to evaluate neonatal sepsis as a risk factor for abnormal neuromotor and cognitive development in very low birth weight preterm infants at 12 months of corrected age.
Methodsthis was a prospective cohort study that followed the neuromotor and cognitive development of 194 very low birth weight preterm infants discharged from a public neonatal intensive care unit. The Bayley Scale of Infant Development (second edition) at 12 months of corrected age was used. The outcomes were the results of the clinical/neurological evaluation and the scores of the psychomotor development index (PDI) and mental development index (MDI) of the Bayley Scale of Infant Development II. The association between neonatal sepsis and neuromotor development and between neonatal sepsis and cognitive development was verified by logistic regression analysis.
Resultsmean birth weight was 1,119g (SD: 247) and mean gestational age was 29 weeks and 6 days (SD: 2). Approximately 44.3%(n=86) of the infants had neonatal sepsis and 40.7% (n=79) had abnormal neuromotor development and/or abnormal psychomotor development index (PDI < 85) at 12 months of corrected age. On the mental scale, 76 (39.1%) children presented abnormal cognitive development (MDI<85). Children with neonatal sepsis were 2.5 times more likely to develop changes in neuromotor development (OR: 2.50; CI: 1.23‐5.10). There was no association between neonatal sepsis and cognitive development impairment.
Conclusionneonatal sepsis was an independent risk factor for neuromotor development impairment at 12 months of corrected age, but not for mental development impairment.
avaliar a sepse neonatal como fator de risco para alterações no desenvolvimento neuromotor e mental de prematuros de muito baixo peso aos 12 meses de idade corrigida.
Métodosestudo de coorte prospectivo que acompanhou o desenvolvimento neuromotor e mental de 194 prematuros de muito baixo peso oriundos de uma UTI neonatal pública no Rio de Janeiro. Utilizou‐se a Escala Bayley de Desenvolvimento Infantil (segunda edição) aos 12 meses de idade corrigida. Os desfechos foram o resultado da avaliação clínica/ neurológica e os resultados da área motora da Escala Bayley e os resultados da área mental (cognitiva) da mesma escala. A associação entre sepse e o desenvolvimento neuromotor e entre sepse e o desenvolvimento mental foi verificada através de regressão logística.
Resultadosa média do peso ao nascer foi 1119g (DP 247) e da idade gestacional 29 semanas e 6 dias (DP 2). Cerca de 44,3% (n=86) das crianças apresentaram sepse neonatal e 40,7% (n=79) apresentaram alteração neuromotora e/ou no índice do desenvolvimento psicomotor (PDI<85) aos 12 meses de idade corrigida. Na escala mental, 76 (39,1%) crianças apresentaram alteração (MDI < 85). As crianças que apresentaram sepse neonatal tiveram 2,5 vezes mais chances de desenvolver alteração do desenvolvimento neuromotor do que as crianças que não apresentaram sepse (OR: 2,50; IC 1,23‐5,10). Porém, não houve associação entre sepse neonatal e alteração cognitiva.
Conclusãoa sepse neonatal foi um fator de risco independente para alteração do desenvolvimento neuromotor, mas não para alteração do desenvolvimento mental.
Sepsis is characterized by systemic manifestations resulting from bacterial invasion and multiplication in the bloodstream, and can lead to high neonatal mortality and morbidity.1 Preterm newborns are at increased risk of developing sepsis. There is evidence that perinatal and neonatal infections are associated with neurodevelopmental impairment in preterm infants.2–6
Some studies indicate sepsis as one of the major risk factors for developmental delay and cerebral palsy, as well as neonatal mortality.7–11 However, there are few studies that assess the age range studied (12 months of corrected age) and, to date, only one Brazilian publication that investigated the association between sepsis and neurodevelopment was retrieved.12 Furthermore, it is necessary to use an appropriate methodology for the control of several confounding factors already established in the literature, such as low gestational age, male gender, bronchopulmonary dysplasia (BPD), and brain injuries that can influence neurodevelopment in this population.
The aim of this study was to evaluate neonatal sepsis as a risk factor for neurodevelopment impairment in preterm infants with very low birth weight at 12 months of corrected age.
MethodsThis prospective cohort study was performed in a tertiary hospital, at a referral unit for high‐risk newborns. Preterm infants (gestational age < 37 weeks) with birth weight less than 1,500g who were born from 2004 to 2010 were included in the cohort. Gestational age was estimated based on the date of the last menstrual period; when that date was uncertain, by early ultrasound and by the New Ballard Score.13 When birth weight was below the 10th percentile for gestational age,14 the infant was classified as small for gestational age. The exclusion criteria were: infants with infection, congenital malformations, genetic syndromes, or those born in other hospitals; neonatal and post‐neonatal deaths were excluded. Children not assessed by the Bayley scale were excluded from the analysis and considered as study loss.
Neonatal sepsis was considered in the presence of a positive blood culture and/or clinical and laboratory signs suggestive of infection.11 Clinical signs included worsening of respiratory distress: tachypnea, sternal and/or subcostal retraction, groaning and cyanosis, apnea, body temperature instability, hyper‐ or hypoglycemia, poor peripheral perfusion, food intolerance, arterial hypotension, and underactive infants.11
Laboratory parameters included: complete blood count with three or more altered parameters according to Rodwell et al.15 and/or C‐reactive protein > 0.5mg/dL; negative or not performed blood culture; no evidence of infection at another site; and established and maintained antimicrobial therapy. Rodwell et al.15 considered the following hematological parameters: leukocytosis (white blood cells [WBC] ≥ 25,000 at birth, or≥30,000 between 12 to 24hours, or >21,000 at over 48hours of life), leukopenia (WBC≤5,000); neutrophilia or neutropenia; increased number of immature neutrophils; increased neutrophilic index; ratio of immature over segmented neutrophils ≥ 0.3; neutrophils with toxic granulation and vacuolization; and thrombocytopenia (<150,000 platelets). These data were collected from medical records.
After being discharged from the neonatal intensive care unit (NICU), the newborns were followed‐up at the outpatient clinic for at‐risk newborns; a clinical and neurological assessment was performed by a pediatrician and a physical therapist monthly until 12 months of age. This consisted of the observation of spontaneous movements and posture, muscle tone in several body segments (cervical, axial, limb, and shoulder girdle), asymmetries and reflexes according to the Amiel‐Tison and Grenier protocol,16 and motor development milestones. Neuromotor impairment was considered when the child showed changes associated with one or more of several body segments at clinical/neurological evaluation, such as: change in muscle tone, abnormal posture, abnormal spontaneous movements, altered neurological examination, and motor developmental delay,17 also considering whether the child reached the age‐appropriate motor milestones.16‐19
The second edition of the Bayley Scale of Infant Development20 was applied by trained psychologists at 12 months of corrected age. The infants were evaluated regarding the mental and psychomotor areas, and the respective development indices were obtained. According to the authors of the scale, the psychomotor development index (PDI) and the mental development index (MDI) are considered normal when the scores are ≥ 85; moderate delay, when the scores are between 70 and 84; significant delay, when the scores are < 70. PDI and MDI were considered to be altered when scores were < 85.
The outcomes of the study were: neuromotor development at 12 months (including clinical/neurological assessment and the motor area results of the Bayley Scale) and mental development. Neuromotor development was considered to be altered when there was a change in the clinical/neurological assessment and/or changes in the PDI.
The neuromotor and mental development at 12 months in children with neonatal sepsis were compared to those without sepsis. The association between the exposure variable (sepsis) and the outcome variables (neuromotor development and mental development) was verified by multivariate analysis (logistic regression). The presence of potential confounding factors was investigated based on the association of covariates with exposure and outcomes.
Demographic data, socioeconomic data, family data, aspects related to the perinatal period, interventions, and neonatal morbidities were considered as covariates. The post‐neonatal information included daycare attendance and hospital admission in the first year of life.
Bronchopulmonary dysplasia (BPD) was defined as oxygen use for 28 days or more.21 Necrotizing enterocolitis (NEC) was considered in cases of need for medical or surgical treatment.22 Patent ductus arteriosus (PDA) was defined as the presence of heart murmur, tachycardia, hyperdynamic precordium, and broad pulse,23 and was confirmed by echocardiography. Serial brain ultrasound examinations were performed in the first two weeks of life and at discharge for brain injury investigation. Data collection was prospective. The information of interest regarding the peri‐ and neonatal periods was collected by one of the researchers in an appropriate file during the infant's hospitalization; post‐neonatal data were collected in the same file at the time of consultations at the follow‐up outpatient clinic.
Statistical analysisData analysis was performed using Epi Info, release 3.5.1 and the Statistical Package for Social Sciences (SPSS), statistical analysis softwares. Statistical tests were used for differences between means (F‐statistics or Kruskal‐Wallis) and proportions (chi‐squared), and the significance level was set at 0.05.
First, a descriptive analysis of data was performed to verify the characteristics of children with and without sepsis. Descriptive analyses were performed separately on the characteristics of children with confirmed sepsis (positive blood culture) and children with clinical sepsis (clinical and laboratory signs of infection in the absence of positive blood culture). Frequencies of sepsis, alterations in PDI and MDI, and major alterations in the neurological examination at 12 months of corrected age were calculated. The association between exposure variables and outcomes was verified by calculating the odds ratio (OR).
To identify potential confounders, the variables associated with both the main exposure ‐ confirmed sepsis, clinical sepsis, and associated sepsis (confirmed and clinical) and the outcomes (neuromotor and mental development) with a significance level < 0.20 were selected. The variations in the magnitude of the association between sepsis and neuromotor development and between sepsis and mental development when adjusted for each of these variables in the study, defined as potential confounders in the previous step, were investigated. Variables whose adjustment led to a change > 10% in the primary exposure variable risk (sepsis) were selected for the multivariate models (logistic regression),24 which were constructed separately for each outcome. In the final model, variables with a significance level of 0.05 were considered.
This study was approved by the Ethics Committee in Research of the Instituto Fernandes Figueira (CAAE 0005.0.008.000‐06). Children's parents or guardians signed an informed consent for participation in the research.
ResultsDuring the study period, 448 newborns with very low weight were admitted. A total of 105 infants were excluded; there were 86 deaths, and nine infants did not attend the first appointment after discharge. Therefore, 248 children began follow‐up at the outpatient clinic. There were six deaths in the first year. The final study population comprised 194 children. The difference of 48 children between the number who started the study and the number who were evaluated through the Bayley scale at 12 months was due to missed appointments or inability of the child to perform the test in the scheduled period. Losses accounted for 19%. There was no statistically significant difference between neonatal characteristics of the study group and those of the losses.
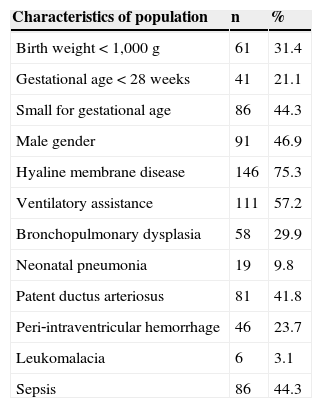
Mean birth weight was 1,119g (median 1,145, SD=247) and mean gestational age was 29 weeks and 6 days (median 30 weeks, SD=2). The neonatal characteristics of the population are shown in Table 1. These data indicate this is a population with high clinical severity, where nearly 50% had sepsis and about one‐third developed BPD. The mean household income was R$ 1,123 (median 800.00, SD=1,210), and the mean maternal level of schooling was 8.9 years (median 8.9 years, SD=3).
Characteristics of the population of 194 very low birth weight premature infants born between 2004 and 2010 and followed‐up at the outpatient clinic for at‐risk newborns.
| Characteristics of population | n | % |
|---|---|---|
| Birth weight < 1,000 g | 61 | 31.4 |
| Gestational age < 28 weeks | 41 | 21.1 |
| Small for gestational age | 86 | 44.3 |
| Male gender | 91 | 46.9 |
| Hyaline membrane disease | 146 | 75.3 |
| Ventilatory assistance | 111 | 57.2 |
| Bronchopulmonary dysplasia | 58 | 29.9 |
| Neonatal pneumonia | 19 | 9.8 |
| Patent ductus arteriosus | 81 | 41.8 |
| Peri‐intraventricular hemorrhage | 46 | 23.7 |
| Leukomalacia | 6 | 3.1 |
| Sepsis | 86 | 44.3 |
Of the studied population, 20% had neuromotor alteration in the clinical/neurological assessment at 12 months, and 37.6% had alterations in the PDI; the mean PDI was 86.6 (median 86, SD=15.3). At 12 months of corrected age, 79 children (40.7%) presented neuromotor alteration at the clinical/neurological assessment and/or changes in PDI. Alterations in the mental development index (MDI) occurred in 39.2% of children. The mean MDI was 86.4 (median 88, SD=13.4).
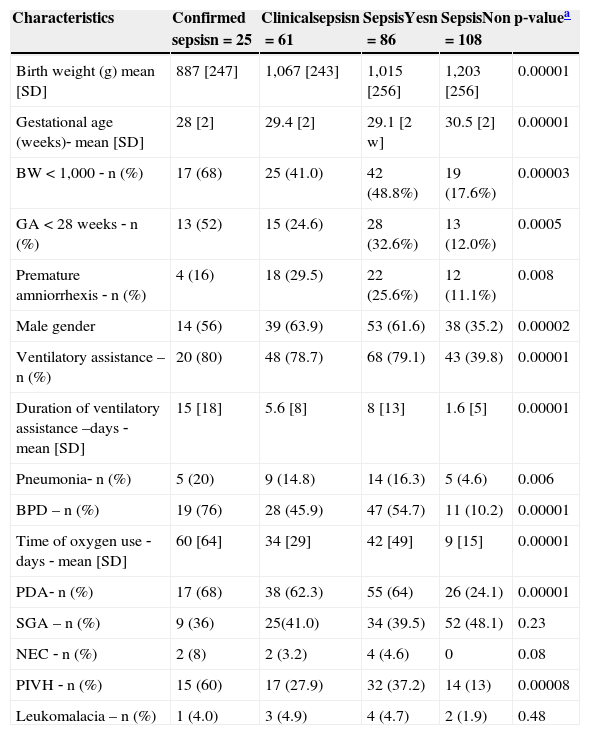
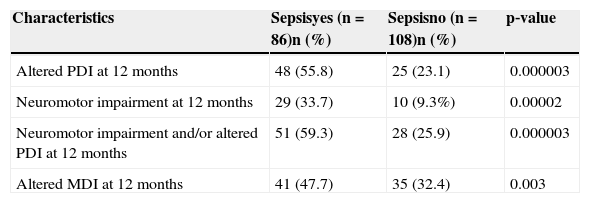
Of the 194 children, 86 (44.3%) developed sepsis, with 25 (12.9%) confirmed cases with positive blood cultures and 61 (31.4%) cases of clinical sepsis. The comparison of neonatal characteristics between the groups with and without sepsis is shown in Table 2. There was a higher frequency of birth weight < 1,000g and gestational age < 28 weeks in patients with sepsis, when compared to those without sepsis (p < 0.00001). Regarding motor development, a two‐fold higher frequency of PDI alterations (< 85) was observed in children with sepsis (55.8%) when compared to those without sepsis (23.1%). There was a significant difference between the means of PDI in children with (81; SD=15.4) and without sepsis (90.8; SD=13.9), as well as a significant difference between the means of MDI in children with (83; SD=14.3) and without sepsis (89.2; SD=12). There was a statistically significant difference between the groups regarding the occurrence of outcomes, with higher frequency in the sepsis group (Table 3).
Comparison of neonatal characteristics of preterm children with very low birth weight who developed sepsis in the neonatal period and those who did not; 2004‐2010.
| Characteristics | Confirmed sepsisn=25 | Clinicalsepsisn=61 | SepsisYesn=86 | SepsisNon=108 | p‐valuea |
|---|---|---|---|---|---|
| Birth weight (g) mean [SD] | 887 [247] | 1,067 [243] | 1,015 [256] | 1,203 [256] | 0.00001 |
| Gestational age (weeks)‐ mean [SD] | 28 [2] | 29.4 [2] | 29.1 [2 w] | 30.5 [2] | 0.00001 |
| BW < 1,000 ‐ n (%) | 17 (68) | 25 (41.0) | 42 (48.8%) | 19 (17.6%) | 0.00003 |
| GA < 28 weeks ‐ n (%) | 13 (52) | 15 (24.6) | 28 (32.6%) | 13 (12.0%) | 0.0005 |
| Premature amniorrhexis ‐ n (%) | 4 (16) | 18 (29.5) | 22 (25.6%) | 12 (11.1%) | 0.008 |
| Male gender | 14 (56) | 39 (63.9) | 53 (61.6) | 38 (35.2) | 0.00002 |
| Ventilatory assistance –n (%) | 20 (80) | 48 (78.7) | 68 (79.1) | 43 (39.8) | 0.00001 |
| Duration of ventilatory assistance –days ‐ mean [SD] | 15 [18] | 5.6 [8] | 8 [13] | 1.6 [5] | 0.00001 |
| Pneumonia‐ n (%) | 5 (20) | 9 (14.8) | 14 (16.3) | 5 (4.6) | 0.006 |
| BPD – n (%) | 19 (76) | 28 (45.9) | 47 (54.7) | 11 (10.2) | 0.00001 |
| Time of oxygen use ‐days ‐ mean [SD] | 60 [64] | 34 [29] | 42 [49] | 9 [15] | 0.00001 |
| PDA‐ n (%) | 17 (68) | 38 (62.3) | 55 (64) | 26 (24.1) | 0.00001 |
| SGA – n (%) | 9 (36) | 25(41.0) | 34 (39.5) | 52 (48.1) | 0.23 |
| NEC ‐ n (%) | 2 (8) | 2 (3.2) | 4 (4.6) | 0 | 0.08 |
| PIVH ‐ n (%) | 15 (60) | 17 (27.9) | 32 (37.2) | 14 (13) | 0.00008 |
| Leukomalacia – n (%) | 1 (4.0) | 3 (4.9) | 4 (4.7) | 2 (1.9) | 0.48 |
BPD, bronchopulmonary dysplasia; BW, birth weight; GA, gestational age; NEC, necrotizing enterocolitis; PDA, patent ductus arteriosus; PIVH, peri‐intraventricular hemorrhage; SD, standard deviation; SGA, small for gestational age.
Comparison of the frequency of neurodevelopmental disorders in preterm infants with very low birth weight who developed sepsis in the neonatal period and those who did not; 2004‐2010.
| Characteristics | Sepsisyes (n=86)n (%) | Sepsisno (n=108)n (%) | p‐value |
|---|---|---|---|
| Altered PDI at 12 months | 48 (55.8) | 25 (23.1) | 0.000003 |
| Neuromotor impairment at 12 months | 29 (33.7) | 10 (9.3%) | 0.00002 |
| Neuromotor impairment and/or altered PDI at 12 months | 51 (59.3) | 28 (25.9) | 0.000003 |
| Altered MDI at 12 months | 41 (47.7) | 35 (32.4) | 0.003 |
Altered MDI, mental development index on the Bayley Scale < 85 at 12 months of corrected age. Altered PDI, psychomotor development index on the Bayley Scale < 85 at 12 months of corrected age.
In the bivariate analysis between the exposure variable (sepsis) and outcomes (neuromotor development and mental development), it was observed that children with sepsis were four times more likely to develop neuromotor development alterations at 12 months than those who did not have sepsis (OR: 4.16; CI: 2.26‐7.65). When verifying the association between the variable confirmed sepsis and the neuromotor outcome, children with confirmed sepsis were approximately three times more likely to have neuromotor alterations at 12 months (OR: 2.99; CI: 1,25‐7.17) than children without confirmed sepsis. In the bivariate analysis between the variable clinical sepsis and outcome, children who had clinical sepsis were 2.72 times more likely to develop neuromotor alteration (CI: 1.46‐5.07).
When analyzing possible confounding factors in the association of sepsis with neuromotor development, it was observed that the following variables were associated with both the exposure and the outcome: birth weight < 1,000g, gestational age < 28 weeks, male gender, neonatal pneumonia, peri‐intraventricular hemorrhage, ventilatory assistance, PDA, and BPD.
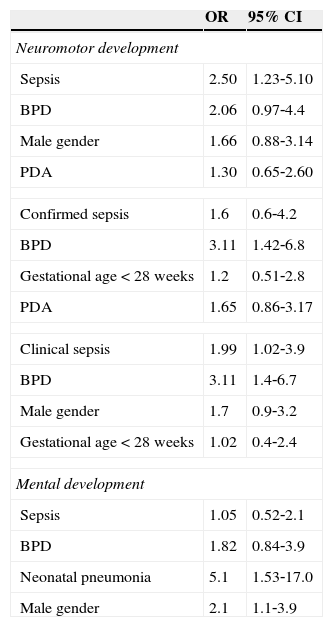
Table 4 shows that for neuromotor development, after adjusting for each of the possible confounding variables, the following variables were selected for the multivariate logistic model: BPD, PDA, and male gender. After controlling these risk factors, neonatal sepsis remained significantly associated with neuromotor development alterations at 12 months of corrected age. Children with sepsis were 2.5 times more likely to develop neuromotor developmental disorders when compared to those without sepsis (OR: 2.50; CI: 1.23‐5.10).
Association of neonatal sepsis with neuromotor and mental development impairment at 12 months of corrected age, adjusted for selected variables.
| OR | 95% CI | |
|---|---|---|
| Neuromotor development | ||
| Sepsis | 2.50 | 1.23‐5.10 |
| BPD | 2.06 | 0.97‐4.4 |
| Male gender | 1.66 | 0.88‐3.14 |
| PDA | 1.30 | 0.65‐2.60 |
| Confirmed sepsis | 1.6 | 0.6‐4.2 |
| BPD | 3.11 | 1.42‐6.8 |
| Gestational age < 28 weeks | 1.2 | 0.51‐2.8 |
| PDA | 1.65 | 0.86‐3.17 |
| Clinical sepsis | 1.99 | 1.02‐3.9 |
| BPD | 3.11 | 1.4‐6.7 |
| Male gender | 1.7 | 0.9‐3.2 |
| Gestational age < 28 weeks | 1.02 | 0.4‐2.4 |
| Mental development | ||
| Sepsis | 1.05 | 0.52‐2.1 |
| BPD | 1.82 | 0.84‐3.9 |
| Neonatal pneumonia | 5.1 | 1.53‐17.0 |
| Male gender | 2.1 | 1.1‐3.9 |
BPD, bronchopulmonary dysplasia; OR, odds ratio; PDA, patent ductus arteriosus; 95% CI, 95% confidence interval.
It can be observed that BPD had borderline statistical significance for the association with neuromotor development alteration (OR: 2.06 CI: 0.97‐4.4). When considering confirmed sepsis as exposure separately, after adjustment for each of the possible confounding variables, the variables selected for the multivariate logistic model were BPD, PDA, and gestational age < 28 weeks. After controlling for these risk factors, confirmed sepsis was not associated with neuromotor development alterations (OR: 1.6 CI: 0.86‐3.17). Considering clinical sepsis as exposure, after controlling for risk factors, it remained significantly associated with neuromotor development alterations (OR: 1.99; CI: 1.02‐3.9).
In the bivariate analysis between the exposure variable (sepsis) and outcome (mental development), it was observed that children with sepsis were 1.9 times more likely to have MDI alterations than those without sepsis (CI: 1.05‐3.40). In the bivariate analysis, the variables confirmed sepsis (OR: 1.82; CI: 0.78‐4.24) and clinical sepsis (OR: 1.50; CI: 0.8‐2.78) were not associated with the outcome of MDI alteration.
Regarding mental development, after adjusting for each of the possible confounding variables, the following variables were selected for multivariate logistic model: BPD, neonatal pneumonia, and male gender. After controlling these risk factors, neonatal sepsis lost statistical significance and did not remain associated with mental development alterations at 12 months of corrected age (OR: 1.05; CI: 0.52‐2.14) (Table 4). The presence of an interaction between the confounders was not identified (Table 4).
DiscussionThis study observed that preterm infants with very low birth weight that had neonatal sepsis are 2.5 times more likely to have altered neuromotor development at 12 months of corrected age, regardless of other risk factors, which supports some results found in the current literature. When analyzing the confirmed sepsis subgroup as a risk factor for neuromotor development alteration, after controlling for other confounding factors, the association lost statistical significance, perhaps due to the small number of confirmed cases. However, clinical sepsis showed to be an independent risk factor for neuromotor alteration.
Other studies that also analyzed children with confirmed and clinical sepsis separately found conflicting results regarding the contribution of each of these groups. In a multicenter study of over 6,000 children, newborns with clinical sepsis had similar chances (OR: 1.6; CI: 1.3‐2.0) to children with confirmed sepsis (OR: 1.5; CI: 1.2‐1.9) of having severe neuromotor alteration between 18 and 22 months.6
Kohlendorfer et al.9 also investigated the association of potential risk factors for neurodevelopmental disorders. Children who developed neonatal sepsis were three times more likely to have neuromotor and cognitive development alterations (PDI and MDI < 85) at 12 months of corrected age.
Conversely, a recent study10 on neurodevelopment alterations in a cohort of preterm infants assessed at 24 months of corrected age included 136 children with confirmed sepsis, 169 with suspected sepsis, and 236 without infection. Children with confirmed sepsis were three times more likely to have cerebral palsy when compared with those without sepsis. It was observed that children with clinical infection were almost twice as likely to have cerebral palsy, but there was no statistical significance. Clinical sepsis and confirmed sepsis showed no association with cognitive delay.10
Although the blood culture is considered to be the gold standard for sepsis diagnosis, its sensitivity is less than 50%.25 In the present study, it was important to include clinical sepsis, as due to the low sensitivity of blood cultures, children who had infection could fail to be included as exposed to sepsis, and thus underestimate its effects on neuromotor development. Several authors have considered clinical sepsis in their recently published articles, either alone or in combination26–28 with confirmed sepsis, and employed similar criteria to those of the present study for the classification of clinical sepsis.6,10,28
Neuromotor alterations in preterm infants with neonatal infection may be mediated by white matter lesions.5,29 However, it is not known which mechanism generates the white matter lesions. It appears that such lesions can be attributed to the susceptibility of oligodendrocyte precursors to inflammation, hypoxia, and ischemia.28 Helmes et al.30 observed no association between late‐onset sepsis and white matter lesions, and found no adverse effects on development at 15 months of age in preterm children.
It should be considered that part of the observed neuromotor alterations may be due to transient neurological abnormalities observed in preterm infants up to 18 months of corrected age. Brandt et al.31 observed abnormal neurological signs, mainly in the first year of life, and concluded that it is not possible to predict whether an early abnormal neurological sign is transient, and thus a longitudinal follow‐up of these children is required, emphasizing the importance of follow‐up in this population.
Gianní et al.32 emphasized the importance of early stimulation and intervention in these children to promote better neuropsychomotor development. In the present study, the families of children with any alteration were instructed to perform exercises at home and/or physical therapy.
The losses that occurred during the follow‐up period can be considered as a study limitation; however, no significant difference was observed between the neonatal characteristics of the losses and the study group. It must be emphasized that the frequency of sepsis was similar between the two groups, indicating that the occurrence of bias is unlikely. It is important to mention that losses are frequent in cohort studies and that those in the present study were similar to the losses observed in others.9,10
The age chosen for the cutoff (12 months of corrected age) aimed at the early detection of neurodevelopmental alterations, and therefore, the earliest and most suitable therapeutic approach, in order to promote proper development. Another important aspect of the study was the systematic monitoring during 12 months in a cohort of 194 children from families of low socio‐economic status, many of them residing in other municipalities. Studies have stressed the importance of long‐term monitoring of this at‐risk population.32 Another reason for the choice of evaluation at 12 months was the scarcity of reports in the literature of sepsis‐related changes at this age range.
ConclusionNeonatal sepsis was an independent risk factor for neuromotor development impairment in premature infants in the studied age range, but there was no association with mental development. is the results suggest that children who develop either confirmed or clinical sepsis in the neonatal period have a differentiated follow‐up of their neuromotor development.
FundingPAPES IV‐ convênio FIOCRUZ/CNPq‐Process number: 400115/2006‐9.
Conflicts of interetThe authors declare no conflicts of interest.
The authors would like to thank Maria de Fátima Junqueira, Juliana Verçosa Rocha Delamônica, and Ana Beatriz Rodrigues Reis for applying the Bayley Scale to the children participating in the study.
Please cite this article as: Ferreira RC, Mello RR, Silva KS. Neonatal sepsis as a risk factor for neurodevelopmental changes in preterm infants with very low birth weight. J Pediatr (Rio J). 2014;90:293–9.
Study performed at Instituto Fernandes Figueira, Fundação Oswaldo Cruz (FIOCRUZ), Avenida Rui Barbosa 716, Flamengo, Rio de Janeiro, RJ, 22250‐020, Brazil.