the present study was conducted to investigate the oxidant-antioxidant status in Egyptian children with sickle cell anemia.
Methodsthe serum levels of total antioxidant capacity (TAO), paraoxonase (PON), vitamin E, nitrite, and malondialdehyde (MDA) were measured in 40 steady state children with homozygous sickle cell anemia (24 males and 16 females) and 20 apparently healthy age- and gender-matched controls.
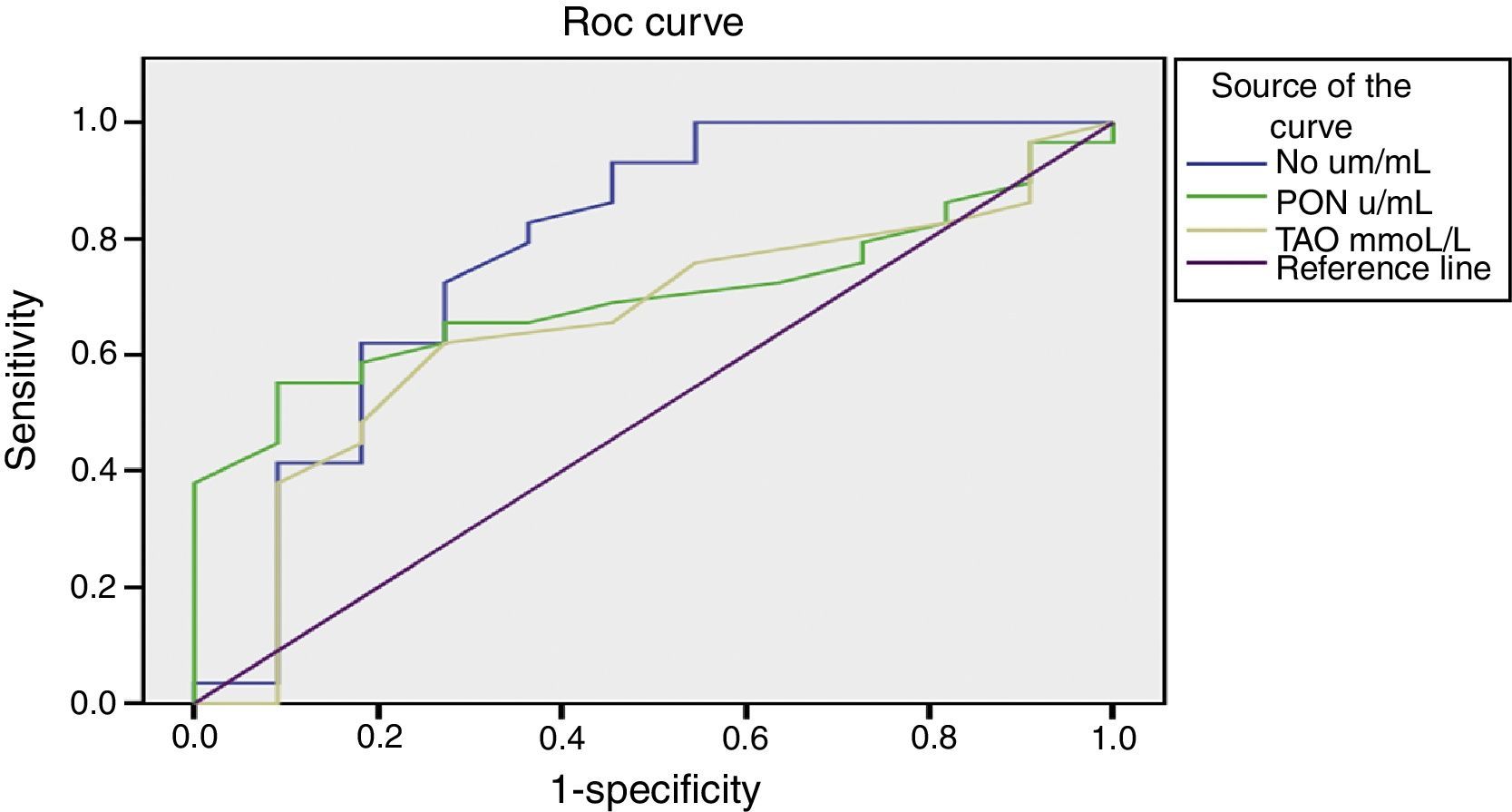
Resultsmean serum TAO, PON, vitamin E, and nitrite levels were significantly lower in the group with sickle cell anemia, whereas mean serum MDA was significantly higher in these children compared to controls. No significant differences in mean levels of TAO, PON, nitrite, vitamin E, and MDA were found in sickle cell anemia patients receiving hydroxyurea when compared with those not receiving hydroxyurea. A significant negative correlation between serum nitrite and the occurrence of vaso-occlusive crises (VOC) was observed (r=−0.3, p=0.04). PON level was found to be positively correlated with patients’ weight and BMI (r=−0.4, p=0.01; r=−0.7, p<0.001, respectively), but not with frequency of VOC. The area under the curve of serum nitrite in predicting occurrence of VOC was 0.782, versus 0.701 for PON, and 0.650 for TAO (p=0.006). Serum MDA was not correlated with nitrite, PON, TAO, or vitamin E levels. No significant correlations were detected between serum nitrite and hemoglobin or antioxidant enzymes.
Conclusionchildren with sickle cell anemia have chronic oxidative stress that may result in increased VOC, and decreased serum nitrite may be associated with increases in VOC frequency. A novel finding in this study is the decrease in PON level in these patients, which is an interesting subject for further research.
o presente estudo foi realizado com o objetivo de investigar o estado oxidante-antioxidante em crianças egípcias com anemia falciforme.
Métodosdosamos os níveis séricos da capacidade antioxidante total (CAT), paraoxonase (PON), vitamina E, nitrito e malondialdeído (MDA) em 40 crianças estáveis com anemia falciforme homozigótica (24 meninos e 16 meninas), e 20 controles pareados por idade/sexo aparentemente saudáveis.
Resultadosos níveis séricos médios da CAT, PON, vitamina E e nitrito foram significativamente menores, ao passo que o nível sérico médio de MDA foi significativamente maior em crianças com anemia falciforme (AF), em comparação aos controles. Não foram encontradas diferenças significativas nos níveis médios de CAT, PON, nitrito, vitamina E e MDA em pacientes com AF em tratamento com hidroxiureia, em comparação aos que receberam hidroxiureia. Encontramos uma correlação negativa significativa entre o nitrito sérico e a ocorrência de crises vaso-oclusivas agudas (CVO) (r=−0,3, p=0,04). Descobrimos que o nível de PON está correlacionado positivamente com o peso e o IMC dos pacientes (r=−0,4; p=0,01; r=−0,7; p<0,001, respectivamente), porém não com a frequência de CVO. A área sob a curva (ASC) do nitrito sérico na previsão da ocorrência de CVO foi 0,782, em comparação a 0,701 para PON e 0,650 para CAT (p=0,006). O MDA não está correlacionado a nitrito, PON, CAT ou vitamina E. Não foram detectadas correlações significativas entre nitrito sérico e hemoglobina ou enzimas antioxidantes.
Conclusãocrianças com AF apresentam estresse oxidativo crônico que pode resultar em aumento das CVO. Em crianças com AF, a redução nos níveis de nitrito sérico pode estar associada a aumentos da frequência de CVO. Um novo achado neste estudo é a redução no nível de PON em pacientes com AF, que é um campo interessante de novas pesquisas.
Sickle cell anemia (SCA) is one of the most common monogenic disorders in the world, predominantly observed in Africa and Southeast Asia. It is a multi-system disease, associated with episodes of acute illness and progressive organ damage.1 SCA results from a p mutation in the genetic code such that glutamic acid is replaced by valine in the globin chain of hemoglobin. This substitution transforms normal adult hemoglobin (HbA) into sickle hemoglobin (HbS). When deoxygenated, HbS polymerizes, and when a critical amount of HbS polymer accumulates within a sickle erythrocyte, cellular injury occurs. A sufficient number of damaged erythrocytes cause the phenotype of sickle cell disease (SCD), characterized by hemolytic anemia and vasoocclusion.2
SCD is emerging as an important model of oxidative stress. Since red blood cells (RBCs) carry oxygen to the body tissues, they are already rich in oxidative fuel. Their distinctive structural features make them susceptible to an oxidant assault. Chronic oxidative stress resulting from an imbalance between the production of reactive oxidant species (ROS) and antioxidant enzymes constitutes a critical factor in endothelial dysfunction, inflammation, and multiple organ damage in SCD. In addition, the disease is characterized by damage to the cell membrane due to increased lipid peroxidation products, such as malondialdehyde (MDA) and the increased consumption of nitric oxide (NO).3,4
Increased ROS production is caused by intrinsic mechanisms of disease, such as increased activity of several oxidases (NADPH oxidase and endothelial xanthine oxidase),5 auto-oxidation of HbS, release of heme iron, increased asymmetric dimethylarginine,6 uncoupling of NO synthase activity, and decreased NO levels.7 This enhanced production of free radicals in SCA and subsequent decreased NO bioavailability inactivate NO-mediated vascular relaxation.8 Impaired vascular relaxation and increased endothelial adherence contribute to the vaso-occlusive phenomena.9
Several reports indicate that SCA patients have lower levels of antioxidants such as NO, total antioxidant capacity (TAO), and vitamin E as compared to normal healthy controls.10–13 Moreover, one study showed a significantly enhanced lipid peroxidation in SCA patients when compared to controls.14 However, limited studies have evaluated the role of oxidants and antioxidant status in children with SCA. To the authors’ knowledge, none have been conducted in patients with SCD in Egypt. The present study aimed to evaluate the oxidant-antioxidant status in Egyptian children with SCA in a steady state through the estimation of serum levels of the lipid peroxidation product MDA, nitrite, PON, vitamin E, and TAO.
Material and methodsThis was a prospective case-control study conducted at the New Children's Hospital of Cairo University, Egypt, and at the Child Health and Medical Biochemistry Departments of the National Research Center, Cairo, Egypt. Forty children with established diagnosis of homozygous (HbSS) SCA (24 males and 16 females aged 10.6±4.5 years) and 20 healthy subjects (age- and gender-matched controls, 12 males and 8 females aged 10.0±2.8 years [p>0.05]) were enrolled in the study after their legal guardians signed the informed consent. All recruited patients were in a steady state attending routine follow-up during the study period (from December 1, 2011 to June 30, 2012). Patients aged > 18 years, those with acute febrile illness within 72hours, or acute vaso-occlusive crises (VOC) within three months prior to enrollment, serious concurrent illness, and those assigned to a regular blood transfusion program were excluded. None of recruited subjects received supplemental antioxidants or vitamins e.g. vitamin E. The study protocol was approved by the Ethics Committee of the Cairo University and by the Ethics Committee of the National Research Center, Cairo, Egypt, according to the Institutional Committee for the Protection of Human Subjects, and adopted by the 18th World Medical Assembly, Helsinki, Finland.
Detailed history-taking and thorough clinical examinations were performed. At enrollment, the number of severe painful episodes in the preceding 12 months was recorded (frequency of VOC per year), with a working definition of a VOC as pain in the extremities, back, abdomen, chest, or head that led to an unscheduled clinic or emergency room visit and required hospitalization, and that could only be explained by SCD, with exclusion of hand-foot syndrome, chest syndrome, osteomyelitis, and any episode of pain that was treated entirely at home.15
Thirty-one patients were on hydroxyurea (HU) therapy with a mean dose of 19.8±3.4 (range 15–30mg/kg/day, given orally once a day). The mean duration of HU was 2.12±1.49 years. Dose escalation was guided by clinical and hematological response with no attempt to reach the maximum tolerated dose (MTD);16 3 subjects were at MTD at time of enrollment.
Blood samples for determination of MDA, nitrite, PON, TAO, and vitamin E were collected and processed as follows: 5mL of blood were collected into plain tubes and allowed to clot for 30min at 25°C; it was then centrifuged at 3,000rpm for 15min at 4°C, and the serum was separated into clean, properly labeled tubes for analysis.
Determination of lipid peroxidation: Lipid peroxidation was assayed by measuring the level of MDA. It was determined by measuring thiobarbituric reactive species using the method of Ruiz-Larrea et al.,17 in which the thiobarbituric acid-reactive substances react with thiobarbituric acid to produce a red colored complex with peak absorbance at 532nm.
Determination of serum nitrite: Serum nitrite (NO2−), as an acceptable surrogate marker to serum NO, was measured using Griess reagent, following the Moshage et al. method;18 nitrite, a stable end-product of NO radical, is commonly used as indicator for the production of NO.19
Determination of PON activity: Arylesterase activity of PON was measured spectrophotometrically in supernatants using phenylacetate as a substrate.20
Measurement of serum TAO levels: Serum TAO levels were determined using an automated measurement method, which is based on the bleaching of the characteristic color of a more stable 2, 2-azino-bis (3-ethylbenz-thiazoline-6-sulfonic acid, [ABTS]) radical cation by antioxidants (Beckman Coulter - Fullerton, CA, USA).21 The ABTS radical cation is decolorized by antioxidants according to their concentrations and antioxidant capacities. The results are expressed in mmol Trolox equivalents/L.
Measurement of vitamin E: Vitamin E as tocopherol was measured by HPLC.22 Freshly-obtained erythrocytes were stored in 2% pyrogallol in ethanol at -70°C. All samples were analyzed within one month of storage using a reverse-phase C-18 column (Waters - Milford, MA, USA), a 95% methanol solvent system, and a UV/VIS detector set at 292nm.22
Statistical analysisPatients’ data were analyzed using the Statistical Package for Social Sciences (SPSS), version 17.0 for Windows. Quantitative variables were expressed by mean±standard deviation (SD), and compared using Student's t-test for unpaired samples and Mann–Whitney's test. Spearman's rank-order test was used for correlating quantitative variables. Qualitative variables were expressed as numbers (frequency) and percentages, and compared between groups using the chi-squared test. Logistic regression analysis was performed, and accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated. A receiver operating characteristic (ROC) curve was made, and area the under the curve (AUC) was calculated. The optimal cut-off was determined for the variables required. p-value was considered to be significant if < 0.05.
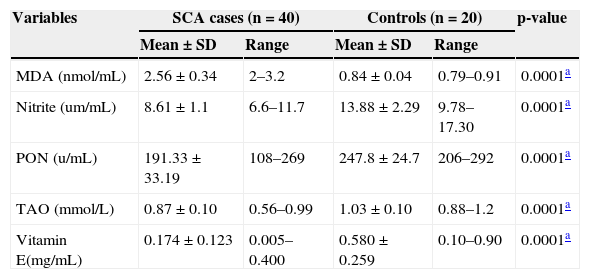
ResultsTable 1 illustrates a comparison of the tested variables between SCA patients and the control group. Mean values of nitrite, PON, TAO, and vitamin E were significantly lower, while the MDA level was significantly higher in SCA patients than in control group. This statistically significant difference in all measured variables was also observed when comparison was made between patients and gender-matched controls. Gender did not appear to affect the oxidant-antioxidant status of SCA patients; there were no significant differences in the mean levels of nitrite, PON, TAO, and MDA in SCD males and females (Table 1).
Comparison of serum malondialdehyde, nitrite, paraoxonase, total antioxidant capacity, and vitamin E in sickle cell anemia patients and controls.
| Variables | SCA cases (n = 40) | Controls (n = 20) | p-value | ||
|---|---|---|---|---|---|
| Mean±SD | Range | Mean±SD | Range | ||
| MDA (nmol/mL) | 2.56±0.34 | 2–3.2 | 0.84±0.04 | 0.79–0.91 | 0.0001a |
| Nitrite (um/mL) | 8.61±1.1 | 6.6–11.7 | 13.88±2.29 | 9.78–17.30 | 0.0001a |
| PON (u/mL) | 191.33±33.19 | 108–269 | 247.8±24.7 | 206–292 | 0.0001a |
| TAO (mmol/L) | 0.87±0.10 | 0.56–0.99 | 1.03±0.10 | 0.88–1.2 | 0.0001a |
| Vitamin E(mg/mL) | 0.174±0.123 | 0.005–0.400 | 0.580±0.259 | 0.10–0.90 | 0.0001a |
| Variables | Males | p-value | Females | p-value | ||
|---|---|---|---|---|---|---|
| Cases (n = 24) | Controls (n = 12) | Cases (n = 16) | Control (n = 8) | |||
| Mean±SD | Mean±SD | Mean±SD | Mean±SD | |||
| MDA (nmol/mL) | 2.62±0.34 | 0.85±0.05 | 0.0001a | 2.46±0.34 | 0.83±0.02 | 0.0001a |
| Nitrite (um/mL) | 8.67±1.07 | 14.48±1.3 | 0.0001a | 8.51±1.17 | 12.98±3.1 | 0.005a |
| PON (u/mL) | 188.08±36.2 | 236.83±18.8 | 0.0001a | 196.1±28.4 | 264.2±24.1 | 0.0001a |
| TAO (mmol/L) | 0.87±0.08 | 1.04±0.11 | 0.0001a | 0.87±0.12 | 1.02±0.09 | 0.005a |
| Vitamin E (mg/mL) | 0.185±0.131 | 0.417±0.175 | 0.001a | 0.158±0.112 | 0.825±0.139 | 0.0001a |
| Variables | SCA patients (n=40) | p-value | |||
|---|---|---|---|---|---|
| Male Cases (n=24) | Female cases (n=16) | ||||
| Mean±SD | Range | Mean±SD | Range | ||
| MDA (nmol/mL) | 2.6±0.34 | 2.1–3.2 | 2.4±0.34 | 2–3.1 | 0.17 |
| Nitrite (um/mL) | 8.67±1.07 | 6.9–11.7 | 8.5±1.17 | 6.6–9.9 | 0.67 |
| PON (u/mL) | 188.08±36.2 | 108–269 | 169.19±28.4 | 129–243 | 0.46 |
| TAO (mmol/L) | 0.87±0.08 | 0.71–0.9 | 0.87±0.12 | 0.56–0.98 | 0.91 |
| Vitamin E (mg/mL) | 0.185±0.131 | 0.005–0.400 | 0.158±0.112 | 0.01–0.40 | 0.580 |
MDA, malondialdehyde; PON, paraoxonase; SCA, sickle cell anemia; SD, standard deviation; TAO, total antioxidant capacity,
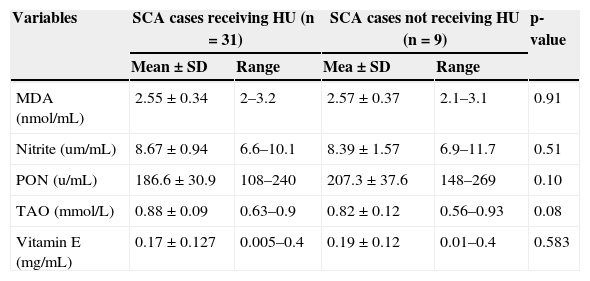
No significant differences in the mean levels of nitrite, PON, TAO, and MDA were observed between SCD patients on HU therapy and those not receiving HU (Table 2). Compared to the mean vitamin E level in studied controls, the prevalence of vitamin E deficiency among SCA patients was 100%.
Comparison of serum malondialdehyde, nitrite, paraoxonase, total antioxidant capacity, and vitamin E in sickle cell anemia patients receiving HU and those not receiving HU.
| Variables | SCA cases receiving HU (n=31) | SCA cases not receiving HU (n=9) | p-value | ||
|---|---|---|---|---|---|
| Mean±SD | Range | Mea±SD | Range | ||
| MDA (nmol/mL) | 2.55±0.34 | 2–3.2 | 2.57±0.37 | 2.1–3.1 | 0.91 |
| Nitrite (um/mL) | 8.67±0.94 | 6.6–10.1 | 8.39±1.57 | 6.9–11.7 | 0.51 |
| PON (u/mL) | 186.6±30.9 | 108–240 | 207.3±37.6 | 148–269 | 0.10 |
| TAO (mmol/L) | 0.88±0.09 | 0.63–0.9 | 0.82±0.12 | 0.56–0.93 | 0.08 |
| Vitamin E (mg/mL) | 0.17±0.127 | 0.005–0.4 | 0.19±0.12 | 0.01–0.4 | 0.583 |
HU, hydroxyurea; MDA, malondialdehyde; PON, paraoxonase; SCA, sickle cell anemia; SD, standard deviation; TAO, total antioxidant capacity.
No significant correlations were detected between the frequency of VOC and levels of MDA, vitamin E, PON, or TAO (p>0.05). However, serum nitrite correlated negatively with the frequency of VOC (r=-0.3, p=0.04), but did not correlate with the levels of hemoglobin, MDA, PON, TAO, or vitamin E (r=0.19, -0.3, 0.08, 0.03, and 0.05, respectively, p>0.05). MDA did not correlate with any of the tested variables, including PON, TAO, and vitamin E (p>0.05).
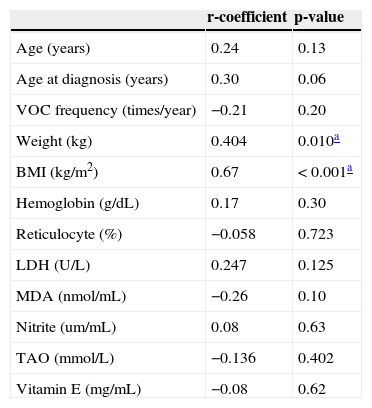
PON level was found to correlate positively with patients’ weight and BMI (r=-0.4, p=0.01; r=-0.7, p<0.001, respectively). No significant correlations were observed between serum PON and frequency of VOC; laboratory indices of hemolysis including hemoglobin, reticulocyte count, or lactate dehydrogenase; or with levels of MDA, nitrite, vitamin E, or TAO (p>0.05) (Table 3).
Correlations of PON level and patients’ clinical and laboratory variables.
| r-coefficient | p-value | |
|---|---|---|
| Age (years) | 0.24 | 0.13 |
| Age at diagnosis (years) | 0.30 | 0.06 |
| VOC frequency (times/year) | −0.21 | 0.20 |
| Weight (kg) | 0.404 | 0.010a |
| BMI (kg/m2) | 0.67 | <0.001a |
| Hemoglobin (g/dL) | 0.17 | 0.30 |
| Reticulocyte (%) | −0.058 | 0.723 |
| LDH (U/L) | 0.247 | 0.125 |
| MDA (nmol/mL) | −0.26 | 0.10 |
| Nitrite (um/mL) | 0.08 | 0.63 |
| TAO (mmol/L) | −0.136 | 0.402 |
| Vitamin E (mg/mL) | −0.08 | 0.62 |
BMI, body mass index; LDH, lactate dehydrogenase; MDA, malondialdehyde; PON, paraoxonase; TAO, total antioxidant capacity; VOC, vaso-occlusive crises.
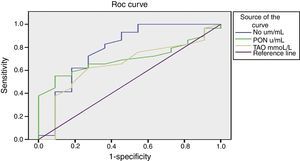
Figure 1 presents the sensitivity and specificity of nitrite, PON, and TAO in predicting the occurrence of VOC at different cut-off values. As there is no gold standard to compare with, nitrite, PON, and TAO were compared by ROC curve. The AUC of nitrite (0.782) was significantly higher when compared to that of PON (0.701) and TAO (0.650) (p=0.006), indicating that the overall predictability of nitrite is significantly higher than that of to PON or TAO. However, when fixing the sensitivity or specificity of nitrite, it was found that either its sensitivity or specificity became unsatisfactory; this makes its adoption as a good predictor of the occurrence of VOC unlikely.
DiscussionThe present data showed that there were decreases in serum nitrite, PON, TAO, and vitamin E levels in SCA children, as well as an increase in oxidative stress represented by MDA level. Gender and HU therapy did not appear to affect the oxidant-antioxidant status of SCA children. Serum nitrite was the only marker that correlated negatively with the frequency of VOC. The overall predictability of nitrite in VOC occurrence was significantly higher when compared to PON and TAO, but an increase in its sensitivity was invariably accompanied by a concomitant decrease in specificity.
Several studies have observed decreases in the activity levels of nitrite,10–12 PON, TAO,11,12 and vitamin E13 in SCD adult patients in steady state. Additionally, increased values of MDA as a lipid peroxidation product were reported as an index of the generation of ROS and oxidative stress in several disorders, including SCD.23 To the authors’ knowledge, this study was the first to investigate the oxidant-antioxidant status in Egyptian SCA children and to report increased oxidative stress in children with SCD.
One of the main findings of the present study is that the nitrite level was comparable in males and females with SCA. This contradicts previous studies reporting reductions of basal and stimulated nitrite production and responses to exogenous NO in male patients with SCA when compared to females.24 Nevertheless, these differences disappear when only children are considered. Gender differences in NO bioavailability are probably caused in part by the protective effects of ovarian estrogen on NO synthase expression and activity in pubertal females.24
HU is described as an inducer of fetal hemoglobin expression, which reduces HbS polymerization in SCA patients, reducing mortality and VOC.25In vitro and animal studies have demonstrated that HU may play an additional role as a NO donor.26 The activation of fetal hemoglobin expression by HU may occur through this NO pathway.27 Clinical studies suggested an antioxidant effect of HU on SCA by measuring glutathione levels and other antioxidants in SCA patients treated with HU and those who were not given this medication.28 However, the small number of patients who were not receiving HU in the present study makes it difficult to draw any conclusions regarding the effects of HU (whether direct or indirect) on the measured oxidant and antioxidant markers.
The present study observed a weak association between the decreases in serum nitrite level and increased frequency of VOC. However, no correlations of serum nitrite level with laboratory indices, such as total hemoglobin level, other antioxidants, or MDA were observed.
SCD is an extremely heterogeneous disease for many reasons, and the occurrence of VOC appears to be multifactorial. Even if low nitrite is one of these factors, it is impossible to conclude that serum nitrite level may provide specific prognostic or clinical information beyond that given by the simple, conventional measurement of hemoglobin concentration. However, it may be reserved as a simple biomarker of oxidative stress in children with SCA in steady state to help in selection and follow-up of those in need for antioxidant supplements.
In the present study, TAO was measured, as its value is more informative than the knowledge of individual antioxidant.12 Its level reflects the collective contribution to the reducing property of non-protein individual antioxidant or electron donating components. However, no correlation was found between TAO and increased frequency of VOC.
An important new observation in the present study was the decrease in PON level in SCA children. PON level was found to increase with body weight and BMI, but it did not correlate with the frequency of VOC or indicators of hemolysis. However, and to the authors’ knowledge, there are no reports on PON level or its impact on the phenotype in SCD patients. PON is a calcium-dependent serum esterase that is synthesized by the liver and is released into the circulation, where it associates mainly with high-density lipoproteins and protects low-density lipoproteins and cellular membranes against lipid peroxidation.29 It is largely believed to have a role in protection against oxidative stress.30 Patients with coronary heart disease showed increased lipid peroxidation and decreased PON activity.31 This may suggest that patients with SCA who showed decreased PON and increased lipid peroxidation (MDA) may be at risk of other forms of vasculopathy, including coronary heart disease, especially with growing up indicating future studies.
The present study demonstrated that oxidative stress is evident in young children with SCA. Thus, it highlights the need for clinical trials examining the value of supplementation with vitamin E or other agents that increase the total antioxidant capacity among these children, and whether this may improve their clinical course.
Children with SCA have chronic oxidative stress that may result in increased VOC. In children with SCA, decreased serum nitrite may be associated with increases in VOC frequency. A novel finding in this study was the decrease in PON levels in SCA patients, which is an interesting field for further research.
FundingEquipment from the Pediatric Hematology Clinic, Cairo University and from the Biochemistry department at the National Research Center were used. This work received no financial assistance from any funding agency in the public, commercial, or non-profit sectors.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank all patients who participated in this study. They would like to express their appreciation to their colleagues and nurses at the Pediatric Hematology and BMT Unit who facilitated this work.
Please cite this article as: El-Ghamrawy MK, Hanna WM, Abdel-Salam A, El-Sonbaty MM, Youness ER, Adel A. Oxidant-antioxidant status in Egyptian children with sickle cell anemia: a single center based study. J Pediatr (Rio J). 2014;90:286–92.