde não foi registrado surto de infecção por ZIKV, a principal causa de microcefalia congênita foram infecções congênitas e o risco atribuível para SCZ na etiologia de microcefalia foi de 5,2%.
In February 2016, the World Health Organization (WHO) declared a Public Health Emergency of International Concern (PHEIC) regarding the Zika virus (ZIKV) infection in Latin America and its possible association with clusters of microcephaly. Earlier, in November 2015, the Brazilian Ministry of Health (MoH) had implemented mandatory reporting of neonatal microcephaly and/or other central nervous system (CNS) abnormalities.1 The association between CNS abnormalities including microcephaly and prenatal infection to ZIKV was proved in 2016,2,3 and a congenital ZIKV syndrome was defined.4
However, congenital microcephaly is a heterogeneous diagnosis based on a measure of head circumference, with a number of genetic and environmental causes described to date, including congenital infections, genetic syndromes, and isolated CNS malformations.1,5,6 Most current guidelines define microcephaly as an occipitofrontal circumference (OFC), or head circumference (HC), more than two standard deviations (SDs) below the population mean, and severe microcephaly as HC <3 SD below the mean. HC is an indirect measure of brain size and may be a predictor of abnormal cerebral development.1,7
The causes of microcephaly often remain unknown. In retrospective studies, no etiology was identified in 40–70% of cases.5,6 Mandatory reporting of microcephaly enables a more in-depth assessment of those cases. However, there is a well-established association between microcephaly and maternal infections, especially those occurring in the first 12 weeks of pregnancy, which can lead to characteristic forms of brain damage. Besides microcephaly, the manifestations include hydrocephalus, cerebral calcifications, ventriculomegaly, cortical migration disorders, white-matter abnormalities, and cerebellar hypoplasia.8
Before 2015, the estimated prevalence of severe microcephaly at birth in Brazil was 0.5 per 10000 live births. After identification of the possible association between ZIKV infection and microcephaly in northeast Brazil, this prevalence rose to around 20 cases per 10000 live births. The northeastern states of Brazil had the highest incidence of ZIKV disease among the general population (34/10000 in the state of Bahia in 2016). Conversely, in Rio Grande do Sul (RS), Brazil's southernmost state, no outbreak occurred, even though autochthonous cases were identified at the beginning of 2016, and the peak incidence was only 1.7 cases per 100000 people.9
MethodsStudy designWe conducted a cross-sectional study where all notifications for congenital microcephaly in the state of Rio Grande do Sul from 1 December 2015 to 31 December 2016 (56 epidemiological weeks) were included.
Case definition and study protocolDuring the first 3 months of the study, notification was mandatory as per the Ministry of Health for all babies born with an HC equal to or less than 32cm. A review for case notification was made by the Ministry of Health in February 2016, when microcephaly was defined by HC<2Z scores below the mean for sex and gestational age (GA), according to the Intergrowth curves.10 Assessment of cases followed the guidelines of the Surveillance and Response Protocol published by the Ministry of Health.1
Case reportingNeonates were reported to the Registry of Public Health Events (Registro de Eventos de Saúde Pública – RESP) by the first physician to identify microcephaly. Clinicians were required to enter data on birth and delivery (including HC, weight, and GA), abnormal clinical and imaging findings, maternal and gestational conditions, and potential infections and exposures during pregnancy, including travels to ZIKV-endemic areas. Cases were subsequently classified by us as (1) “non-confirmed” cases of microcephaly: proportionate premature babies with no other anomalies (they were excluded as microcephaly cases when adjusting sex and gestational age by applying the intergrowth charts); small HC in proportionately small-for-gestational-age babies, with normal development and no associated anomaly; HC of 32cm at birth (criteria for inclusion only in the first three months of mandatory report) with no other anomalies and normal development at the follow-up; (2) “confirmed” or true cases of microcephaly: all remaining reported cases. Confirmed cases were subsequently classified by etiology; congenital infections were diagnosed using the following criteria: (1) ZIKV – confirmed by maternal history of traveling to endemic areas, cutaneous rash, and joint pain; and neonatal history of CNS imaging findings, physical examination findings, or a ZIKV polymerase chain reaction (PCR) result (in blood) consistent with the Zika virus disease; (2) CMV – confirmed by positive PCR in urine; (3) toxoplasmosis and rubella – confirmed by positive immunoglobulin (IgM) serology in blood; (4) syphilis – confirmed by positive Venereal Disease Research Laboratory (VDRL) screening in a neonatal blood or cerebrospinal fluid (CSF) sample or a positive fluorescent treponemal antibody (FTAAbs) test.
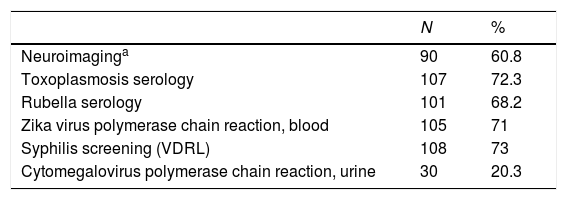
Toxoplasmosis and rubella serology tests and ZIKV PCR in maternal and neonatal samples were performed at the State Central Laboratory (Laboratório Central do Estado, LACEN). Syphilis screening by VDRL, CMV PCR and neuroimaging (ultrasound, CT, or MRI) were performed at the patients’ municipalities of origin. Table 1 describes the distribution of diagnostic tests performed in the sample.
Laboratory and imaging investigation of 148 notified cases of microcephaly in Rio Grande do Sul, Brazil, from 01 Dec 2015 to 31 Dec 2016.
| N | % | |
|---|---|---|
| Neuroimaginga | 90 | 60.8 |
| Toxoplasmosis serology | 107 | 72.3 |
| Rubella serology | 101 | 68.2 |
| Zika virus polymerase chain reaction, blood | 105 | 71 |
| Syphilis screening (VDRL) | 108 | 73 |
| Cytomegalovirus polymerase chain reaction, urine | 30 | 20.3 |
Maternal and neonatal data were analyzed by the Rio Grande do Sul State Health Surveillance Center (Centro Estadual de Vigilância em Saúde, CEVS). Records were reviewed jointly by the CEVS staff and the Teratogen Information Service (Serviço de Informações sobre Agentes Teratogênicos, SIAT). The SIAT is staffed with a multidisciplinary team of specifically trained medical geneticists, pediatricians, and nurses – including some of the authors of the present report – and is affiliated with the Medical Genetics Service at Hospital de Clínicas de Porto Alegre (HCPA), a tertiary care center and major referral hospital in Rio Grande do Sul.
Patient careAll patients initially received care at their municipalities of origin. All cases of confirmed microcephaly were referred to our hospital (HCPA) for clinical evaluation and, when necessary, additional investigation.
Investigation of genetic causes or isolated CNS malformationsNeonates with a family history of genetic anomalies, consanguinity, abnormal imaging findings, or clinical manifestations (morphological changes) suggestive of genetic conditions underwent specific genetic testing (including karyotyping and screening for inborn errors of metabolism) and a detailed physical examination, performed by a medical geneticist, in tandem with the investigation of potential infectious causes.
HC percentiles were calculated using the Intergrowth charts, corrected by sex and GA. Analysis of the sample was carried out in SPSS version 20.0. The prevalence of microcephaly was calculated using the number of live births from December 2015 to December 2016. This study was approved by the HCPA Research Ethics Committee (protocol n. 16-0577).
ResultsOverall, 148 neonates were reported in the state of Rio Grande do Sul during the 56 epidemiological weeks of interest, among 153744 registered live births (prevalence: 9.6/10000). Neuroimaging scan was performed in 60.8% of the cases, and the tests for congenital infections were conducted from 20% to 73% of the neonates (Table 1).
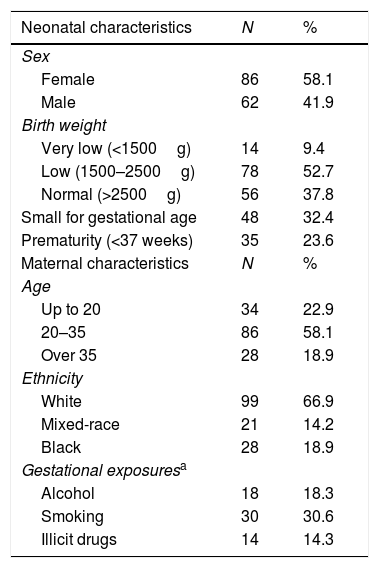
Maternal demographic and newborn anthropometric characteristics are presented in Table 2. Overall, 18 mothers (18.3%) reported having consumed alcohol during pregnancy (of those, 5 had given birth to neonates with CNS abnormalities of undefined etiology), and 30 reported smoking; 14 reported use of illegal drugs. Seventy-seven neonates (62.1%) had low birth weight, 35 (23.6%) were born premature, and 48 (32.4%) were small for gestational age. Neuroimaging findings were abnormal in 48.9% of cases (n = 44/90), when available.
Maternal and neonatal characteristics of 148 notified cases of microcephaly in Rio Grande do Sul, Brazil, from 01 Dec 2015 to 31 Dec 2016.
| Neonatal characteristics | N | % |
|---|---|---|
| Sex | ||
| Female | 86 | 58.1 |
| Male | 62 | 41.9 |
| Birth weight | ||
| Very low (<1500g) | 14 | 9.4 |
| Low (1500–2500g) | 78 | 52.7 |
| Normal (>2500g) | 56 | 37.8 |
| Small for gestational age | 48 | 32.4 |
| Prematurity (<37 weeks) | 35 | 23.6 |
| Maternal characteristics | N | % |
| Age | ||
| Up to 20 | 34 | 22.9 |
| 20–35 | 86 | 58.1 |
| Over 35 | 28 | 18.9 |
| Ethnicity | ||
| White | 99 | 66.9 |
| Mixed-race | 21 | 14.2 |
| Black | 28 | 18.9 |
| Gestational exposuresa | ||
| Alcohol | 18 | 18.3 |
| Smoking | 30 | 30.6 |
| Illicit drugs | 14 | 14.3 |
A detailed analysis of the 148 reported babies led to 90 (60.8%) cases considered as “non-confirmed” microcephaly: 20 with an HC of 32cm at birth and normal development at follow-up; and 70 born with an HC of less than 32cm but excluded after the Intergrowth charts (adjusted for gestational age and sex) were applied, or proportionately small for gestational age and without any brain or neurological abnormality detected. In this group, some risk factors known for being related to prematurity/small for gestational age were detected, such as HIV infection (2 cases), alcohol intake (5 cases), use of crack cocaine (2 cases), smoking tobacco, and maternal hypertension; however, a definite cause could not be established.
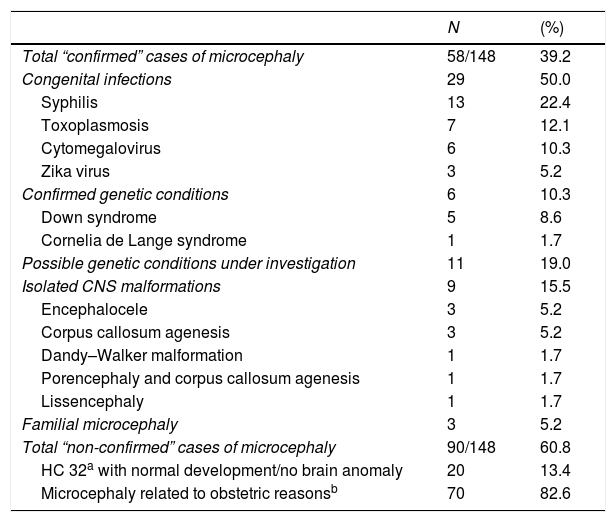
In the 58 confirmed cases of microcephaly (prevalence = 3.8/10000 live births), congenital infections were the leading causal factor identified (n = 29; 50.0%): syphilis (n = 13; 22.4%), toxoplasmosis (n = 7; 12.1%), CMV (n = 6; 10.3%), and ZIKV (n = 3; 5.2%). Nine cases (15.5%) were classified as isolated CNS malformations (Table 3) and consisted of encephalocele (5.2%) and corpus callosum agenesis (5.2%), among others. Genetic syndromes were diagnosed in six patients (10.3%), but in 11 cases a genetic condition was suspected without confirmation of any specific syndrome.
Description of causes of 148 notified cases of microcephaly in Rio Grande do Sul, Brazil, from 01 Dec 2015 to 31 Dec 2016.
| N | (%) | |
|---|---|---|
| Total “confirmed” cases of microcephaly | 58/148 | 39.2 |
| Congenital infections | 29 | 50.0 |
| Syphilis | 13 | 22.4 |
| Toxoplasmosis | 7 | 12.1 |
| Cytomegalovirus | 6 | 10.3 |
| Zika virus | 3 | 5.2 |
| Confirmed genetic conditions | 6 | 10.3 |
| Down syndrome | 5 | 8.6 |
| Cornelia de Lange syndrome | 1 | 1.7 |
| Possible genetic conditions under investigation | 11 | 19.0 |
| Isolated CNS malformations | 9 | 15.5 |
| Encephalocele | 3 | 5.2 |
| Corpus callosum agenesis | 3 | 5.2 |
| Dandy–Walker malformation | 1 | 1.7 |
| Porencephaly and corpus callosum agenesis | 1 | 1.7 |
| Lissencephaly | 1 | 1.7 |
| Familial microcephaly | 3 | 5.2 |
| Total “non-confirmed” cases of microcephaly | 90/148 | 60.8 |
| HC 32a with normal development/no brain anomaly | 20 | 13.4 |
| Microcephaly related to obstetric reasonsb | 70 | 82.6 |
Case 1: female, born in 2015 from a nonconsanguineous healthy young couple; family history negative for congenital anomalies; gestational age (GA) 38 weeks; weight, 2,436g; length, 44cm; HC, 27cm (<3 Z-score). The mother had traveled to northeast Brazil (Recife, Pernambuco) during her second month of pregnancy, when she had pruriginous rash, conjunctivitis, and arthralgia. She was not tested, since at the time she had the infection, ZIKV was not considered an infection of concern. Clinical examination of the baby was performed by our team at three months of age, when we observed a significant craniofacial disproportion with small head circumference and prominent occipital bone, collapsed skull, redundant skin in the scalp and nuchal region, spasticity, hyperactivity, swallowing difficulties, and inconsolable crying. Eye fundus was not evaluated since the patient was lost for follow-up. RT-PCR was negative in the baby's LCR and serum. Neuro Magnetic Resonance: microcalcifications, ventricular enlargement, pachygyria, cortical atrophy. STORCH was negative. Case concluded as CZS based on clinical and epidemiological criteria.
Case 2: male, born in 2015 from a nonconsanguineous healthy young couple; family history negative for congenital anomalies; gestational age (GA) 37 weeks; weight, 2740g; length, 43cm; HC, 31cm (<2 Z-score). Mother traveled to Campinas, São Paulo, and immediately after her return she had a pruriginous rash and arthralgia but was not tested by PCR. Clinical examination by our team was performed at 20 months of age. At that time HC was 39.5cm (<3 Z-scores). We observed marked craniofacial disproportion, narrow forehead with bifrontal depression, overlapped sutures, epicanthal folds, strabismus, club feet, and arthrogryposis in the knees. Marked hypertonia, irritability and neuromotor delay were noted at neurological examination. Neuro Magnetic Resonance: calcifications, ventricular enlargement, polymicrogyria. STORCH was negative. Case concluded as CZS based on clinical and epidemiological criteria.
Case 3: female, born in 2016 from a nonconsanguineous healthy young couple; family history negative for congenital anomalies; gestational age (GA) 37 weeks; weight, 2100g; length, 43cm; HC, 26.5cm (<3 Z-score). Mother reported mild fever, pruriginous rash, and arthralgia on her second month of pregnancy. She did not travel but was in a town where dengue fever is endemic. Not tested during her pregnancy. Clinical examination by our team was performed at 11 months of age: HC, 33cm (<3 Z-score) craniofacial disproportion, narrow forehead with bifrontal depression, prominent occipital bone, collapsed skull, scalp skin folds, hands with contractures, arthrogryposis. Neurological examination: spasticity, irritability, swallowing difficulties and inconsolable crying. Delayed neurodevelopment. Eye fundus normal. RT-PCR was negative in the baby's LCR and serum. CT scan: bilateral brain calcifications, ventricular enlargement, simplified gyral pattern. STORCH was negative. Case concluded as CZS based on clinical and epidemiological criteria.
DiscussionIn Rio Grande do Sul, Brazil's southernmost state, the autochthonous circulation of ZIKV was restricted to some geographical regions and to the summer period, since this part of Brazil registers average winter temperatures below 20°C, thus inhibiting Aedes egyptii activity. However, the prevalence of microcephaly in the present study was 3.8/10000, which far exceeds the pre-2015 Brazilian estimate of 0.5 cases per 10000 live births.9 For comparative purposes, in a study that evaluated live births from January 2015 to January 2016, the highest prevalence rates were observed in states in northeast Brazil: Pernambuco (16.6/10000 CI = 12.3–17.2) and Paraíba (10.8/10.000; CI = 8.86–13.04) versus 0.60/10000 (CI 0.22–1.31) in four states without ZIKV transmission.11 Therefore, those observations show that there was an increase in reports of microcephaly compared to previous years in Rio Grande do Sul, albeit not as high as in the Northeast, where there was a true ZIKV epidemics. The increase in reports in Rio Grande do Sul is most probably related to the broader definition of microcephaly (HC <2 Z-scores below the mean) as well as to the active search and reporting of newborns with a small head, irrespective of its etiology. This hypothesis is consistent with observations in hospitals participating in the ECLAMC (Latin American Study of Congenital Malformations) in the period between 2005 and 2014, where a population base prevalence for microcephaly was estimated at 3.0/10000 (CI 95 2.7–3.4/10000).12
In the present study, 50.0% of the microcephaly cases were infection-related, but only 10.4% were secondary do ZIKV. A nationwide study investigated the prevalence of infection-related microcephaly in Brazil in 2015–2016 using secondary information from the Brazilian's Ministry of Health Surveillance Systems. It concluded that among 5968 cases of microcephaly reported in Brazil 1950 (32.7%) were infection-related (30.3% were still under investigation). ZIKV infection was the etiology in 15.6% of cases, predominantly in the northeast. STORCH infections could be only confirmed in 5.4% of cases, and this small percentage is probably due to lack of availability of laboratory tests.11 In Piauí, northeast Brazil, a prevalence of 13.6/10000 cases of microcephaly during the Zika virus epidemic was observed in 2015–2016, with 45.3% (34/75) of them possibly related to Zika, since STORCH in those was negative.13
Syndromic genetic conditions accounted for 10% of cases in the present study; 19% were still under investigation for a possible genetic condition; and 15% were associated to central nervous system malformations. A similar rate was reported in the USA, in a sample of newborns with microcephaly, with 13% of cases attributable to confirmed genetic conditions.14 In South American hospitals, in the ECLAMC study,12 23% of the microcephalic babies were diagnosed with a genetic syndrome, and 12% of cases were associated with neural malformations, not too different from our study.
In our series, 61% were discarded on clinical grounds. In their work analyzing the first 1501 livebirths with complete investigation in Brazil, Franca et al.15 also discarded 59% of the notifications, most frequently because they had no obvious abnormalities such as neurological symptoms, or because they were proportionately small newborn babies (e.g. with low birthweight). One of the reasons of this high percentage of exclusion on clinical grounds is the adoption of the <2 Z-scores as a threshold for notification, which is less specific to detect congenital anomalies and includes many “normal” babies with HC in the lowest distribution of the normal curve. Before the ZIKV emergence, the clinical definition of microcephaly was generally restricted to <3 Z-scores.12
This study is subjected to several limitations. First, due to the nature of the ZIKV health emergency, the occasional unavailability of some diagnostic modalities, and the inability of some patients to travel to HCPA, not all clinical assessments were performed by the same team. Second, HC measurement may not have been performed in a consistently correct manner, and neuroimaging was only available to approximately 60% of newborns. Third, retrospective recall bias applies to some maternal conditions, including treatments and exposures during pregnancy (especially concerning alcohol intake). Fourth, CMV serology was available for only 29% of cases, and herpes screening was not included at all.
Finally, the unavailability of truly reliable diagnostic tests for ZIKV is another limitation of this study. As noted in other studies, ZIKV PCR in neonatal or maternal serum is often negative as little as 7 days after infection. Immunological tests for population screening are susceptible to cross-reactivity with other flavivirus infections, and were not performed systematically in our sample. Within this context, a thorough, targeted clinical examination based on the textbook case description of congenital Zika syndrome plays a decisive role in the diagnosis.8,16,17 Thus, less severe cases of ZIKV infection may have been underreported.
Despite those limitations, this study provides an important estimate of the etiology of microcephaly in a region adjacent to but not directly affected by a ZIKV outbreak.
In conclusion, congenital infections have the highest impact in the etiology of microcephaly in Rio Grande do Sul, although the contribution of ZIKV was comparatively lower than that observed in northeast Brazil. Therefore, despite efforts by the Brazilian Ministry of Health to improve care and prevention of gestational diseases, much work remains to be done in terms of education and public health to improve the efficacy of preventive measures and ensure that they reach the majority of the population.
FundingThis work was funded by the Fundo de Incentivo à Pesquisa de Eventos do Hospital de Clínicas de Porto Alegre (FIPE-HCPA) and the Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq).
Conflicts of interestThe authors declare no conflicts of interest.
We thank CEVS for its support in case assessment; SIAT for its work on data investigation; the management of HCPA, which served as the referral hospital for these cases; and the Graduate Program in Medical Sciences at Universidade Federal do Rio Grande do Sul.
Please cite this article as: Herber S, Silva AA, Sanseverino MT, Friedrich L, Ranieri TM, Favreto C, et al. Prevalence and causes of congenital microcephaly in the absence of a Zika virus outbreak in southern Brazil. J Pediatr (Rio J). 2019;95:600–6.