The objective of this study was to evaluate the effects of two low-dose combined oral contraceptives on bone metabolism in adolescents for one year.
MethodsThis was a quasi-experimental study. The adolescents were divided into three groups: oral contraceptives 1 (n=42) (20μg EE/150μg desogestrel), oral contraceptives 2 (n=66) (30μg EE/3mg drospirenone), and a control group (n=70). Adolescents underwent anthropometric assessment and densitometry (dual-energy X-ray). Bone age and bone formation markers (osteocalcin and bone alkaline phosphatase) were evaluated. The oral contraceptives users were evaluated again after 12 months. Linear regression analysis was used to indirectly study the effect of each additional year of chronological age on anthropometric and densitometric variables as well as on bone markers in the control group.
ResultsAt study entry, no significant differences were observed between the oral contraceptives 1, oral contraceptives 2, and controls in the analyzed variables. Linear regression analysis showed an increase in bone mineral density and bone mineral content for each additional year. There was a significant reduction in bone alkaline phosphatase levels; no significant difference was observed for osteocalcin in control individuals. Comparison of dual-energy X-ray variables at baseline and after one year showed no significant differences in the oral contraceptives 1 or oral contraceptives 2 groups. A significant reduction in bone alkaline phosphatase and osteocalcin levels was observed in both the oral contraceptives 1 and oral contraceptives 2 groups.
ConclusionAdolescent women gain peak bone mass during this phase of life. Two low-dose combined oral hormonal contraceptives were associated with lower bone gain and lower bone formation markers than in untreated controls.
O objetivo deste estudo foi avaliar os efeitos de dois contraceptivos orais combinados de baixa dosagem por um ano sobre o metabolismo ósseo em adolescentes.
MétodosEste foi um estudo quase experimental. As adolescentes foram divididas em três grupos: contraceptivos orais 1 (n=42) (20μg de EE/150μg de desogestrel), contraceptivos orais 2 (n=66) (30μg EE/3mg de drospirenona) e grupo controle (n=70). As adolescentes foram submetidas à avaliação antropométrica e densitometria (raio-X de dupla energia). Foram avaliados a idade óssea e os marcadores de formação óssea (osteocalcina e fosfatase alcalina óssea). As usuárias de contraceptivos orais foram novamente avaliadas após 12 meses. A análise de regressão linear foi utilizada para estudar, indiretamente, o efeito de cada ano adicional da idade cronológica sobre as variáveis antropométricas e densitométricas e sobre os marcadores ósseos no grupo de controle.
ResultadosNo início do estudo, não foram observadas diferenças significativas nas variáveis analisadas entre as usuárias de contraceptivos orais 1, contraceptivos orais 2 e o grupo controle. A análise de regressão linear mostrou um aumento na densidade mineral óssea e no conteúdo mineral ósseo para cada ano adicional. Houve uma redução significativa nos níveis de fosfatase alcalina óssea e não foi observada diferença significativa para osteocalcina nos indivíduos controles. A comparação das variáveis do raio-X de dupla energia no início e após um ano não mostrou diferença significativa no grupo de contraceptivos orais 1 ou contraceptivos orais 2. Foi observada uma redução significativa nos níveis de fosfatase alcalina óssea e osteocalcina nos dois grupos contraceptivos orais 1 e contraceptivos orais 2.
ConclusãoAs adolescentes atingiram o pico de massa óssea durante essa fase da vida. Duas formulações de contraceptivos hormonais orais de baixa dosagem, após um ano de uso, se associaram a menor incremento na densidade mineral óssea e menor concentração de marcadores de formação óssea quando confrontados com resultados de adolescentes não usuárias de contraceptivos.
Adequate bone mass acquisition during childhood and adolescence has been indicated as a protective factor of skeletal health in adulthood.1 One risk factor for osteopenia/osteoporosis and fragility fractures during adulthood and old age is low bone mineral density (BMD) or the lack of its preservation during childhood and adolescence. It seems virtually impossible to increase peak bone mass in adulthood, thus the growing individual must gain the necessary bone mass at the appropriate time. In this respect, about 40–45% of the bone mass present in adulthood is acquired during adolescence.2
In this context, it should be remembered that contraceptives are prescribed at increasingly younger ages to protect young people against unintended pregnancies at the initiation of sexual activity.3 This practice has raised questions as to whether the amount of estrogen in the formulation is adequate for this age group regarding bone development and attaining an adequate bone mass peak. Much of the published research on the subject includes a wide range of women of different ages, including adolescents, young people, and even young adults, as well as diverse moments of growth and bone mineralization, creating difficulties when interpreting the results of increases in bone mass.
Recent studies involving young boys and girls have demonstrated a relationship between bone markers as predictors of the development of bone mineral content (BMC) and BMD during the period of greatest bone mass gain. The results showed that the concentrations of markers of bone formation and resorption are higher in the early years of puberty than in advanced stages of pubertal development.4–7 The possible effects of contraceptives on bone mass gain can cause bone remodeling disorders.8,9
The importance of hormonal contraceptives for bone mass gain and their participation in bone remodeling highlight the need to conduct prospective studies specifically addressing adolescence – a period characterized by greatest bone mass acquisition – to evaluate the effects of those drugs on bone health in this age group and possible future outcomes in adulthood and old age.
Thus, the objective of the present study was to prospectively evaluate whether there would be any effects and possible differences between the use of two types of low-dose oral contraceptives (OC) containing 20μg ethinylestradiol (EE)/150μg desogestrel or 30μg EE/3mg drospirenone for one year on the bone metabolism of adolescents, and compare the results with those obtained for healthy adolescents who did not use OCs.
MethodsThis was a quasi-experimental study approved by the Research Ethics Committee of the Botucatu School of Medicine, São Paulo, Brazil. The sample consisted of healthy adolescents with a height between the 5th and 95th percentiles for age and a body mass index (BMI) between the 5th and ≤95th percentiles according to the Centers for Disease Control and Prevention.10 Adolescents who had an active sex life and wanted to start a contraceptive method were included in the OC exposure groups, being offered two types of OCs. Those who did not require OCs were directed to the control group.
The participants were divided into three groups. Group 1 (OC1) consisted of healthy adolescent girls aged 12 full years to 20 incomplete years in post-menarche, late puberty.11 The adolescents were registered at the Adolescent Medicine outpatient clinic of the University Hospital and were referred to guidance and prescription of a contraceptive method due to an active sexual life. The subjects (n=42) received a low-dose combined oral contraceptive (OC) containing 20μg ethinylestradiol (EE) and 150μg desogestrel. Group 2 (OC2) consisted of adolescent girls of the same age range and with the same characteristics as those of OC1. Those subjects (n=66) received another formulation of low-dose combined OC containing 30μg EE and 3mg drospirenone. The control group consisted of 70 adolescent girls matched to the other two groups and who did not use OCs. All adolescents of the study and control groups were in post-menarche, had regular menstrual cycles, and had no oligomenorrhea/amenorrhea. All participants were non-smokers and non-drinkers and did not participate in sporting activities besides the 2-h physical activity performed once a week during school hours. The health-related exclusion criteria adopted for the study were: a history of prematurity or low birth weight; prolonged steroid treatment or use of calcium or iron supplements during the 12 months prior to the study; diabetes mellitus; acute or chronic malnutrition; congenital or acquired bone diseases; gastrointestinal malabsorption; a history of nephropathy with or without chronic renal failure; endocrinopathies; early or late puberty; chronic drug use; cystic fibrosis; and celiac disease. Other exclusion criteria were the use of medications known to negatively affect bone metabolism, previous use of an OC or other hormonal contraceptive method including depot medroxyprogesterone acetate, switching methods during the study, being vegetarian or consuming a high-fiber diet, and daily consumption of more than 300mg of caffeine or more than 500mL of cola-based soft drinks.
All adolescents underwent an anthropometric assessment. The BMI was calculated and the pubertal stage was evaluated using the Tanner criteria.11 Bone age was determined by the Greulich and Pyle method12 at baseline and after 12 months of follow-up.
Adolescents who completed all previous steps were submitted to bone mass evaluation at the beginning of OC use and at the end of follow-up (after 1 year). Bone mass was measured densitometrically by dual-energy X-ray absorptiometry (DXA) with a Hologic QDR 4500 Discovery A (Hologic, Inc., Bedford, MA). Osteocalcin and bone alkaline phosphatase (BAP) biomarkers were evaluated before and after 12 months of OC use. The dosages were measured using an assay from Metra™ Biosystems, with intra- and inter-assay coefficients of variation of 8% and 7.6%, respectively.
The study proposed to perform a follow-up of the three groups over a period of 1 year; however, there were difficulties at follow-up concerning adolescents who composed the control group, at which point they were to undergo new bone mineral densitometry and blood collections, procedures similar to those proposed for the users of contraceptives. Difficulties included: many did not return on the scheduled day for the blood collection or performance of densitometry; others started using medication that was among the exclusion criteria; and finally, a large percentage requested prescription of a contraceptive method. For this reason the decision was made to exclude the control group and perform a linear regression analysis with a normal response with independent observations among the adolescents of the control group, allowing an estimation of the annual effect on our outcomes, and compensating for the absence of a one-year follow-up among adolescents in the control group. Using this technique, we were able to indirectly compare groups during one year of follow-up.
Statistical analysisThe Kruskal–Wallis test was used to compare baseline variables between the control, OC1, and OC2 groups, and the Mann–Whitney test was used to compare the OC1 and OC2 groups. The ANOVA test was used for the variables BMI, Z-score BMI, and percentile BMI (values presented as mean) as these variables presented symmetrical distribution.
The effects of age on anthropometric and densitometric variables as well as on the concentrations of bone markers in the control group were evaluated by simple linear regression with a normal response. The Wilcoxon test was used to compare anthropometric and densitometric variables, as well as bone markers at baseline and after 12 months in the OC1 and OC2. For BMI, Z-score BMI, and BMI percentile, the paired test was used. Differences and associations were considered significant when p<0.05.
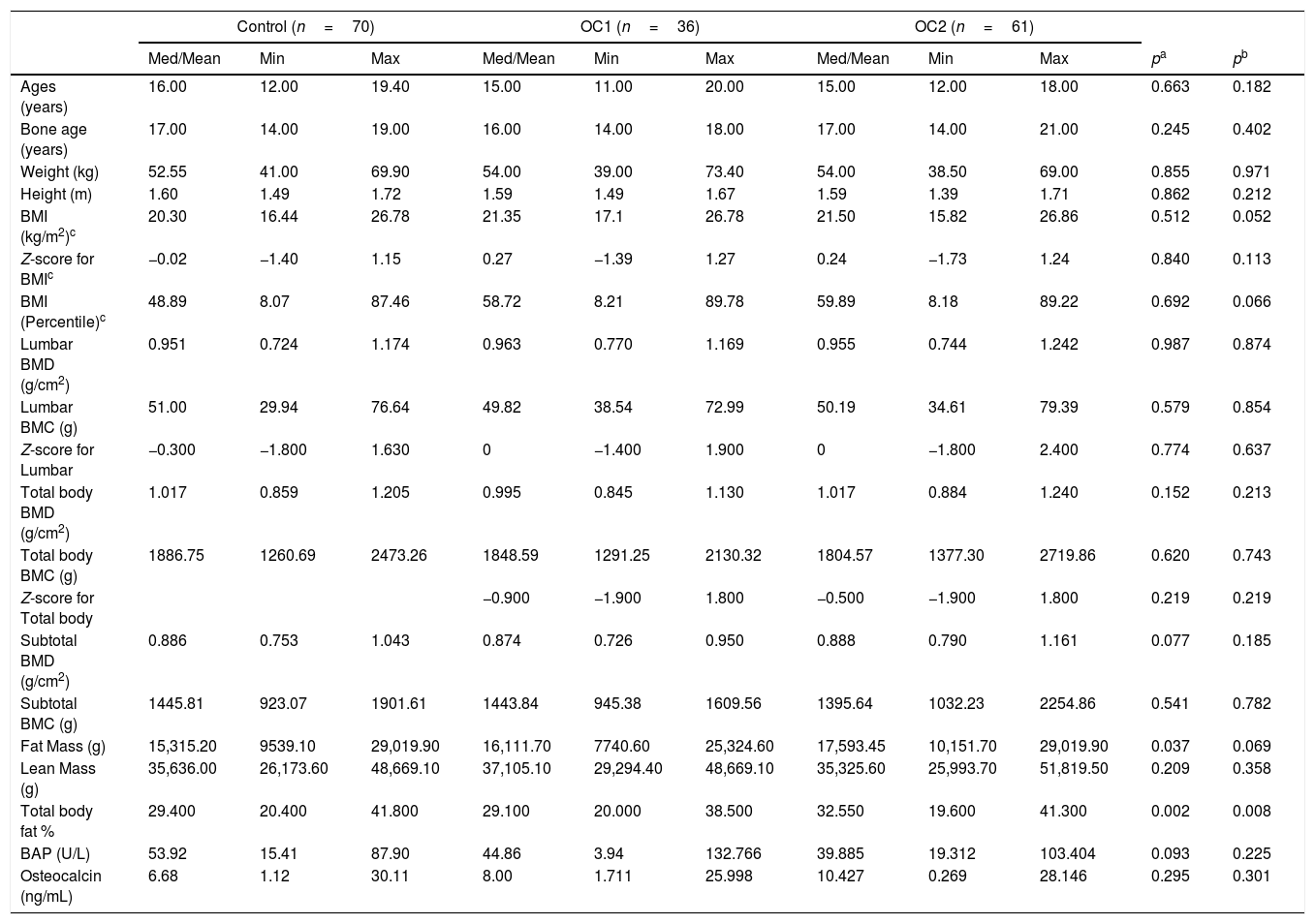
ResultsIn the OC1 group, 36 subjects completed the study and six were excluded because they chose to discontinue the OC due to personal reasons. Of the 66 subjects included in the OC2 group, five were excluded for the same personal reasons. Comparison of anthropometric and densitometric variables, body composition obtained by densitometry, and concentrations of BAP and osteocalcin at baseline revealed no significant differences between the three groups, indicating the homogeneity between the groups at the moment of inclusion, except for body fat percentage. This variable was higher in the OC2 group compared to the control and OC1 groups. However, none of the participants were obese (Table 1).
Comparison of anthropometric and densitometric variables and bone formation markers at baseline between adolescents receiving low-dose oral contraceptives and the control group.
| Control (n=70) | OC1 (n=36) | OC2 (n=61) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Med/Mean | Min | Max | Med/Mean | Min | Max | Med/Mean | Min | Max | pa | pb | |
| Ages (years) | 16.00 | 12.00 | 19.40 | 15.00 | 11.00 | 20.00 | 15.00 | 12.00 | 18.00 | 0.663 | 0.182 |
| Bone age (years) | 17.00 | 14.00 | 19.00 | 16.00 | 14.00 | 18.00 | 17.00 | 14.00 | 21.00 | 0.245 | 0.402 |
| Weight (kg) | 52.55 | 41.00 | 69.90 | 54.00 | 39.00 | 73.40 | 54.00 | 38.50 | 69.00 | 0.855 | 0.971 |
| Height (m) | 1.60 | 1.49 | 1.72 | 1.59 | 1.49 | 1.67 | 1.59 | 1.39 | 1.71 | 0.862 | 0.212 |
| BMI (kg/m2)c | 20.30 | 16.44 | 26.78 | 21.35 | 17.1 | 26.78 | 21.50 | 15.82 | 26.86 | 0.512 | 0.052 |
| Z-score for BMIc | −0.02 | −1.40 | 1.15 | 0.27 | −1.39 | 1.27 | 0.24 | −1.73 | 1.24 | 0.840 | 0.113 |
| BMI (Percentile)c | 48.89 | 8.07 | 87.46 | 58.72 | 8.21 | 89.78 | 59.89 | 8.18 | 89.22 | 0.692 | 0.066 |
| Lumbar BMD (g/cm2) | 0.951 | 0.724 | 1.174 | 0.963 | 0.770 | 1.169 | 0.955 | 0.744 | 1.242 | 0.987 | 0.874 |
| Lumbar BMC (g) | 51.00 | 29.94 | 76.64 | 49.82 | 38.54 | 72.99 | 50.19 | 34.61 | 79.39 | 0.579 | 0.854 |
| Z-score for Lumbar | −0.300 | −1.800 | 1.630 | 0 | −1.400 | 1.900 | 0 | −1.800 | 2.400 | 0.774 | 0.637 |
| Total body BMD (g/cm2) | 1.017 | 0.859 | 1.205 | 0.995 | 0.845 | 1.130 | 1.017 | 0.884 | 1.240 | 0.152 | 0.213 |
| Total body BMC (g) | 1886.75 | 1260.69 | 2473.26 | 1848.59 | 1291.25 | 2130.32 | 1804.57 | 1377.30 | 2719.86 | 0.620 | 0.743 |
| Z-score for Total body | −0.900 | −1.900 | 1.800 | −0.500 | −1.900 | 1.800 | 0.219 | 0.219 | |||
| Subtotal BMD (g/cm2) | 0.886 | 0.753 | 1.043 | 0.874 | 0.726 | 0.950 | 0.888 | 0.790 | 1.161 | 0.077 | 0.185 |
| Subtotal BMC (g) | 1445.81 | 923.07 | 1901.61 | 1443.84 | 945.38 | 1609.56 | 1395.64 | 1032.23 | 2254.86 | 0.541 | 0.782 |
| Fat Mass (g) | 15,315.20 | 9539.10 | 29,019.90 | 16,111.70 | 7740.60 | 25,324.60 | 17,593.45 | 10,151.70 | 29,019.90 | 0.037 | 0.069 |
| Lean Mass (g) | 35,636.00 | 26,173.60 | 48,669.10 | 37,105.10 | 29,294.40 | 48,669.10 | 35,325.60 | 25,993.70 | 51,819.50 | 0.209 | 0.358 |
| Total body fat % | 29.400 | 20.400 | 41.800 | 29.100 | 20.000 | 38.500 | 32.550 | 19.600 | 41.300 | 0.002 | 0.008 |
| BAP (U/L) | 53.92 | 15.41 | 87.90 | 44.86 | 3.94 | 132.766 | 39.885 | 19.312 | 103.404 | 0.093 | 0.225 |
| Osteocalcin (ng/mL) | 6.68 | 1.12 | 30.11 | 8.00 | 1.711 | 25.998 | 10.427 | 0.269 | 28.146 | 0.295 | 0.301 |
Note. Med (Median).
OC1, adolescents receiving oral contraceptive containing 20μg EE/150g desogestrel; OC2, adolescents receiving oral contraceptive containing 30μg EE/3mg drospirenone.
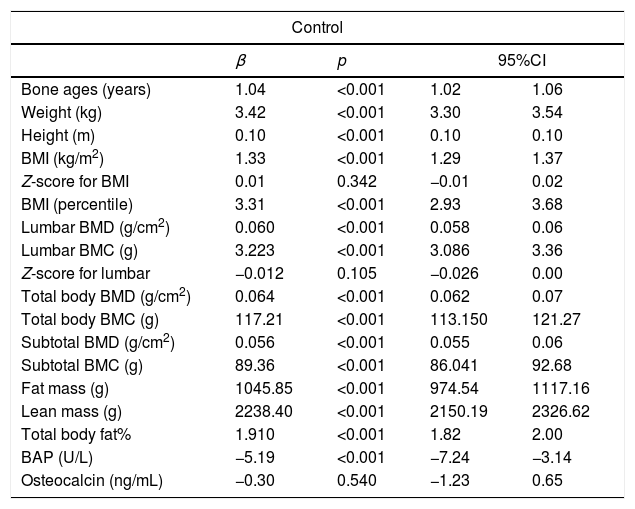
Simple linear regression analysis was used to study the effect of each additional year of chronological age on all variables in the control group. For each additional year of chronological age, bone age increased by 1.04 years, weight by 3.42kg, and BMI by 1.33kg/m2. The statistically significant annual effect on height (increase of 0.1m per year) does not agree with the biologically expected effect. Thus, this effect was reevaluated using a linear regression model with insertion of an intercept, which resulted in a biologically more sensible annual effect on height of practically zero (p=0.953) (Table 2).
Linear regression analysis to evaluate the effect of age on anthropometric and densitometric variables and bone formation markers in adolescents not using oral contraceptives (control group).
| Control | ||||
|---|---|---|---|---|
| β | p | 95%CI | ||
| Bone ages (years) | 1.04 | <0.001 | 1.02 | 1.06 |
| Weight (kg) | 3.42 | <0.001 | 3.30 | 3.54 |
| Height (m) | 0.10 | <0.001 | 0.10 | 0.10 |
| BMI (kg/m2) | 1.33 | <0.001 | 1.29 | 1.37 |
| Z-score for BMI | 0.01 | 0.342 | −0.01 | 0.02 |
| BMI (percentile) | 3.31 | <0.001 | 2.93 | 3.68 |
| Lumbar BMD (g/cm2) | 0.060 | <0.001 | 0.058 | 0.06 |
| Lumbar BMC (g) | 3.223 | <0.001 | 3.086 | 3.36 |
| Z-score for lumbar | −0.012 | 0.105 | −0.026 | 0.00 |
| Total body BMD (g/cm2) | 0.064 | <0.001 | 0.062 | 0.07 |
| Total body BMC (g) | 117.21 | <0.001 | 113.150 | 121.27 |
| Subtotal BMD (g/cm2) | 0.056 | <0.001 | 0.055 | 0.06 |
| Subtotal BMC (g) | 89.36 | <0.001 | 86.041 | 92.68 |
| Fat mass (g) | 1045.85 | <0.001 | 974.54 | 1117.16 |
| Lean mass (g) | 2238.40 | <0.001 | 2150.19 | 2326.62 |
| Total body fat% | 1.910 | <0.001 | 1.82 | 2.00 |
| BAP (U/L) | −5.19 | <0.001 | −7.24 | −3.14 |
| Osteocalcin (ng/mL) | −0.30 | 0.540 | −1.23 | 0.65 |
Analysis of the densitometric variables in the control group showed statistically significant differences in lumbar spine BMD, total body BMD, and subtotal BMD, with increases of 0.060g/cm2, 0.064g/cm2, and 0.056g/cm2 for each additional year, respectively. Increases were also observed in lumbar spine BMC, total body BMC, and subtotal BMC (3.22g, 117.21g, and 89.36g, respectively; p<0.001). Fat mass and lean mass increased by 1045.85g and 2238.40g, respectively (Table 2).
Regarding bone biomarkers, there was a statistically significant reduction in the concentration of BAP (−5.19U/L) with each additional year of chronological age in the control group. No significant difference was observed for osteocalcin (Table 2).
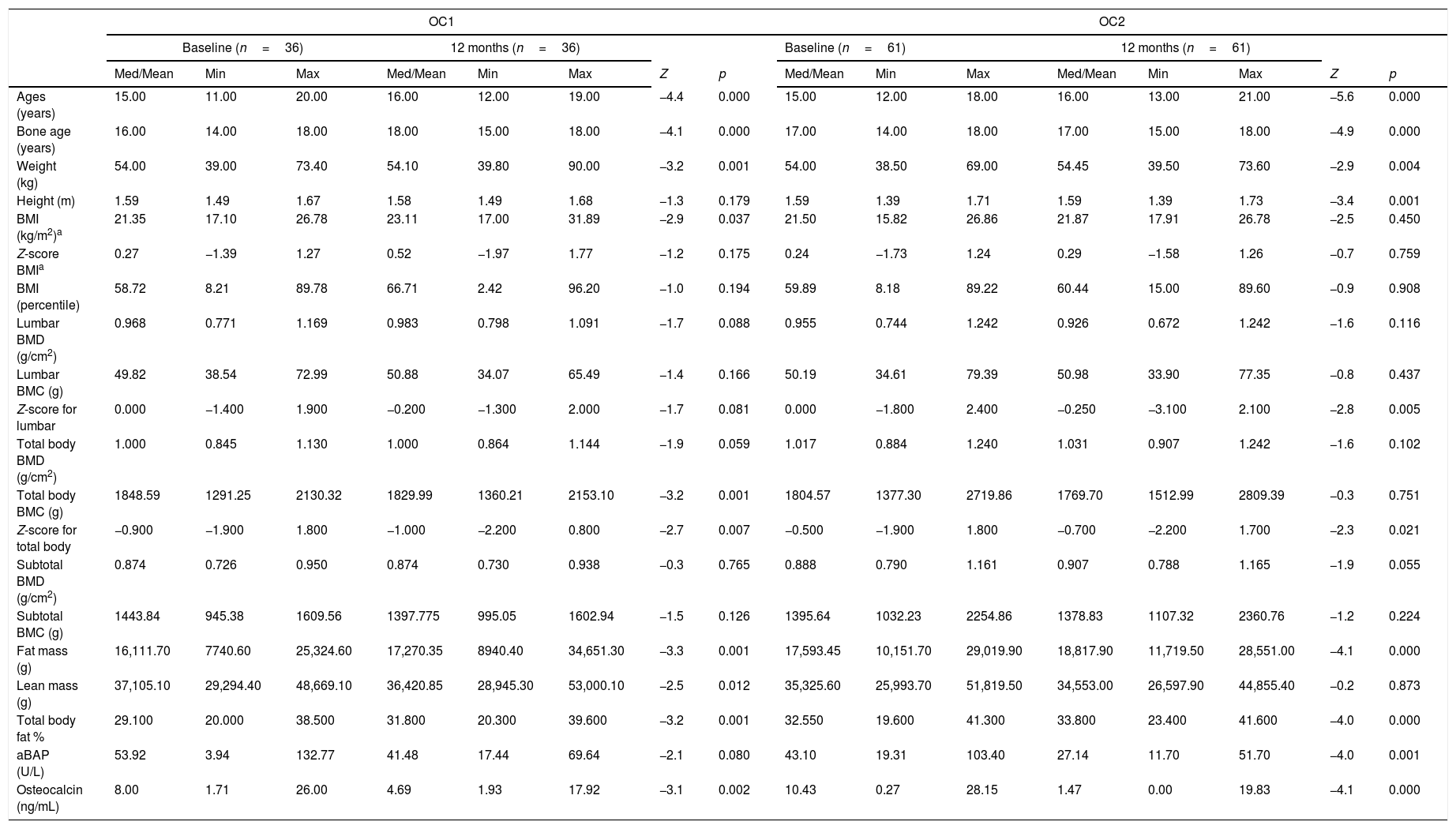
No significant differences were found for the following variables: lumbar BMD, lumbar BMC, total body BMD, total body BMC, subtotal BMD, and subtotal BMC in the OC2 group; the same was found in the OC1 group, except for total body BMC (p=0.001). Significant differences were observed in fat mass and body fat percentage between baseline and 12 months for the two contraceptive formulations. No significant difference in lean mass was observed between baseline and after 12 months in the OC2 group (Table 3).
Comparison of anthropometric and densitometric variables and bone formation markers between baseline and after 12 months in the two groups receiving oral contraceptives.
| OC1 | OC2 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (n=36) | 12 months (n=36) | Baseline (n=61) | 12 months (n=61) | |||||||||||||
| Med/Mean | Min | Max | Med/Mean | Min | Max | Z | p | Med/Mean | Min | Max | Med/Mean | Min | Max | Z | p | |
| Ages (years) | 15.00 | 11.00 | 20.00 | 16.00 | 12.00 | 19.00 | −4.4 | 0.000 | 15.00 | 12.00 | 18.00 | 16.00 | 13.00 | 21.00 | −5.6 | 0.000 |
| Bone age (years) | 16.00 | 14.00 | 18.00 | 18.00 | 15.00 | 18.00 | −4.1 | 0.000 | 17.00 | 14.00 | 18.00 | 17.00 | 15.00 | 18.00 | −4.9 | 0.000 |
| Weight (kg) | 54.00 | 39.00 | 73.40 | 54.10 | 39.80 | 90.00 | −3.2 | 0.001 | 54.00 | 38.50 | 69.00 | 54.45 | 39.50 | 73.60 | −2.9 | 0.004 |
| Height (m) | 1.59 | 1.49 | 1.67 | 1.58 | 1.49 | 1.68 | −1.3 | 0.179 | 1.59 | 1.39 | 1.71 | 1.59 | 1.39 | 1.73 | −3.4 | 0.001 |
| BMI (kg/m2)a | 21.35 | 17.10 | 26.78 | 23.11 | 17.00 | 31.89 | −2.9 | 0.037 | 21.50 | 15.82 | 26.86 | 21.87 | 17.91 | 26.78 | −2.5 | 0.450 |
| Z-score BMIa | 0.27 | −1.39 | 1.27 | 0.52 | −1.97 | 1.77 | −1.2 | 0.175 | 0.24 | −1.73 | 1.24 | 0.29 | −1.58 | 1.26 | −0.7 | 0.759 |
| BMI (percentile) | 58.72 | 8.21 | 89.78 | 66.71 | 2.42 | 96.20 | −1.0 | 0.194 | 59.89 | 8.18 | 89.22 | 60.44 | 15.00 | 89.60 | −0.9 | 0.908 |
| Lumbar BMD (g/cm2) | 0.968 | 0.771 | 1.169 | 0.983 | 0.798 | 1.091 | −1.7 | 0.088 | 0.955 | 0.744 | 1.242 | 0.926 | 0.672 | 1.242 | −1.6 | 0.116 |
| Lumbar BMC (g) | 49.82 | 38.54 | 72.99 | 50.88 | 34.07 | 65.49 | −1.4 | 0.166 | 50.19 | 34.61 | 79.39 | 50.98 | 33.90 | 77.35 | −0.8 | 0.437 |
| Z-score for lumbar | 0.000 | −1.400 | 1.900 | −0.200 | −1.300 | 2.000 | −1.7 | 0.081 | 0.000 | −1.800 | 2.400 | −0.250 | −3.100 | 2.100 | −2.8 | 0.005 |
| Total body BMD (g/cm2) | 1.000 | 0.845 | 1.130 | 1.000 | 0.864 | 1.144 | −1.9 | 0.059 | 1.017 | 0.884 | 1.240 | 1.031 | 0.907 | 1.242 | −1.6 | 0.102 |
| Total body BMC (g) | 1848.59 | 1291.25 | 2130.32 | 1829.99 | 1360.21 | 2153.10 | −3.2 | 0.001 | 1804.57 | 1377.30 | 2719.86 | 1769.70 | 1512.99 | 2809.39 | −0.3 | 0.751 |
| Z-score for total body | −0.900 | −1.900 | 1.800 | −1.000 | −2.200 | 0.800 | −2.7 | 0.007 | −0.500 | −1.900 | 1.800 | −0.700 | −2.200 | 1.700 | −2.3 | 0.021 |
| Subtotal BMD (g/cm2) | 0.874 | 0.726 | 0.950 | 0.874 | 0.730 | 0.938 | −0.3 | 0.765 | 0.888 | 0.790 | 1.161 | 0.907 | 0.788 | 1.165 | −1.9 | 0.055 |
| Subtotal BMC (g) | 1443.84 | 945.38 | 1609.56 | 1397.775 | 995.05 | 1602.94 | −1.5 | 0.126 | 1395.64 | 1032.23 | 2254.86 | 1378.83 | 1107.32 | 2360.76 | −1.2 | 0.224 |
| Fat mass (g) | 16,111.70 | 7740.60 | 25,324.60 | 17,270.35 | 8940.40 | 34,651.30 | −3.3 | 0.001 | 17,593.45 | 10,151.70 | 29,019.90 | 18,817.90 | 11,719.50 | 28,551.00 | −4.1 | 0.000 |
| Lean mass (g) | 37,105.10 | 29,294.40 | 48,669.10 | 36,420.85 | 28,945.30 | 53,000.10 | −2.5 | 0.012 | 35,325.60 | 25,993.70 | 51,819.50 | 34,553.00 | 26,597.90 | 44,855.40 | −0.2 | 0.873 |
| Total body fat % | 29.100 | 20.000 | 38.500 | 31.800 | 20.300 | 39.600 | −3.2 | 0.001 | 32.550 | 19.600 | 41.300 | 33.800 | 23.400 | 41.600 | −4.0 | 0.000 |
| aBAP (U/L) | 53.92 | 3.94 | 132.77 | 41.48 | 17.44 | 69.64 | −2.1 | 0.080 | 43.10 | 19.31 | 103.40 | 27.14 | 11.70 | 51.70 | −4.0 | 0.001 |
| Osteocalcin (ng/mL) | 8.00 | 1.71 | 26.00 | 4.69 | 1.93 | 17.92 | −3.1 | 0.002 | 10.43 | 0.27 | 28.15 | 1.47 | 0.00 | 19.83 | −4.1 | 0.000 |
Note. Med (median).
OC1, adolescents receiving oral contraceptive containing 20μg EE/150g desogestrel; OC2, adolescents receiving oral contraceptive containing 30μg EE/3mg drospirenone.
Wilcoxon test for comparison between time points in OC1 and OC2.
There was a reduction in median BAP levels from 53.92U/L at baseline to 41.48U/L after 12 months of use in the OC1 group (p=0.080), and from 43.10U/L to 27.14U/L in the OC2 group (p<0.001). Reductions in osteocalcin concentrations were observed from 8.0ng/mL at baseline to 4.69ng/mL after 12 months in the OC1 group (p=0.002), and from 10.43ng/mL to 1.47ng/mL in the OC2 group (p<0.001) (Table 3).
DiscussionHealthy adolescents in the age range of 12 full years to 20 incomplete years who did not use oral hormonal contraceptives exhibited a significant increase in bone mass when analyzed by bone densitometry at the different skeletal sites proposed, according to the linear regression analysis used to verify the effect of one year of chronological age on the analyzed variables. With respect to bone formation markers, an increase in concentrations was observed, which coincided with the time of peak height velocity (PHV), confirming the parallelism between these events. This increase was followed by a decrease in biomarker levels, which accompanied the expected deceleration of PHV. Thus, although BMD values increased with the advancement of maturation events, the concentrations of biomarkers reduced with age at the end of adolescence.7,13
At baseline, the three studied groups were homogeneous in terms of anthropometric and densitometric characteristics, as well as markers of bone formation. Users of the two types of OC exhibited lower bone increment between baseline and 12 months than what was estimated for the control group, according to the results obtained through the linear regression analysis, thus demonstrating a lower increase in bone mass in adolescents who took the two contraceptive formulations. It should be emphasized that this period comprising the second decade of life is considered unique and fundamental for future bone health, since 92% of total bone mass is acquired by about the age of 18 years and 99% by the age of 26 years.14–16
Women using OCs are known to lack the high concentrations of endogenous estrogen observed at mid-cycle, a characteristic of spontaneous cycles, as well as the oscillations seen during the different phases of menstrual cycles.16 The efforts to reduce side effects, especially cardiovascular complications resulting from the use of OCs, has led to a gradual reduction in the concentrations of estrogen in contraceptive formulations, a fact that may have caused a deleterious effect on BMD. Due to the lower concentration of sex steroids in the circulation, a smaller increase in peak bone mass acquired in adolescence was observed,17 although other organs and systems were protected by this reduction in EE concentrations in contraceptives.
When we compare the results of the present study with data in the literature we can confirm that the use of OCs precipitates a lower increase in bone mass in adolescents, since linear regression analysis showed an increase in BMD and BMC at all skeletal sites analyzed in control subjects. These results agree with those reported in the study by Polatti et al.18 in which BMD increased by 7.8% in controls.18,19 Conversely, in the present study, analysis of bone mass gain through densitometry revealed a very discrete increase among OC users after 12 months compared to baseline, but the difference was not statistically significant.
Cromer et al.20 evaluated the evolution of bone mass in 370 adolescents aged 12–18 years, divided into users and non-users of OCs, over a period of 12 months. Users of medroxyprogesterone acetate exhibited an average reduction of 1.4% in lumbar spine BMD (p<0.001), while those using OC containing 20μgEE and 100μg levonorgestrel gained 2.3% of BMD in the lumbar spine. However, this increase was 3.8% in the control group. The increase in BMD found in users of OCs observed both in the study by Polatti et al.18 and in the present study is noteworthy; however, this increase was lower than that expected for the group of non-users of OC. These results suggest that the moderate gains in BMD in adolescents using low-dose OCs allow some bone mass gain, but not the peak bone mineralization necessary to serve as a “bone bank” for adulthood and old age.9,18–20
In addition to the effects related to estrogen, the impact of progestogens on bone mass should also be taken into consideration, with different underlying mechanisms. First, well-established effects confirm that high doses of progesterone act on the hypothalamus-pituitary-gonadal axis, causing hypoestrogenism, accelerated bone remodeling and rapid bone mass loss.21,22 Another possibility is that progesterone exerts a stimulatory effect through progesterone and androgen receptors.23 Progesterone and androgen receptors are expressed in osteoblasts and osteoclasts, and the activation of those two receptors has been demonstrated by an increase in bone mass, thus increasing osteoblast activity and reducing bone resorption resulting from osteoclast stimulation.18,24
Many progestogens committed to androgen receptors, such as drospirenone and desogestrel found in the OCs used in the present study, activate androgen receptors by competitive inhibition, thus blocking endogenous androgenic action. This mechanism of action results in important antiandrogenic effects on bone metabolism since testosterone plays a fundamental role in bone mass acquisition. In view of the small number of studies investigating the influence of progestogens present in OC formulations, it is difficult to distinguish whether the effects on bone metabolism arise from progesterone or from the reduced levels of estrogen.23,24
An increase in bone markers is observed during the period of growth acceleration in girls, corresponding to Tanner stage 2. Bone mass also increases, with the concentration peak of the markers coinciding with PHV observed in Tanner stage 3. Subsequently, at the time of growth deceleration, markers of bone metabolism decline, while bone mass continues to increase, probably due to elevated levels of sex steroids and insulin-like growth factor 1. This phenomenon was reported by Tobiume et al.25 In view of these considerations, and since all adolescents of the present sample were in late puberty, we believe that the reduction in concentrations of bone formation markers was influenced by the use of OCs. Thus, the bone marker results obtained in this study do not appear to be influenced only by age, but also by the different concentrations of EE in the two formulations.
With respect to bone formation markers, the present study demonstrated a reduction in the serum concentrations of these markers in the two groups after 12 months of OC use, with reductions of 20–37% in BAP and 40–86% in osteocalcin. Similar results were reported in the study by Paoletti et al.,26 which compared 28 women aged 20–30 years who used OC containing 30μg EE/3mg drospirenone with non-users of the same age range. Serum concentrations of the bone formation markers analyzed decreased by 20–30% in OC users, but remained unchanged in controls.
The reduction observed in the biomarkers of bone formation can be attributed to the use of OCs, which was confirmed by the demonstration of significant differences in BAP and osteocalcin levels in the adolescents after one year of follow-up.
The limitations of this study were the short follow-up period of OC users and the small number of participants. In addition, we had difficulties accompanying the adolescents in the control group, since a large contingent did not return on the scheduled dates to perform the proposed evaluations.
On the other hand, the strengths of the study are its prospective design and the fact that bone densitometry and bone biomarker measurements were performed by a single technician blinded to group assignment. Moreover, the use of the two formulations of low-dose OCs for one year was associated with a lower increase in bone mass, with BMD and BMC gains below those estimated for this age group. There is evidence that the introduction of these contraceptives in the second decade of life is related to consistently lower levels of bone formation markers, confirming the lower increase in bone mass demonstrated by densitometry. Following up these adolescents for a longer period of exposure to combined OCs is necessary to better understand the effects of these drugs on bone mass and bone formation biomarkers.
FundingFAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo). Grants (07/07731-0, 2011/05991-0, and 2015/04040-2) and Pro-Rector for Research at UNESP.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Rizzo AC, Goldberg TB, Biason TP, Kurokawa CS, Silva CC, Corrente JE, et al. One-year adolescent bone mineral density and bone formation marker changes through the use or lack of use of combined hormonal contraceptives. J Pediatr (Rio J). 2019;95:567–74.