The prevalence of obesity is increasing. The aim of this study was to investigate if there is endothelial dysfunction in children with normal or excess weight, and whether the metabolic profile, adipokines, and endothelial dysfunction would be more strongly associated with physical fitness or with physical activity levels.
MethodCross-sectional study involving children aged 5–12 years. The evaluation included venous occlusion plethysmography, serum levels of adiponectin, leptin and insulin, lipid profile, physical activity score (PAQ-C questionnaire), and physical fitness evaluation (Yo-Yo test).
ResultsA total of 62 children participated in this study. Based on the body mass index, 27 were eutrophic, 10 overweight and 25 obese. Triglycerides, LDL cholesterol, HOMA-IR, and leptin were higher in the obese and excess-weight groups compared to the eutrophic group (p<0.01). HDL cholesterol and adiponectin levels were higher in the eutrophic group compared to the obese and excess-weight groups (p<0.01). Flow-mediated vasodilation after hyperemia was higher in the eutrophic group in comparison to obese and excess-weight subjects (p<0.05). There was no difference in the physical activity levels among groups measured by PAQ-C. The Yo-Yo test was significantly associated with HDL cholesterol (rho=−0.41; p=0.01), and this association remained after adjusting for body mass index z-score (rho=0.28; p=0.03).
ConclusionThis study showed that endothelial dysfunction is already present in obese children, suggesting a predisposition to atherosclerotic disease. Moreover, HDL cholesterol levels were correlated with physical fitness, regardless of body mass index.
A prevalência da obesidade está aumentando. O objetivo deste estudo foi investigar se há disfunção endotelial nas crianças com peso normal ou excesso de peso e se o perfil metabólico, as adipocinas e a disfunção endotelial seriam mais fortemente associados à aptidão física ou aos níveis de atividade física.
MétodoEstudo transversal que envolve crianças de 5-12 anos. A avaliação incluiu pletismografia de oclusão venosa, níveis séricos de adiponectina, leptina, insulina e lipidograma, escore de atividade física (questionário PAQ-C) e avaliação da aptidão física (teste Yo-yo).
ResultadosUm total de 62 crianças participou deste estudo. Com base no índice de massa corporal, 27 eram eutróficos, 10 estavam acima do peso e 25 estavam obesos. Os níveis de triglicerídeos, colesterol LDL, HOMA-RI e leptina estavam mais elevados nas crianças obesas e com excesso de peso que o grupo de eutróficos (p<0,01). Os níveis de colesterol HDL e adiponectina estavam mais elevados no grupo de eutróficos em comparação ao grupo de obesos e com excesso de peso (p<0,01). A vasodilatação mediada pelo fluxo após hiperemia foi maior no grupo de eutróficos em comparação aos indivíduos obesos e com excesso de peso (p<0,05). Não houve nenhuma diferença nos níveis de atividade física entre os grupos medidos pelo PAQ-C. O teste de ida e volta foi significativamente associado ao colesterol HDL (ró=– 0,41; p=0,01) e essa associação continuou após ajustar o escore z do índice de massa corporal (ró=0,28; p=0,03).
ConclusãoEste estudo mostrou que a disfunção endotelial já está presente nas crianças obesas, sugeriu uma predisposição à doença aterosclerótica. Além disso, os níveis de colesterol HDL foram correlacionados à aptidão física, independentemente do índice de massa corporal.
The prevalence of obesity is increasing worldwide and is considered a public health problem due to its epidemic form.1 The causes for this increment in prevalence seem to be a progressive reduction in the level of physical activity2 and changes in dietary habits, with an increase of energy intake.3 One of the main concerns with obesity is its association with several risk factors for developing cardiovascular disease, such as hypertension, dyslipidemia, hyperinsulinemia and glucose intolerance, culminating with type 2 diabetes mellitus (DM2).4 Even though obesity affects all age groups, for many years it was thought that complications were found only in obese adults. Several studies have now demonstrated that childhood obesity has negative effects on children's health.
Cardiovascular disease is mainly diagnosed at an old age, but the insidious processes of atherosclerosis, the basis for cardiovascular disease, may begin in early childhood. Endothelial dysfunction not only is the precursor but can also be used as a marker of the atherosclerotic process as it predicts cardiovascular mortality and morbidity.5,6 This has been demonstrated in adults as well as in obese children and adolescents.7
Physical activity is a behavior defined as any movement produced by the skeletal muscles that results in a substantial increase in energy expenditure beyond resting levels.8 The body adaptation to physical activity has two main components: physical fitness and the actual practice of physical activity. The practice of physical activity, with its variables (regularity, length/duration, and intensity), is the principal determinant of physical fitness, although it is not the only one. Physical fitness represents the body response to physical activity and is under the influence of other variables, such as age, gender, health, genetics, and environmental factors such as diet, stress, cigarette smoking, alcohol consumption, and personal background, meaning that someone who was very active in the past could have better physical fitness in the present, even if not presently active. For most individuals, increasing physical activity improves physical fitness,9 and high levels of both are related to lower rates of morbidity and mortality from various causes, including cardiovascular diseases.10
In adults, it is a well-known fact that the regular practice of physical activity improves endothelial function, reducing cardiovascular mortality and morbidity.11 In children, the regular practice of moderate to vigorous physical activity affects endothelial function.12 Longitudinal studies demonstrated that, for obese children, physical training might improve endothelial function regardless of changes in body mass index (BMI) or body weight.13,14
It is crucial to better understand the physiology of increased cardiovascular risk beginning in childhood. In children, is the regular practice of physical activity a direct protector of endothelial function, regardless of weight, or does the mechanism by which physical activity protects the endothelial function only happen through weight control? Furthermore, there is the physical fitness factor, which needs to be taken into consideration.
The aim of this study was to investigate, in children with normal or excess weight, if there is endothelial dysfunction and whether the metabolic profile, adipokines, and endothelial function would be more strongly associated with physical fitness or with habitual physical activity levels.
Material and methodsSubjectsThis was a cross-sectional study involving children aged 5–12 years, prepubertal or in early puberty (Tanner 2 pubertal stage). Children were recruited from the general pediatric clinics and from schools. Children were classified as overweight or obese based on the WHO criteria. Children with a BMI z-score between+1 e+2 were considered overweight, and those with values above+2 were considered obese. For statistical analysis purposes, overweight and obese children integrated a sole group referred to as “excess weight”. The z-score was calculated with the use of the Anthro Plus version 1.04 (WHO, Geneva, Switzerland). The exclusion criteria included diseases or conditions that could interfere in the evaluation of the microcirculation, inflammatory markers or with the test to evaluate the physical conditioning, such as renal, hematologic, hepatic, rheumatologic, cardiovascular, respiratory, infectious, and endocrine diseases. Recent exposure to traumas or surgeries would also exclude subjects from the study. The study was approved by the Ethics and Research Committee from the institution, and an informed consent form was signed by the parent/guardian.
ProceduresThe children were evaluated at the Child Obesity Clinic from the Pediatric Endocrinology Service of the University Hospital. The evaluation included a medical-nutritional assessment. Blood pressure (Tycos, Welch Allyn Company, Arden, NC, USA) was assessed with the child in a sitting position, following standard protocol. Weight was assessed with a digital scale (Filizola®, São Paulo, SP, Brazil) with a resolution of 100g. Height was measured with a wall-mounted stadiometer (Tonelli®, Criciúma, SC, Brazil) with a resolution of 1mm. All children were weighed and measured wearing light clothes and no shoes. Waist circumference was measured at the midpoint between the last costal arch and the iliac crest. The hip circumference was measured in most subjects, and the waist/hip ratio was calculated. The circumference of the abdomen was measured two fingers below the umbilicus. All circumferences were measured with a non-distensible tape.
All blood samples were collected in the morning, after a 12-h fast. Samples were immediately centrifuged, stored and frozen at −80°C for posterior analysis. Insulin was measured in gamma-C12 equipment with a Coat-A-Count radioimmunoassay solid phase 1251 (DPC, Los Angeles, CA, USA). Leptin and adiponectin were measured by radioimmunoassay on gamma-C12 equipment with kits using the PEG double antibody method (Linco Research, St. Charles, MO, USA). Serum glucose, total cholesterol (TC), high-density lipoprotein (HDL), and triglycerides (TG) were analyzed immediately after collection. HOMA-IR was calculated by multiplying the value of fasting glucose (mmol/L) by fasting insulin (μIU/mL) and dividing by 22.5.15
On a separate visit, venous occlusion plethysmography was performed after at least a 4-h fast and abstinence from caffeine and exercise. Subjects remained in a controlled-temperature room (20–22°C) at supine position. Forearm blood flow (FBF), in mL/min/100mL tissue, was measured using VOP (Hokanson, EC6, D.E., Bellevue, WA, USA) in the non-dominant forearm or leg (based on the size of the child), kept at heart level, with a mercury-in-silastic strain gauge placed on the proximal third of the member at its maximum circumference. Measurements of the baseline flow and during hyperemia response after a 3-min forearm arterial occlusion were performed. It has been demonstrated that there is an initial (1–2min) rise in forearm volume, with a small and slow rise with continuation of the ischemia. Since this study dealt with young children, and to decrease the discomfort of the exam, a 3-min occlusion time was used. There was a 20-min interval between the reactive hyperemia response and the second baseline flow measurement. The mean of the first 4 measurements in each recording period was used for analysis.
The Dual-energy X-ray absorptiometry (DXA) (GE Healthcare®, San Francisco, CA, USA), assessed whole-body and regional fat as well as lean tissue mass. The child was oriented not to use medications and to wear clothing without metal accessories.
The physical activity scores were assessed by the Physical Active Questionnaire (PAQ-C), which investigates – by asking parents and the child – the frequency and intensity of physical activity practiced in the previous seven days (including the weekend).16 The questionnaire consists of nine questions about playing sports and games; physical activities at school and as leisure, including weekends. Questions have values ranging from 1 to 5, and final scores are attained by averaging responses, from very sedentary (1) to very active (5). This questionnaire was translated into Portuguese and validated for the Brazilian population and age group, and used in different studies.17 Children with a score <4.0 were considered sedentary, and children with a score ≥4.0 were considered active. The questionnaire also assesses the number of hours spent watching television per day.
The evaluation of physical fitness was made by Hardens Yo-Yo Intermittent Endurance Test Level 1,18 which consists of two bouts, of 20m each, of progressive speed shuttle running, interspersed with 5s of active recovery. The subject had to run following the beeps emitted by a CD player. The signal was based on the speed in km/h, starting at 8km/h and increasing 0.5km/h each minute. The aim was for the subject to arrive at the other cone before the next beep. The run was terminated when there was voluntary interruption by the participant because of exhaustion or two consecutive delays. All children were oriented to use light clothing and tennis shoes to the test. When the subjects could not run more than 240m (five complete laps) they were classified as not fit, and when they could run more than 240m they were considered fit.
Statistical analysisPreliminary calculations showed that for a Statistical Power of 80% the desirable sample size would have been 99 individuals.
Data normality and homoscedasticity were tested by Kolmogorov–Smirnov and Shapiro–Wilk tests. Some minor distributional problems were found; therefore, the data was analyzed in a nonparametric approach. Differences in the anthropometric and physiological parameters between the three groups were tested through nonparametric Kruskal–Wallis ANOVA. The Dunn post hoc test was used to identify specific differences between groups.
Comparisons between the eutrophic and excess weight groups (overweight+obese groups) were carried out through the Mann–Whitney U test. Correlations between variables among the groups were obtained through Spearman's Rho, as well as partial correlation analyses. The descriptive and comparative statistics were performed with Statistica version 8 (StatSoft Inc., Tulsa, OK, USA), and correlation tests were performed with the PPCOR package for the R Language and Environment for Statistical Computing version 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria). Statistical significance level was set to alpha 0.05.
ResultsSixty-two children participated in this study; 31 were boys. Based on the BMI, 27 were eutrophic, 10 were overweight, and 25 were obese.
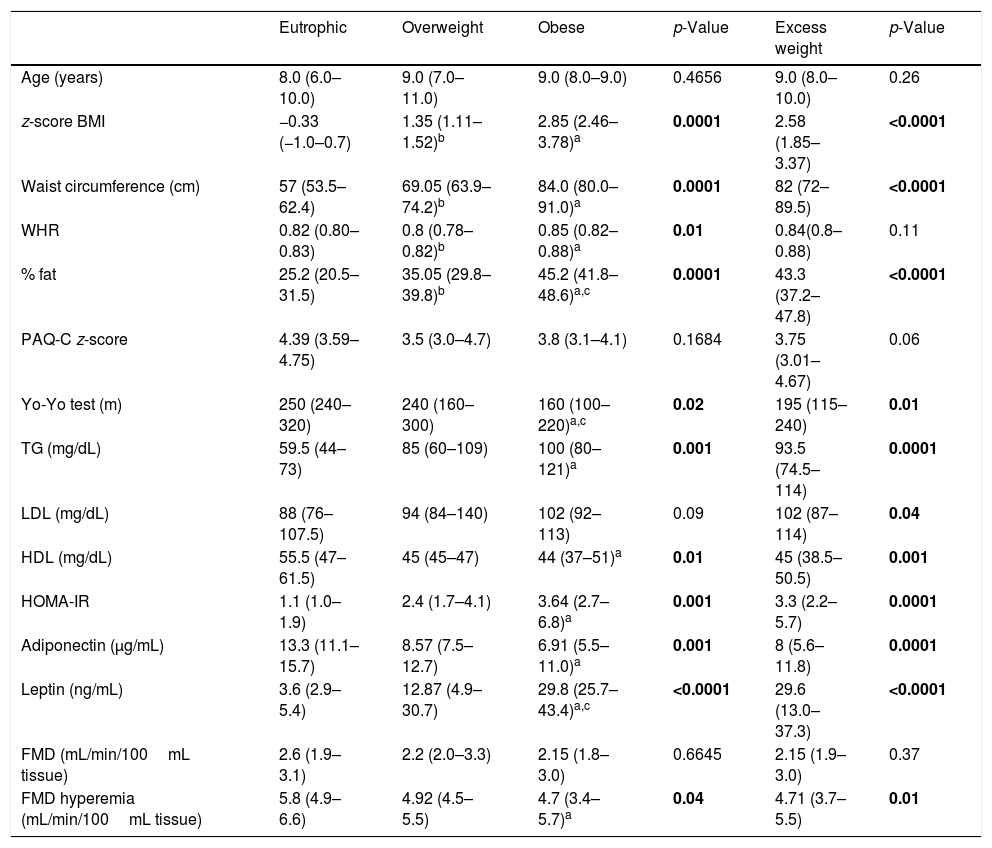
As expected, the anthropometric variables (BMI z-score, waist circumference, waist-hip ratio and body fat), triglycerides, LDL cholesterol, HOMA-IR, and leptin were significantly higher in the obese and excess weight groups (individually or grouped together as the excess weight group) compared to the eutrophic group (p<0.01). The HDL cholesterol and adiponectin levels were higher in the eutrophic group compared to the obese and excess weight groups (p<0.01) (Table 1).
Comparison between eutrophic, overweight and obese children in relation to anthropometric data, physical activity, physical fitness, metabolic profile, adipokines, and FMD.
| Eutrophic | Overweight | Obese | p-Value | Excess weight | p-Value | |
|---|---|---|---|---|---|---|
| Age (years) | 8.0 (6.0–10.0) | 9.0 (7.0–11.0) | 9.0 (8.0–9.0) | 0.4656 | 9.0 (8.0–10.0) | 0.26 |
| z-score BMI | −0.33 (−1.0–0.7) | 1.35 (1.11–1.52)b | 2.85 (2.46–3.78)a | 0.0001 | 2.58 (1.85–3.37) | <0.0001 |
| Waist circumference (cm) | 57 (53.5–62.4) | 69.05 (63.9–74.2)b | 84.0 (80.0–91.0)a | 0.0001 | 82 (72–89.5) | <0.0001 |
| WHR | 0.82 (0.80–0.83) | 0.8 (0.78–0.82)b | 0.85 (0.82–0.88)a | 0.01 | 0.84(0.8–0.88) | 0.11 |
| % fat | 25.2 (20.5–31.5) | 35.05 (29.8–39.8)b | 45.2 (41.8–48.6)a,c | 0.0001 | 43.3 (37.2–47.8) | <0.0001 |
| PAQ-C z-score | 4.39 (3.59–4.75) | 3.5 (3.0–4.7) | 3.8 (3.1–4.1) | 0.1684 | 3.75 (3.01–4.67) | 0.06 |
| Yo-Yo test (m) | 250 (240–320) | 240 (160–300) | 160 (100–220)a,c | 0.02 | 195 (115–240) | 0.01 |
| TG (mg/dL) | 59.5 (44–73) | 85 (60–109) | 100 (80–121)a | 0.001 | 93.5 (74.5–114) | 0.0001 |
| LDL (mg/dL) | 88 (76–107.5) | 94 (84–140) | 102 (92–113) | 0.09 | 102 (87–114) | 0.04 |
| HDL (mg/dL) | 55.5 (47–61.5) | 45 (45–47) | 44 (37–51)a | 0.01 | 45 (38.5–50.5) | 0.001 |
| HOMA-IR | 1.1 (1.0–1.9) | 2.4 (1.7–4.1) | 3.64 (2.7–6.8)a | 0.001 | 3.3 (2.2–5.7) | 0.0001 |
| Adiponectin (μg/mL) | 13.3 (11.1–15.7) | 8.57 (7.5–12.7) | 6.91 (5.5–11.0)a | 0.001 | 8 (5.6–11.8) | 0.0001 |
| Leptin (ng/mL) | 3.6 (2.9–5.4) | 12.87 (4.9–30.7) | 29.8 (25.7–43.4)a,c | <0.0001 | 29.6 (13.0–37.3) | <0.0001 |
| FMD (mL/min/100mL tissue) | 2.6 (1.9–3.1) | 2.2 (2.0–3.3) | 2.15 (1.8–3.0) | 0.6645 | 2.15 (1.9–3.0) | 0.37 |
| FMD hyperemia (mL/min/100mL tissue) | 5.8 (4.9–6.6) | 4.92 (4.5–5.5) | 4.7 (3.4–5.7)a | 0.04 | 4.71 (3.7–5.5) | 0.01 |
Results expressed as median (lower–upper quartile). Excess weight group represents the overweight and obese children combined.
FMD, flow-mediated vasodilation; BMI, body mass index; WHR, waist–hip ratio; PAQ-C, physical activity questionnaire for older children; TG, triglycerides; LDL, low density lipoprotein; HDL, high density lipoprotein; IL-6, interleukin-6.
Comparison between Eutrophic×obese.
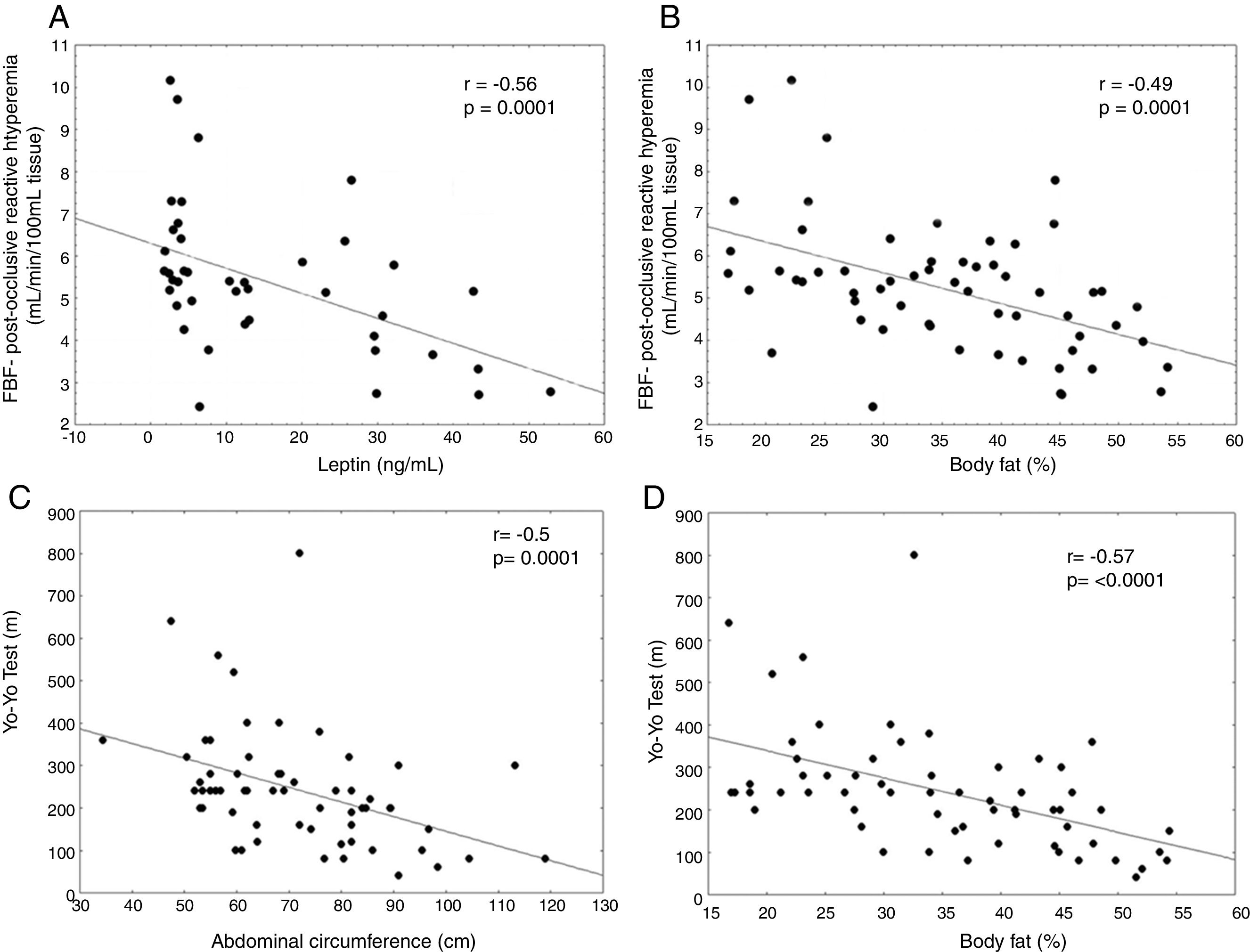
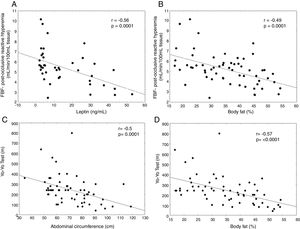
In the analysis of endothelial function, flow-mediated vasodilatation (FMD) after hyperemia was higher in the eutrophic group in comparison to the obese and excess weight subjects, demonstrating an impaired endothelial function in the latter (p<0.05) (Table 1). Changes in the FMD after hyperemia strongly correlated negatively with leptin (rho=−0.56; p=0.0001) and body fat percentage (rho=−0.49, p=0.0001), as shown in Fig. 1A and B, respectively, demonstrating that the higher the leptin levels or body fat was, the lower was the endothelial response to hyperemia.
There was no difference in physical activity levels among groups measured by PAQ-C. Thirty children were classified as active (8 were obese) and 30 as sedentary. Two children did not answer the PAQ-C questionnaire. Based on the Yo-Yo test, thirty-two children were considered physically fit (6 obese) and 28 unfit. Two children did not complete the Yo-Yo test. There was no statistically significant correlation between PAQ-C or the Yo-Yo test and FMD reactive hyperemia. There was a strong negative correlation between the Yo-Yo test and waist circumference (rho=−0.50, p=0.0001) as well as body fat percentage (rho=−0.57, p<0.0001) (Fig. 1C and D, respectively).
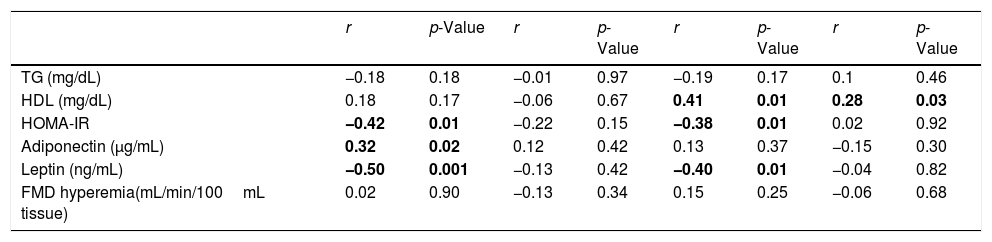
Since one of the questions entailed trying to differentiate between the influence of body weight and of physical fitness and the practice of physical activity, a 3-way correlation analysis was performed. Firstly, we investigated if there was correlation between the metabolic profile or inflammatory markers and physical fitness or the practice of physical activity. Subsequently, we adjusted it for the BMI z-score to see if the correlation persisted. HOMA-IR and leptin were inversely correlated with the PAQ-C z-score, while adiponectin was positively correlated. After adjustments for BMI z-score (adding the BMI z-score to the correlation analysis as a third variable), those correlations were not sustained, demonstrating that the initial correlations were probably dependent of the BMI z-score (Table 2).
Correlation between PAQ-C, Yo-Yo test and metabolic profile, adipokines and FMD hyperemia. Partial correlation with PAQ-C z-score. Adjustment to BMI z-score Partial correlation with Yo-Yo test Adjustment to BMI z-score.
| r | p-Value | r | p-Value | r | p-Value | r | p-Value | |
|---|---|---|---|---|---|---|---|---|
| TG (mg/dL) | −0.18 | 0.18 | −0.01 | 0.97 | −0.19 | 0.17 | 0.1 | 0.46 |
| HDL (mg/dL) | 0.18 | 0.17 | −0.06 | 0.67 | 0.41 | 0.01 | 0.28 | 0.03 |
| HOMA-IR | −0.42 | 0.01 | −0.22 | 0.15 | −0.38 | 0.01 | 0.02 | 0.92 |
| Adiponectin (μg/mL) | 0.32 | 0.02 | 0.12 | 0.42 | 0.13 | 0.37 | −0.15 | 0.30 |
| Leptin (ng/mL) | −0.50 | 0.001 | −0.13 | 0.42 | −0.40 | 0.01 | −0.04 | 0.82 |
| FMD hyperemia(mL/min/100mL tissue) | 0.02 | 0.90 | −0.13 | 0.34 | 0.15 | 0.25 | −0.06 | 0.68 |
FMD, flow-mediated vasodilation.
Significance level (p<0.05).
In the correlation analysis using the Yo-Yo test with metabolic profile and adjusting for BMI z-score, HDL correlated with the Yo-Yo test (rho=0.41; p=0.01), and the correlation remained, although weaker, when it was adjusted to BMI z-score (rho=0.28; p=0.03), demonstrating that obesity explains some of the correlation between the distance ran in the Yo-Yo test and HDL, but it is not the only factor influencing that correlation. When analyzing the Yo-Yo test results and the HOMA-IR (rho=−0.38; p=0.01) (rho=0.02; p=0.92), as well as leptin (rho=−0.40; p=0.01) (rho=−0.04; p=0.82) there was an initial correlation, but it did not remain with the adjustment to BMI z-score (Table 2), thus demonstrating that the initial correlation was probably dependent on BMI z-score.
DiscussionThis study evaluated the relationship between physical fitness, physical activity, metabolic profile, and endothelial function in obese young children.
Preliminary calculations showed that the desirable sample size would have been 99 individuals; due to different factors, only 56 individuals participated in the study, which leaves us with a power of 55%. However, even with a smaller-than-the-ideal sample size, this study showed differences in physical fitness levels between obese and non-obese children, and that obese young persons already have a predisposition to atherosclerotic disease.
The LDL cholesterol, HOMA-IR, and leptin, known markers of cardiovascular disease, were higher in the obese group. At the same time, serum levels of HDL cholesterol and leptin were lower. This metabolic profile indicates that even at a young age these children already have a greater risk of developing a cardiovascular disease in the future.19
Endothelial dysfunction is considered the key to the development of cardiovascular disease, and its early detection may be important to develop strategies to intervene in children who have a higher risk for atherosclerotic disease.20 In our findings, there were changes in the endothelial function in obese children in comparison with other groups. Fig. 1A and B reiterate those findings, showing a strong inverse correlation between FMD and leptin, a marker of obesity, and FMD versus corporal fat. Young children with increased body fat or leptin (marker of obesity) already have an impaired endothelial function. An observational study about the relationship of the physical activity levels in children aged 5–10 years, classified as moderately active, found a correlation between physical activity levels and FMD, concluding that it would be important to start a program of physical activity in childhood to influence arterial health.21 Some studies of intervention have showed the efficiency of physical exercise in improving endothelium-dependent vasodilatation of obese children and adolescents,22,23 increasing the nitric oxide availability.24
The concept of “fat but fit” states that in adults the physical fitness may be as important as the amount of fat mass in terms of cardiovascular risk. The difficulty to investigate this concept in children is that the literature shows that obese children spend less time practicing some sort of physical activity than non-obese children,25 and, as expected, this seems to affect their body composition and to reduce their physical fitness.26 A study with children aged 7–11 years found that obesity negatively affected endurance, speed, and agility, and those were inversely associated with fitness.27 On the other hand, it seems that the sedentary lifestyle itself increases the risks of prematurely developing atherosclerosis and other diseases,28 as one study showed that even healthy and normal weight children who were classified as having low cardiorespiratory fitness had worse metabolic and inflammatory profiles than those with better fitness.29
Even though in our study there was no difference in physical fitness or physical activity when comparing children with excess weight and eutrophic children, we found an inverse correlation between the distance ran in the Yo-Yo test and the percentage of corporal fat and abdominal waist circumference, demonstrating that physical fitness, more than the practice of physical activity, already influences body composition at a young age.
To try to determine what was more important to cardiovascular health in children – adiposity or sedentarism – we verified correlations between physical activity or physical fitness and markers of CVD, adjusting for BMI z-score. There was an initial, partial correlation between physical activity and HDL, HOMA-IR, adiponectin, and leptin, but this correlation was not maintained when adjusted for BMI z-score, suggesting a probably stronger influence of body mass on those variables. The same was observed for physical fitness in relation to the variables HOMA-IR and leptin. Similarly to our findings, in one study the cardiorespiratory fitness of obese and normal-weight children was inversely associated with HOMA-IR, but this correlation disappeared when adjusted for age, sex and fat mass.30 However, in our study, the correlation with HDL was maintained even after adjustment for body mass, suggesting that the influence of physical fitness on the lipid profile is more important than that of weight excess.
Our study did not show differences in physical activity levels between obese and non-obese children, although it did show differences in physical fitness. This study also showed that obese children had higher LDL cholesterol and leptin, lower HDL cholesterol and adiponectin, and already presented signs of endothelial dysfunction, strongly suggesting a predisposition to atherosclerotic disease. Even though some of those differences were mainly related to excess weight, HDL cholesterol levels correlate to physical fitness, regardless of BMI. There was no correlation between those markers and habitual physical activity.
FundingThis work was supported by a grant from the Carlos Chagas Filho Foundation for Research Support in the state of Rio de Janeiro – FAPERJ. Jociene Terra da Penha was supported by a fellowship from the National Research Council of Brazil (CNPq).
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Penha JT, Gazolla FM, Carvalho CN, Madeira IR, Rodrigues-Junior F, Machado EA, et al. Physical fitness and activity, metabolic profile, adipokines and endothelial function in children. J Pediatr (Rio J). 2019;95:531–7.