To evaluate the possible effects of the introduction of the pneumococcal conjugate 10-valent vaccine schedule in the state of Parana on pneumococcal meningitis cases and to assess the distribution of serotypes among cases.
MethodCross-sectional study with retrospective data collection of cases of pneumococcal meningitis in the state of Paraná reported to Sistema de Informação de Agravos de Notificação (SINAN), from 1998 to 2011. A total of 1,339 cases of pneumococcal meningitis were analyzed; 1,205 cases from the pre-vaccine period (1998-2009) were compared to 134 cases from the post-vaccine period (2010-2011). Descriptive and comparative statistical analyses (chi-squared test and prevalence ratio) were performed using JMP 5.1.2 statistical software (JMP Statistical Discovery, North Carolina, USA) and EPI INFO 6 (Centers for Disease Control and Prevention, Georgia, EUA).
ResultsThere was a significant reduction in the mean rates of incidence and mortality in the general population. The analysis of cases in the pre- and post-vaccination periods in the age groups covered by vaccination (younger than 2 years) showed significant reductions in incidence rates (6.01 cases/100,000 to 2.49 cases/100,000 individuals) and mortality (1.85 cases/100,000 population to 0.47 cases/100,000 population), while the mean lethality rate did not change significantly. There was a significant reduction in cases whose serotypes are included in the vaccine (80.7% to 53.3%).
ConclusionEven after a short time of use, the 10-valent pneumococcal conjugate vaccine has already had a significant impact in reducing the incidence and mortality of meningitis cases among infants, as well as the reduction of cases whose serotypes are included in the vaccine.
Avaliar os possíveis efeitos da introdução da vacina pneumocócica conjugada 10 valente no calendário vacinal no Paraná sobre os casos de meningite pneumocócica; avaliar a distribuição dos sorotipos dentre os casos.
MétodoEstudo observacional, transversal, com coleta de dados retrospectiva dos casos de meningite pneumocócica no Estado do Paraná, notificados ao SINAN, no período de 1998 a 2011. Foram analisados 1339 casos de meningite pneumocócica e comparados os 1205 casos do período pré-vacina (1998 a 2009) com os 134 do período pós-vacina (2010 a 2011). A análise estatística descritiva e comparativa (teste qui-quadrado e razão de prevalência) foi realizada no software de estatística JMP 5.1.2 (JMP Statistical Discovery, Carolina do Norte, EUA) e no Programa EPI INFO 6.
ResultadosObservou-se redução significativa das taxas médias de incidência e mortalidade na população geral. A análise dos casos nos períodos pré e pós-vacina nas faixas etárias contempladas pela vacinação (menores de 2 anos) mostrou reduções significativas das taxas de incidência (6,01 casos/100.000 para 2,49 casos/100.000 habitantes), mortalidade (1,85 casos/100.000 habitantes para 0,47 casos/100.000 habitantes), enquanto que a letalidade média não apresentou variação significativa. Houve redução significativa dos casos cujos sorotipos estão incluídos na vacina (80,7% para 53,3%).
ConclusãoMesmo com um tempo reduzido de uso, a vacina pneumocócica conjugada 10 valente já apresentou um impacto relevante na diminuição dos coeficientes de incidência e mortalidade dos casos de meningite entre os lactentes, além de redução de casos cujos sorotipos estão incluídos na vacina.
Streptococcus pneumoniae (pneumococcus) can be found in the nasopharynx and oropharynx mucosa of healthy humans, and is important due to its morbidity and mortality related to diseases such as meningitis, pneumonia, and septicemia, among others.1 In Brazil, it is the second-leading causative agent of bacterial meningitis, following Neisseria meningitidis.2
Pneumococcal disease prevention is primarily based on active immunization. A total of 93 pneumococcus serotypes3 have been identified, according to the antigenicity and immunogenicity of the polysaccharide capsule, the main bacterial virulence factor. The polysaccharide antigens induce a serotype-specific immunological response, which is very useful for the composition of pneumococcal vaccines. The 23-valent vaccine consists of purified capsular polysaccharides of 23 serotypes of Streptococcus pneumoniae, produces thymus-independent immune response, and is therefore indicated only for children older than 2 years of age. When the polysaccharides are individually conjugated to protein carriers, there is immunogenicity improvement, as they are capable of triggering the immune memory response (thymus-dependent) and can be administered to children younger than 2 years of age, the main age group affected by invasive pneumococcal disease.1,4,5
The pneumococcal conjugate vaccines released by regulatory agencies and currently marketed in Brazil are the 10-valent and 13-valent, which protect against ten and 13 pneumococcal serotypes, respectively. The 10-valent pneumococcal conjugate vaccine became part of the National Immunization Program (NIP) schedule from 2010 for children younger than 24 months. The 23-valent pneumococcal polysaccharide vaccine is available at the Special Immunobiological Reference Centers (Centros de Referências de Imunobiológicos Especiais–CRIE) to patients older than 2 years of age considered at risk for invasive pneumococcal disease, and institutionalized elderly patients older than 60 years.6
The objective of this study was to evaluate the effect of the introduction of the 10-valent pneumococcal conjugate vaccine into the NIP vaccination schedule on the epidemiological indicators and serotypes of pneumococcal meningitis in the state of Paraná, Brazil.
MethodsThis was an observational, cross-sectional study with retrospective data collection of cases of meningitis caused by Streptococcus pneumoniae, demonstrated through laboratory tests, which occurred in the state of Paraná and were notified to the Notifiable Diseases Information System (Sistema de Informação de Agravos de Notificação–SINAN), from January of 1998 to December of 2011.
The analysis of cases of pneumococcal meningitis was performed by comparing the periods according to the introduction of 10-valent pneumococcal conjugate vaccine in the NIP. The pre-vaccine period included the years 1998 to 2009, and the post-vaccine period, the years 2010 and 2011.
The indicators used for the analysis of cases over the years, or in the pre- and post-vaccine periods, based on population data from the Brazilian Institute of Geography and Statistics (Instituto Brasileiro de Geografia e Estatística–IBGE) were as follows:
- •
Mean incidence rate: (mean number of meningitis cases in the period/mean population of the period)×100,000
- •
Mean mortality rate: (mean number of deaths due to pneumococcal meningitis in the period/mean population of the period)×100,000.
- •
Mean lethality: (mean number of deaths by pneumococcal meningitis in the period/mean number of cases of pneumococcal meningitis in the period)×100.
The term “vaccination coverage” was used for the frequency of the serotypes available in the conjugate vaccines of the studied sample.
Data were recorded in a Microsoft Excel® 2010 (Microsoft, New York, USA) spreadsheet, verified, and then exported to JMP Statistical Discovery Software 5.1.2 (JMP Statistical Discovery, North Carolina, USA) and EPI INFO 6 (Centers for Disease Control and Prevention, Georgia, USA). The estimate of the difference between categorical variables was performed by chi-squared test, with a minimum significance level of 5%. The prevalence ratio was used in the comparison of cases in children younger than 1 year between the pre- and post-vaccine periods, with a confidence interval of 95%.
The study was approved by the Research Ethics Committee of the Department of Health of the State of Paraná/Hospital do Trabalhador, on August 26, 2010, Case No. 218/2010.
ResultsBetween January of 1998 and December of 2011, 1,354 cases of pneumococcal meningitis were reported to SINAN in the state of Paraná. Of this population, 15 cases were excluded due to inconsistency of information and duplicate notifications, totaling 1,339 cases, with 1,205 in the pre-vaccine and 134 in the post-vaccine period.
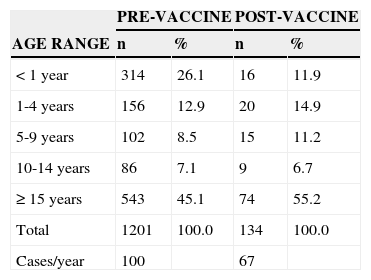
The distribution of cases according to age range in both periods is shown in Table 1. When analyzing the pre-vaccine and post-vaccine periods, a 54% reduction can be observed in the frequency of cases in the age group<1 year (26.1% to 11.9%, respectively). The prevalence ratio among cases of pneumococcal meningitis in children younger than 1 year between the pre- and post-vaccine periods was 2.68 (95% CI: 1.62 to 4.44, p<0.01).
Distribution of cases of pneumococcal meningitis in the periods pre- (1998-2009) and post-vaccination (2010-2011), according to age range. Paraná, 1998-2011.
| PRE-VACCINE | POST-VACCINE | |||
|---|---|---|---|---|
| AGE RANGE | n | % | n | % |
| < 1 year | 314 | 26.1 | 16 | 11.9 |
| 1-4 years | 156 | 12.9 | 20 | 14.9 |
| 5-9 years | 102 | 8.5 | 15 | 11.2 |
| 10-14 years | 86 | 7.1 | 9 | 6.7 |
| ≥ 15 years | 543 | 45.1 | 74 | 55.2 |
| Total | 1201 | 100.0 | 134 | 100.0 |
| Cases/year | 100 | 67 | ||
Obs: Four cases had no data on age in the pre-vaccine period.
1. Evaluation of epidemiological indicators in the pre and post-vaccine periods:
When comparing the epidemiological indicators between the two studied periods in the general population, there was a significant decrease. The mean incidence rate decreased by 36.0% (1.00 cases/100,000 inhabitants to 0.64 cases/100,000 inhabitants; p<0.001) and the mean mortality rate decreased by 65.5%, from 0.29 cases/100,000 inhabitants to 0.10 cases/100,000 inhabitants (p<0.001).
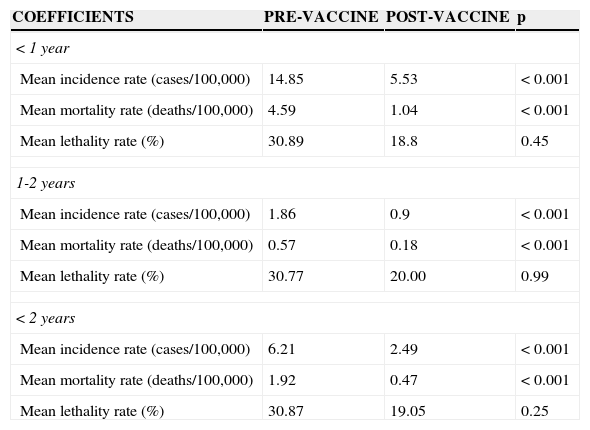
When analyzing the profile indicators between pre-and post-vaccine periods, specifically in the population of children younger than 2 years, there were significant reductions in mean incidence and mortality rates (Table 2). The mean incidence rate decreased by 59.9% (6.21 cases/100,000 to 2.49 cases/100,000 inhabitants; p<0.01), and the mean mortality rate decreased by 75.5% (1.92 deaths/100,000 to 0.47 deaths/100,000; p<0.01). Similar results were obtained in the analysis of the age range younger than 1 year and between 1 and 2 years. Although lethality rates also decreased between the two periods, the differences were not statistically significant.
Epidemiological indicators for pneumococcal meningitis in children younger than 2 years. Paraná, 1998-2011.
| COEFFICIENTS | PRE-VACCINE | POST-VACCINE | p |
|---|---|---|---|
| < 1 year | |||
| Mean incidence rate (cases/100,000) | 14.85 | 5.53 | < 0.001 |
| Mean mortality rate (deaths/100,000) | 4.59 | 1.04 | < 0.001 |
| Mean lethality rate (%) | 30.89 | 18.8 | 0.45 |
| 1-2 years | |||
| Mean incidence rate (cases/100,000) | 1.86 | 0.9 | < 0.001 |
| Mean mortality rate (deaths/100,000) | 0.57 | 0.18 | < 0.001 |
| Mean lethality rate (%) | 30.77 | 20.00 | 0.99 |
| < 2 years | |||
| Mean incidence rate (cases/100,000) | 6.21 | 2.49 | < 0.001 |
| Mean mortality rate (deaths/100,000) | 1.92 | 0.47 | < 0.001 |
| Mean lethality rate (%) | 30.87 | 19.05 | 0.25 |
2. Distribution of serotypes identified in the pre- and post-vaccine periods:
In the pre-vaccine period, 46.3% (558/1205) of the cases were serotyped. Of these, 58.1% corresponded to cases of which pneumococcus serotype was included in the 10-valent conjugate vaccine (1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, 23F). In the post-vaccine period, 45.5% (61/134) of the cases were serotyped, of which 47.5% were caused by serotypes included in the vaccine. When the possible cross-protection between serotypes 6A and 6B is considered, in the pre- and post-vaccine periods, the identification of 62.2% and 52.5% of the serotypes included in the 10-valent vaccine are observed, respectively.
When analyzing the most frequent serotypes between the periods in the general population, an increase in the frequency of cases with serotype 3, 10A, and 4 in the post-vaccination period is observed, with the first two serotypes not included in the 10-valent conjugate vaccine.
3. Distribution of serotypes in the age range younger than 2 years:
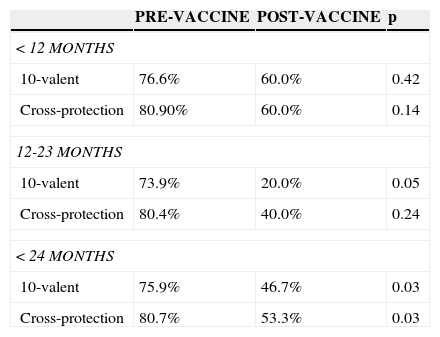
The analysis of serotyped cases in the age group younger than 2 years (covered by the vaccine distributed by the NIP) showed that of the 202 cases analyzed (187 in the pre-vaccine and 15 in the post-vaccine period), the percentage of serotyped cases caused by serotypes included in the 10-valent conjugate vaccine had decreased from 75.9% to 46.7% between the pre- and post-vaccine periods. Considering only the age range of 12-23 months, the decrease was more significant (from 73.9% to 20%). The comparison between the pre- and post-vaccine periods in children younger than 2 years, with the expansion of cross-protection of serotype 6A, shows a significant decrease in cases of which serotypes are included in the vaccine (from 80.7% to 53.3%, p=0.030; Table 3).
Coverage of the 10-valent pneumococcal conjugate vaccine in the pre- and post-vaccine periods according to age.
| PRE-VACCINE | POST-VACCINE | p | |
|---|---|---|---|
| < 12 MONTHS | |||
| 10-valent | 76.6% | 60.0% | 0.42 |
| Cross-protection | 80.90% | 60.0% | 0.14 |
| 12-23 MONTHS | |||
| 10-valent | 73.9% | 20.0% | 0.05 |
| Cross-protection | 80.4% | 40.0% | 0.24 |
| < 24 MONTHS | |||
| 10-valent | 75.9% | 46.7% | 0.03 |
| Cross-protection | 80.7% | 53.3% | 0.03 |
Obs: Cross-protection with serotype 6 A.
The analysis of the effects of the introduction of the 10-valent conjugate vaccine in the NIP in the Brazil must be carefully considered. The 10-valent pneumococcal conjugate vaccine was introduced into the routine immunization program in Brazil from March to September of 2010.7
In the present study, when comparing the pre-vaccine (1998-2009) with the post-vaccine period (2010 and 2011), and even including the year 2010, which was a transition period when municipalities were progressively implementing pneumococcal vaccine at the basic health units, a reduction of 54% in cases of meningitis in children younger than 1 year was found, with the highest prevalence of cases in this age group in the pre-vaccine period. When analyzing the general population, the incidence rates and the mean mortality rates decreased by 36.0% and 65.5%, respectively. For the age group covered by the vaccine, i.e., younger than 2 years, significant reductions in the mean incidence and mortality rates (reduction of 59.9% and 75.5%, respectively) were also observed.
Since the Kaiser Permanent Study Center,8 studies have shown the benefits of using conjugate pneumococcal vaccines.9,10
The use of the 7-valent pneumococcal conjugate vaccine (no longer marketed) showed 97% efficacy against invasive pneumococcal diseases caused by vaccine serotypes and 89% against invasive pneumococcal diseases in general. The impact of this vaccine was demonstrated by the reduction of invasive diseases, from 79% to 100%, and in cases of medical consultations for acute otitis media, with an impact of 13% to 43%.11 Regarding the 10-valent pneumococcal conjugate vaccine, Afonso et al. found significant reductions in hospitalizations for pneumonia in Belo Horizonte, Curitiba, and Recife, after its introduction in the Brazilian immunization schedule.12 This vaccine also showed to be effective in studies performed in Finland13 and Quebec,14 with reductions in the incidence rates of invasive pneumococcal diseases.
Although the post-vaccine period in Brazil still comprises a short time period, with a three-dose vaccine coverage in the first year of life of 35.36% in 2010, reaching 94.19% in 2011,15 a reduction in epidemiological indicators can be observed, which is in agreement with studies that showed benefits with the use of pneumococcal conjugate vaccines within 15 months after its introduction.14 The impact of the vaccine use and indirect benefits of the herd effect, i.e., decreased cases in unvaccinated individuals by reducing nasopharyngeal colonization and transmission of vaccine serotypes by vaccinated children16 would be too early at this stage of the analysis. It is expected that data will be compatible with those found in effectiveness studies after the introduction of the 7-valent pneumococcal conjugate vaccine, in which the indirect effects were responsible for a 19% to 62% reduction in the overall incidence of invasive pneumococcal diseases in individuals aged>18 years, and 81% to 92% of cases related to vaccine serotypes in the same age group.11
When the main pneumococcal serotypes identified in cases of meningitis in this research were compared with serotypes included in the 10-valent conjugate vaccine, a lower rate was observed (58.1% pre-vaccine) in relation to other reported studies. However, considering the possible cross-protection between serotypes 6A and 6B, there would be an identification of 62.2% in the pre-vaccine period.
Rates found in Brazil were 77.6% for the 10-valent conjugate vaccine and 86% for the 13-valent conjugate vaccine for invasive pneumococcal diseases;17,18 data from Africa show rates of 70% to 84% for the 10-valent conjugate vaccine and from 79% to 88% for the 13-valent conjugate vaccine.19 For meningitis, the coverage of the 10-valent and 13-valent conjugate vaccines would be 82.8% and 94.2%, respectively.2
In this study, when comparing the periods before and after the introduction of the 10-valent conjugate vaccine in the Brazilian NIP, a significant reduction was observed in rates of serotypes included in the vaccine as the cause of diseases in the age range<2 years (from 80.7% to 53.3%), which could be explained by the routine use of this immunobiological agent.
When making a projection, comparing the coverage of pneumococcal conjugate vaccines available in Brazil, there is better coverage (serotypes included in the vaccines coincident with the isolates in the state) with the 13-valent vaccine, when compared to the 10-valent, both in the pre-vaccine (69.2% and 58.1%, respectively, p<0.01) and the post-vaccine period (68.9% and 47.5%, respectively, p=0.017). If the 10-valent vaccine coverage were extended with the cross-protection of serotype 6A, this difference would not be maintained in the post-vaccine period (13-valent: 68.9% and 10-valent: 52.5%, p=0.064), but rather, only in the pre-vaccine period (13-valent: 69.2% and 10-valent: 62.2%, p=0.014).
The effect of serotype replacement, i.e., increase in cases by serotypes not included in the conjugate vaccines through a reduction in the circulation of vaccine serotypes in a vaccinated population,20 appears to be premature, in addition to the fact that fluctuations in the frequency of the serotypes can occur without the need for selective pressure of vaccines.21 In the present study, an increase in the number of cases in the post-vaccine period was observed due to serotypes 3 and 10A, not included in the 10-valent conjugate vaccine. In Europe and the United States, after using the 7-valent pneumococcal conjugate vaccine, the main non-vaccine serotypes found in invasive pneumococcal diseases were 1, 3, 7F, 15, 19A, 27F, and 33F.22
The analysis of cases of pneumococcal meningitis in the pre- and post-vaccine periods showed a reduction of cases in the age range<1 year, significant reductions in incidence and overall mortality coefficients, and of the same coefficients in the age groups covered by the vaccine. The coverage of pneumococcal vaccines for meningitis cases was lower than that described in the literature, but with a reduction of the vaccine serotypes in the post-vaccine period.
One limitation of this study was the source of data for analysis, which is based on notification data. Although notification of meningitis is compulsory in Brazil, the lack of some information, the divergence of data, and the lack of proper completion of forms at times prevented a more complete analysis of the available data, information that could not be included in this research. Nevertheless, the development of new studies that follow the scenario of invasive pneumococcal diseases in the state (not only of meningitis), the effects of the introduction of pneumococcal conjugate vaccine into the NIP, and the maintenance of surveillance on serotypes circulating in the population are encouraged.
Permanent surveillance of cases of pneumococcal meningitis and the most prevalent serotypes allows the choice of conjugate vaccines that are best adapted to the geographical region and its population.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank the staff of the Department of Epidemiological Surveillance (DEVE), the Division of Communicable Diseases Surveillance (DVVTR), and the State Secretariat of Paraná, particularly Ms. Marlene Sera Wille, for their collaboration with data collection.
Please cite this article as: Hirose TE, Maluf EM, Rodrigues CO. Pneumococcal meningitis: epidemiological profile pre- and post-introduction of the pneumococcal 10-valent conjugate vaccine. J Pediatr (Rio J). 2015;91:130–5.
Study conducted at Universidade Federal do Paraná (UFPR), Curitiba, PR, Brazil.