To analyze the association between parental tobacco consumption and the prevalence of psychomotor development disorders in children between 6 and 22 months of age.
MethodOne hundred and nine mothers, fathers, and their babies participated in the study. The sociodemographic and clinical conditions were assessed using questionnaires. Tobacco consumption was assessed using the Fagerström Test for Nicotine Dependence (FTND). Child development was evaluated using the Scale of Psychomotor Development in Early Childhood.
ResultsThere was a significant negative correlation between the father's morning smoking (FTND) and the child's language development quotient; r=-0.41, p=0.005, r2=0.15. The children of mothers without nicotine dependence had a higher mean language development quotient than children of mothers with nicotine dependence; F(1, 107)=5.51, p=0.021, ηp2=0.05.
ConclusionParental smoking appears to have a detrimental effect on child development.
Analisar a relação entre o consumo de tabaco parental e a prevalência de distúrbios no desenvolvimento psicomotor em crianças entre os seis e os vinte e dois meses de idade.
MétodoCento e nove mães, pais e seus bebês participaram no estudo. As circunstâncias sociodemográficas e clínicas foram avaliadas com recurso a questionários. O consumo de tabaco foi avaliado utilizando o Teste de Fagerström para a Dependência Tabágica (Heatherton, Kozlowski, Frecker, & Fagerström, 1991).O desenvolvimento infantil foi avaliado utilizando a Escala do Desenvolvimento Psicomotor da Primeira Infância (Brunet & Lézine, 1951).
ResultadosHá uma correlação negativa significativa entre o fumo matinal (FTND) do pai e o quociente de desenvolvimento de linguagem da criança, r=-0,41, p=0,005, r2=0,15. As crianças de mães sem dependência tabágica têm em média um quociente de desenvolvimento de linguagem superior às crianças de mães com dependência tabágica, F(1,107)=5,51, p=0,021, ηp2=0,05.
ConclusãoO consumo de tabaco parental parece ter um efeito prejudicial para o desenvolvimento da criança.
Development in the first years of life is essential. Several environmental factors, such as the parental consumption of substances, can increase the likelihood of developmental difficulties in childhood,1 especially in the emotional, educational,2 social, behavioral, and psychological levels.3 Tobacco consumption, specifically–which has high prevalence in Portugal (22%)4–is an important public health problem, and has been associated with difficulties in self-regulation; increased excitability and activation in the neonatal period;5 lower birth weight;6,7 learning difficulties;6 lower volume of the frontal and cerebellar lobes–responsible for emotional functioning, impulse control, and attention;8 smaller head circumference;9 cognitive and language neurodevelopmental disorders;10,11 and childhood emotional and behavioral disorders.6 This association can be explained by the fact that during childhood, the brain continues to develop and is particularly sensitive to environmental pollutants,6 or by brain disorders resulting from exposure to nicotine during pregnancy.8
As environmental risk factors appear to be related to children's developmental disorders, especially motor, language, social, cognitive, behavioral, and psychological disorders, the study of psychomotor development associated to these factors is the aim of this study. Although there is a great deal of literature related to child development, the association between tobacco use by both parents and psychomotor development is still largely unknown. Therefore, this study differs from previous studies due to the fact that (1) it analyzes several aspects of child development - posture, language, visual-motor coordination, and social–rather than being limited to overall development; (2) most studies focus on the effects of this consumption on children's health, and/or during pregnancy and not after it; (3) both parents were considered.
MethodsParticipantsParticipants were recruited from four day care centers located in the city of Funchal-Madeira, Portugal, after authorization by the Direção Regional de Educação (Regional Education Board). Most of the participants were of Portuguese origin (94.3%) and white (98.0%).
Participation in the study was proposed to 124 mothers and 124 fathers; 87.9% agreed to participate, 9.3% refused to participate due to lack of free time, and 2.8% were not interested in participating. Thus, the sample consisted of 109 mothers, 109 fathers, and 109 babies. The sample inclusion criterion was: (1) to be the mother/father of a child aged between 6 and 22 months; the exclusion criteria were: (1) illiteracy and (2) the existence of diseases in the babies. The study was performed during 2011 and the data collection phase lasted three months.
ToolsSociodemographic and clinical dataA questionnaire was used to collect social and demographic data (age, gender, marital status, years of education, professional status, physical and psychological diseases, medical or psychological treatment, number of pregnancies, number of miscarriages, number of children, age of children, and children's physical and psychological diseases), as well as clinical information on the pregnancy and newborn (pregnancy planning, prenatal care, risk pregnancy, gestational age, type of delivery, type of anesthesia, Apgar score, weight and height, head circumference, reanimation, health problems at birth, current sleep pattern–monophasic (long periods of continuous sleep) vs. biphasic (alternation between periods of sleep and wakefulness), and type of feeding.
Nicotine dependenceFagerström Test for Nicotine Dependence (FTND)12,13This test was developed to compensate for the psychometric limitations of the Fagerström Tolerance Questionnaire,12 and aims to measure the nicotine dependence of an individual.13 It consists of six items related to smoking habits and behaviors, rated on a Likert scale ranging from 0-3 points. Higher results indicate a greater smoking dependence,14 in which a score of 0-3 indicates absence of nicotine dependence, 4-6 indicates moderate nicotine dependence, and ≥ 7 indicates severe nicotine dependence. The Portuguese version has acceptable psychometric properties, with Cronbach's α=0.66.13 Test-retest reliability was ensured by correlation values of the original scale of 0.99. Factor analysis showed the existence of two factors: (1) cigarette consumption–daily consumption patterns–and (2) morning smoking - degree of urgency to restore the level of nicotine after the nighttime abstinence.13 In the present study, this tool had an acceptable internal consistency, with Cronbach's α=0.73.
Psychomotor developmentScale of Psychomotor Development in Early Childhood15This scale allows the evaluation of the child's developmental level (1-30 months) in each of the following areas: postural–child's movements such as rolling over, sitting, and walking; visual-motor coordination–manipulation of objects, visual-manual coordination, and solving problems; language–expression and understanding; and social–social and personal relations, especially adaptation to social situations, games, and relations with others.15
It consists of 150 items, which are distributed in levels (1-30). This scale allows the calculation of the Developmental Quotient (DQ=DA×100/CA), in which DA represents the developmental age and CA the chronological age. A DQ ≤ 75 indicates lower development, whereas DQ of 76 to 100 indicates normal development, and>100 demonstrates higher development.15
The scale validity criterion is 0.68, with a test-retest correlation coefficient of 0.85.15
ProceduresAll evaluation procedures were performed and approved by the ethics committee of the institution. Mothers and fathers received an explanation on the purpose and procedures of the study, and after the informed consent was signed, they were asked to complete a socio-demographic/clinical questionnaire and the FTND12,13 (approximate duration of 20-25minutes).
The Scale of Psychomotor Development in Early Childhood15 was used to assess the infant's psychomotor development. This assessment was always performed by the same investigator, appropriately trained and familiarized with the scale application procedures. Each baby was observed individually and only at a specific time (approximately 30 to 40minutes), while the daycare professional was present, but away from the baby's visual range. The questions pertaining to each subtest were answered by the day care professional at the end of each observation.
Statistical proceduresPearson's Correlation test was used to assess whether there was a correlation between the degree of nicotine dependence (FTND) of the mother and the father and the child's postural, visual-motor, language, social, and overall development quotient.16
The significance of nicotine dependence factor (FTND ≥ 7) of the mother and father on the composite of the variables child's postural, visual-motor, language, and social development quotient was evaluated by several Multivariate Analysis of Variance (MANOVAs).17 The significance of the difference between the child's overall development quotient according to nicotine dependence of the mother and father (FTND ≥ 7) was evaluated by using the t-test for independent samples.16
The chi-squared test was used to assess whether there was an association between child's overall developmental quotient (lower, normal, upper) and the nicotine dependence (FTND ≥ 7) of the mother and father.16
Effect sizes of each of the analyses performed and interpreted based on the classification proposed by Maroco,17 Kinnear and Gray,18 and Cohen.19
ResultsMost pregnancies were planned (65.1%) and desired (97.2%). Most mothers had prenatal care (98.2%) and a normal pregnancy (79.8%), lasting 37 weeks or more (94.7%, M=39.34, SD=1.70). Most babies were born by vaginal delivery (62.3%), and 37.7% were born by cesarean section, with most mothers receiving epidural anesthesia (73.4%). Apgar score values ranged from 3-10 (M=8.96, SD=1.06) at the first minute of life (97.2% ≥ 7) and between 7-10 (M=9.74, SD=0.57) at the fifth minute. At birth, weight varied between 1.990kg and 4.530kg (M=3.31, SD=0.45), and most had a birth weight ≥ 2.500kg (99.1%), whereas height ranged from 36.00-59.05cm (M=48.85, SD=3.06), and head circumference ranged between 30-37cm (M=34.64, SD=1.22). Most babies did not require reanimation at birth (97.2%) and had no health problems (95.4%). Most had a pattern of biphasic sleep (59.6%). Most mothers breastfed (81.7%) for a period of time ranging from one month to 22 months (M=7.29, SD=5.29).
Maternal age ranged between 19 and 45 years (M=33.17, SD=5.88) and paternal age ranged between 20 and 50 years (M=36.29, SD=6.03). The sociodemographic characteristics are shown in Table 1.
Sociodemographic data.
| Mother (n=109) | Father (n=109) | |
|---|---|---|
| % | % | |
| Age | ||
| < 36 | 58.7 | 45.0 |
| ≥ 36 | 41.3 | 55.0 |
| Years of schooling | ||
| < 9 | 20.2 | 31.2 |
| ≥ 9 | 79.8 | 68.8 |
| Marital status | ||
| Married | 82.6 | 82.6 |
| Single | 11.9 | 11.9 |
| Separated/Divorced | 5.5 | 5.5 |
| Professional status | ||
| Student | 1.8 | - |
| Employed | 74.3 | 82.6 |
| Unemployed | 19.3 | 17.4 |
| Homemaker | 4.6 | - |
| Physical diseases | ||
| Yes | 1.8 | 7.3 |
| No | 98.2 | 92.7 |
| Psychological diseases | ||
| Yes | 0.9 | - |
| No | 99.1 | 100 |
| Medical/psychological treatment | ||
| Yes | 7.3 | 5.5 |
| No | 92.7 | 94.5 |
| Number of children | ||
| 1 | 40.4 | 40.4 |
| 2 | 35.8 | 38.5 |
| 3 or more | 23.8 | 21.1 |
| M (SD) | ||
| Number of previous pregnancies | 2.23 (1.27) | |
| Number of previous miscarriages | 0.27 (0.63) | |
Babies were aged between 6 and 22 months, with most being aged ≥ 12 months (M=14.50, SD=4.62). Approximately half were males (50.5%; Table 1).
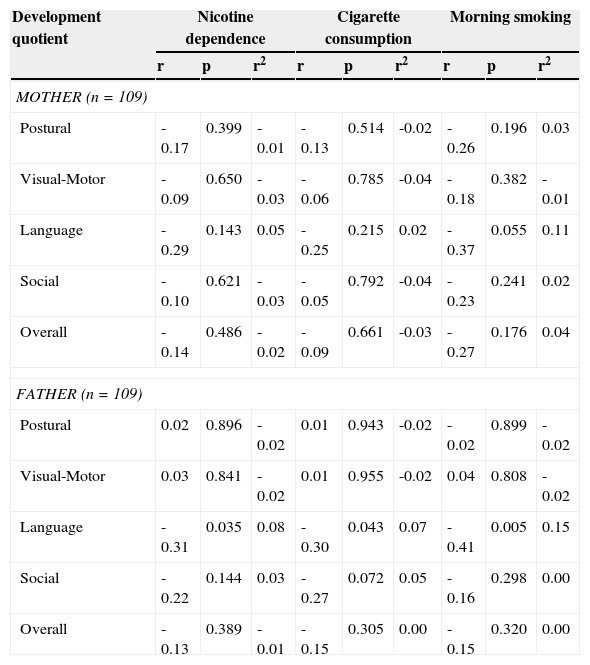
Association between Maternal and Paternal Nicotine Dependence (FTND) and Child Development QuotientThere was no statistically significant correlation between the mother's nicotine dependence and cigarette smoking (FTND) and the postural, visual-motor, language, social, and overall child development quotient, with small effect size (r2 ≤ 0.1). There was no statistically significant correlation between the mother's morning smoking (FTND) and the postural, visual-motor, language, social, and overall child development quotient, with small effect size (r2 ≤ 0.1). However, there was a negative and marginally significant correlation between the mother's morning smoking (FTND) and the child's language development quotient, with medium-sized effect size (r2=0.11) (Table 2). Thus, a higher degree of maternal morning smoking was associated with a lower language development quotient.
Association between nicotine dependence, cigarette consumption, morning tobacco consumption, and child development quotient.
| Development quotient | Nicotine dependence | Cigarette consumption | Morning smoking | ||||||
|---|---|---|---|---|---|---|---|---|---|
| r | p | r2 | r | p | r2 | r | p | r2 | |
| MOTHER (n=109) | |||||||||
| Postural | -0.17 | 0.399 | -0.01 | -0.13 | 0.514 | -0.02 | -0.26 | 0.196 | 0.03 |
| Visual-Motor | -0.09 | 0.650 | -0.03 | -0.06 | 0.785 | -0.04 | -0.18 | 0.382 | -0.01 |
| Language | -0.29 | 0.143 | 0.05 | -0.25 | 0.215 | 0.02 | -0.37 | 0.055 | 0.11 |
| Social | -0.10 | 0.621 | -0.03 | -0.05 | 0.792 | -0.04 | -0.23 | 0.241 | 0.02 |
| Overall | -0.14 | 0.486 | -0.02 | -0.09 | 0.661 | -0.03 | -0.27 | 0.176 | 0.04 |
| FATHER (n=109) | |||||||||
| Postural | 0.02 | 0.896 | -0.02 | 0.01 | 0.943 | -0.02 | -0.02 | 0.899 | -0.02 |
| Visual-Motor | 0.03 | 0.841 | -0.02 | 0.01 | 0.955 | -0.02 | 0.04 | 0.808 | -0.02 |
| Language | -0.31 | 0.035 | 0.08 | -0.30 | 0.043 | 0.07 | -0.41 | 0.005 | 0.15 |
| Social | -0.22 | 0.144 | 0.03 | -0.27 | 0.072 | 0.05 | -0.16 | 0.298 | 0.00 |
| Overall | -0.13 | 0.389 | -0.01 | -0.15 | 0.305 | 0.00 | -0.15 | 0.320 | 0.00 |
r2, size effect coefficient determinant.
Pearson's correlation test.
There was no statistically significant correlation between nicotine dependence, the morning smoking and cigarette smoking (FTND) of the father, and the postural, visual-motor, social, and overall child development quotient, with small effect size (r2 ≤ 0.1). However, there was a negative and statistically significant correlation between paternal nicotine dependence, smoking and morning smoking (FTND), and the child's language development quotient, with small effect size (r2 ≤ 0.1), for nicotine dependence and medium-sized (r2=0.15) for morning smoking (Table 2). Thus, higher paternal nicotine dependence, cigarette smoking and morning smoking were associated with a lower language development quotient.
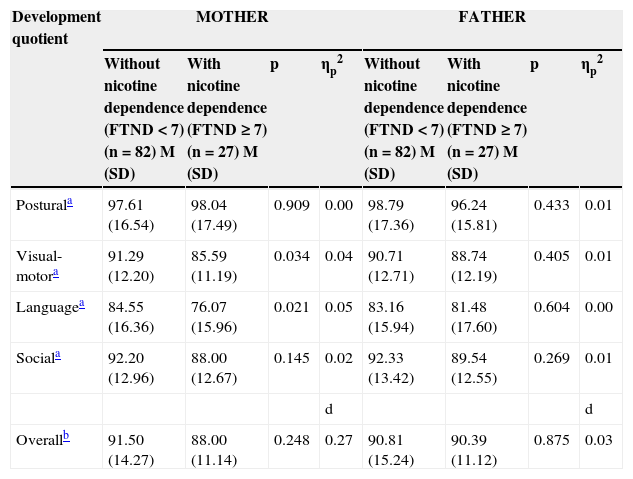
Impact of Maternal and Paternal Nicotine Dependence (FTND) on the Child's Development QuotientThe maternal nicotine dependence factor (FTND<7 vs. FTNP ≥ 7) had a statistically significant effect on the multivariate composite of child development quotient, λ=0.91, F(4, 104)=2.59, p=0.041, ηp2=0.09, with the eta value suggesting a medium-sized effect. Univariate analysis showed that children of mothers without nicotine dependence had a higher mean visual-motor development, F(1, 107)=4.61, p=0.034 and language quotient F(1, 107)=5.51, p=0.021, than children of mothers with nicotine dependence, with the eta value suggesting small effect size (Table 3). There were no significant differences between children of mothers with nicotine dependence vs. children of mothers with no nicotine dependence in terms of overall development quotient, t (107)=1.16, p=0.248; however, Cohen's d-value suggests a medium-sized effect (Table 3). The children of mothers with no nicotine dependence had a higher mean overall development quotient than children of mothers with nicotine dependence.
Parental nicotine dependence and child development quotient.
| Development quotient | MOTHER | FATHER | ||||||
|---|---|---|---|---|---|---|---|---|
| Without nicotine dependence (FTND<7) (n=82) M (SD) | With nicotine dependence (FTND ≥ 7) (n=27) M (SD) | p | ηp2 | Without nicotine dependence (FTND<7) (n=82) M (SD) | With nicotine dependence (FTND ≥ 7) (n=27) M (SD) | p | ηp2 | |
| Posturala | 97.61 (16.54) | 98.04 (17.49) | 0.909 | 0.00 | 98.79 (17.36) | 96.24 (15.81) | 0.433 | 0.01 |
| Visual-motora | 91.29 (12.20) | 85.59 (11.19) | 0.034 | 0.04 | 90.71 (12.71) | 88.74 (12.19) | 0.405 | 0.01 |
| Languagea | 84.55 (16.36) | 76.07 (15.96) | 0.021 | 0.05 | 83.16 (15.94) | 81.48 (17.60) | 0.604 | 0.00 |
| Sociala | 92.20 (12.96) | 88.00 (12.67) | 0.145 | 0.02 | 92.33 (13.42) | 89.54 (12.55) | 0.269 | 0.01 |
| d | d | |||||||
| Overallb | 91.50 (14.27) | 88.00 (11.14) | 0.248 | 0.27 | 90.81 (15.24) | 90.39 (11.12) | 0.875 | 0.03 |
FTND, Fagerström Test for Nicotine Dependence; M, mean; SD standard deviation; ηp2, eta squared–size effect for MANOVA; d, Cohen's d-value–size effect for t-test.
Paternal nicotine dependence factor (FTND<7 vs. FTNP ≥ 7) did not have a statistically significant effect on the multivariate composite of child development quotient, λ=0.99, F(4, 104)=0.32, p=0.865, ηp2=0.01, with the eta value suggesting a small effect size. There were no significant differences between children of fathers with nicotine dependence vs. children of fathers with no nicotine dependence in terms of overall development quotient, t (107)=0.16, p=0.875, with Cohen's d-value suggesting a small effect size (Table 3).
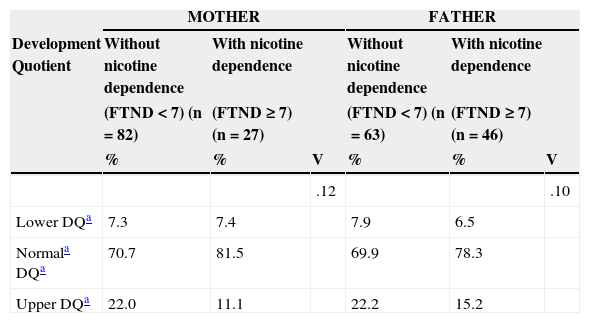
Association between Maternal and Paternal Nicotine Dependence (FTND) and Child Development QuotientThere was no significant association between the overall development quotient of the child and the maternal nicotine dependence (FTND<7 vs. FTNP ≥ 7), χ2(2)=1.56, p=0.459, and the paternal dependence, χ2(2)=1.01, p=0.605, with small effect size (Table 4).
Association between parental nicotine dependence and child development quotient.
| MOTHER | FATHER | |||||
|---|---|---|---|---|---|---|
| Development Quotient | Without nicotine dependence | With nicotine dependence | Without nicotine dependence | With nicotine dependence | ||
| (FTND<7) (n=82) | (FTND ≥ 7) (n=27) | (FTND<7) (n=63) | (FTND ≥ 7) (n=46) | |||
| % | % | V | % | % | V | |
| .12 | .10 | |||||
| Lower DQa | 7.3 | 7.4 | 7.9 | 6.5 | ||
| Normala DQa | 70.7 | 81.5 | 69.9 | 78.3 | ||
| Upper DQa | 22.0 | 11.1 | 22.2 | 15.2 | ||
DQ, development quotient; FTND, Fagerström test for nicotine dependence; V, Cramer's V–size Effect for the chi-squared test.
This study was conducted to analyze the association between parental consumption of tobacco and child development. The results show that there was such an association, with parental daily consumption pattern, nicotine dependence, and the urgency of consumption after a nocturnal abstinence period demonstrating association with language difficulties; there was an especially significant association between maternal nicotine dependence and language, visual-motor, and global development. These results are especially important because they show that the effect of parental nicotine dependence is harmful not only during pregnancy, as other studies had demonstrated10 but also after delivery. In fact, this study does not clarify the differential effect of prenatal or postnatal maternal nicotine dependence on child development. However, when considering the effect of paternal smoking dependence on child development, it can be assumed that this refers to the postnatal period, which draws attention to the vulnerability of the newborn to passive exposure to parental smoking. The mechanisms that explain the effect of tobacco consumption during pregnancy on the fetus have been well studied in recent years, and there is some evidence indicating that the consumption of nicotine and other tobacco components influence gestational duration,20 have toxic effects on fetal brain development during pregnancy,10 and are associated with fetal hypoxia, changes in the serotonin uptake, changes in the dopaminergic systems, and changes in DNA and RNA synthesis in the brain.17 Nicotine appears to target specific neurotransmitter receptors in the fetal brain, causing abnormalities in cell proliferation and differentiation, resulting in cell number deficits and changes in synaptic activity,21 impairing the fetal-placental development and metabolism22 with developmental implications for the newborn.23 As a consequence, there are significant changes in the brain physiology responsible for basic perceptual skills;24 loss of central nervous system cells in the postnatal period; reduction of the frontal lobe and cerebellar volumes responsible for attention, emotion, and impulse control;8 as well as a smaller head circumference in newborns.9 These physiological alterations may be responsible for developmental disorders found in these children, such as self-regulatory difficulties, increased excitability,5 decrease in cognitive functions, and learning and memory deficits.10,11 Overall developmental difficulties observed in this study, with emphasis on language and visual-motor skills, are consistent with previous studies that indicate an association between parental consumption of tobacco and child development disorders,25 especially psychomotor disorders.26 Regarding the effect of tobacco consumption by the parents, the effect is caused by exposure after birth, which indicates the newborn's specific vulnerability to exposure to toxins released by tobacco consumption.27 Because of the implications, parental smoking during the perinatal period is an important public health problem, and therefore intervention programs in perinatal health that target smoking cessation, not only for future mothers but also for future fathers, should be considered a priority to ensure a better quality of life for families.
Despite the limitations of this study–especially because it did not consider a detailed analysis of the behavior associated with tobacco consumption or regarding parental consumption history–it should be considered an important contribution to the study of the effect of parental smoking, as it helps to better understand the effect of consumption not only by mothers, but also by fathers, thus encompassing both parents in order to analyze the individual effect on psychomotor development.
Future studies should assess not only the differential effect of consumption by each parent, but also the differential effect of prenatal and postnatal tobacco consumption on child development.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank the Regional Director of Education for allowing the study to be carried out in Região Autónoma da Madeira (RAM) day care centers. The authors are also very grateful to the heads of educational establishments, teachers, and kindergarten assistants, and the parents who allowed the observation of the psychomotor development of their babies and spent part of their precious time to answer the questionnaire.
This study was developed as part of the project “The role of genotype-environment interaction on the resilience and vulnerability to developmental and mental health problems in the first 18 months of life” (PTDC/PSI-PCL/119152/2010) funded by the Fundação para a Ciência e Tecnologia (Foundation for Science and Technology).
Please cite this article as: Santos NF, Costa RA. Parental tobacco consumption and child development. J Pediatr (Rio J). 2015;91:366–72.