Evaluate the association between inflammatory process, adiposity, and vitamins A, D, and E in adolescents, according to gender.
MethodsCross-sectional study with adolescents aged 12–19 years old of both genders attending public schools in Recife. A questionnaire was used to collect data on socioeconomic level, lifestyle, and food intake of adolescents. Then, an anthropometric evaluation and a blood sampling were performed to analyze serum concentrations of α-1-acid glycoprotein, retinol, β-carotene, α-tocopherol, and 25-hydroxy-vitamin D.
ResultsThe levels of α-1-acid glycoprotein were higher for abdominal obesity in both genders. Male adolescents with insufficient serum α-tocopherol levels had low levels of α-1-acid glycoprotein (p=0.03) and an increased risk of 25-hydroxy-vitamin D and β-carotene deficiency in relation to total and abdominal fat; female adolescents had an increased risk of insufficient β-carotene with abdominal obesity (PR: 1.33; 95% CI: 1.2–1.5).
ConclusionAbdominal adiposity implies a higher risk of inflammation and causes different changes to the levels of fat-soluble vitamins according to gender.
Avaliar a associação entre processo inflamatório, adiposidade e as vitaminas A, D e E em adolescentes, segundo o sexo.
MétodosEstudo transversal com adolescentes de 12 a 19 anos de ambos os sexos de escolas públicas de Recife. Foi utilizado um questionário para coleta de dados socioeconômicos de estilo de vida e de consumo alimentar dos adolescentes. Em seguida, realizou-se a avalição antropométrica e coleta de sangue para análise das concentrações séricas de α-1-glicoproteína ácida, retinol, β-caroteno, α-tocoferol e 25-hidroxivitamina D.
ResultadosOs níveis de α-1-glicoproteína ácida foram maiores na obesidade abdominal de ambos os sexos. Os meninos com níveis séricos insuficientes de α-tocoferol expressaram níveis reduzidos de α-1-glicoproteína ácida (p = 0,03) e apresentaram um maior risco de deficiência de 25-hidroxivitamina D e β-caroteno na adiposidade total e abdominal, enquanto as meninas mostraram maior risco de insuficiência de β-caroteno com a obesidade abdominal (RP 1,33; IC 95% 1,2-1,5).
ConclusãoA adiposidade abdominal reflete maior risco de inflamação e causa alterações distintas nas concentrações das vitaminas lipossolúveis, de acordo com o sexo.
Obesity has been considered the most important nutritional disorder1 and called a worldwide epidemic due to the high occurrence in children, adolescents, and young adults in recent years.2 Excess weight is implicated in a wide variety of health problems due to the increase in body fat proportion and disorders associated with the increased mechanical load of adipose tissue.3
Evidence suggests that, in the presence of obesity, there may be a decrease in the levels of fat-soluble vitamins in plasma, due to a lower intake of these nutrients and/or higher deposition in adipose tissue, which, because they are soluble in fat, are deposited in the adipocytes, thus decreasing their bioavailability in individuals with excess adiposity.4 In addition, vitamin-carrier proteins would behave as negative proteins in the acute phase of inflammation,5 thus decreasing their availability in plasma during the chronic inflammatory processes found in obesity.
It has been observed that obese children and adolescents show a higher risk of low levels of fat-soluble vitamins when compared to those with adequate weight.6,7 Since current nutritional recommendations do not consider the bioavailability of fat-soluble vitamins in the presence of obesity-related metabolic inflammation, a chronic inadequacy state for these micronutrients may place adolescents with excess weight at risk of nutritional disorder. Thus, the present study aimed to evaluate the association between inflammatory process, adiposity, and vitamins A, D, and E in adolescents, according to gender.
MethodsStudy design and sampleThis was a cross-sectional study of adolescents aged between 12 and 19 years, of both genders, carried out from March to April 2013, nested in a prospective cohort performed between 2007 and 2013, with randomly recruited adolescents using a multi-stage sampling process in public schools of Recife, northeastern Brazil. Adolescents receiving vitamin A, D, and E supplements or multivitamins in the last 3 months were excluded.
The sample size was based on an estimated prevalence (p) of α-tocopherol deficiency of 25%, a sampling error (d) of 5.5%, a 95% confidence level (z), and a design effect (c) of 2.1, considering that sampling was carried out by clusters. Using the formula n=(z2×p×q×c)/d2, corrected for a finite population, that resulted in a minimum sample of 370 individuals. To correct for any losses, a percentage of 11% [100/(100−11)] was added. The final sample comprised 411 adolescent students.
Evaluation methods and techniquesThe application of the food intake questionnaire used in the study, blood collection, and anthropometric measurements were performed by trained technicians who had knowledge of the procedures and study development routine.
Dietary variablesFood consumption was assessed by a semiquantitative food frequency questionnaire for adolescents (FFQA), developed and validated by Slater et al.,8 which was adapted for foods commonly used in the region. The analysis was performed through the DietSys software version 4.01 (National Cancer Institute – Bethesda, MD, USA). The results of vitamin A, D, and E consumption were compared with the dietary reference intakes (DRI's) proposed by the Institute of Medicine9,10 and classified according to the estimated average requirement (EAR). An adequate vitamin A intake for boys was considered: >630μg/day (9–13 years), >485mg/day (14–18 years); and vitamin A intake for girls: >625mg/day (9–13 years), >500μg/day (14–18 years); vitamin E intake: >12mg/day; and vitamin D >10μg/day.
Anthropometric variablesWeight and height measurements were carried out according to the original technique recommended by Lohman et al.,11 and waist circumference (WC) was obtained according to the criteria of Taylor et al.12 The adolescents’ nutritional diagnosis was defined according to the World Health Organization (WHO) body mass index (BMI) curves.13 The indicators BMI for age (BMI/A) and height for age (H/A), expressed in Z-scores, were used to classify the nutritional status of the students. For the H/A indicator, the following were considered: short stature:Z-score −2 and ≤1; overweight: >Z-score +1 and ≤+2; obesity: >Z-score +2.12 Overweight individuals were grouped with their obese peers, being classified in the excess weight category. The diagnosis of excess weight was obtained when it was greater than +1 SD. In the diagnosis of abdominal obesity, the cutoff point for the classification of WC was the one used by Taylor et al.,12 and the cutoff used for the waist-to-height ratio (WHtR) was that recommended by Li et al.14
Biochemical variablesA 10mL blood sample was collected for the biochemical analyses after a 10- to 12-hour fast. The tubes were stored and transported for sample processing at laboratory of clinical analyses (LAPAC), where the concentrations of α-1-acid glycoprotein (AGA) and 25-hydroxyvitamin D (25(OH)D) were analyzed. A serum aliquot (2mL) was frozen and then sent to the Micronutrient Investigation Center (CIMICRON) of the Universidade Federal da Paraíba (UFPB), to determine the serum levels of retinol, β-carotene, and α-tocopherol.
α-1-Acid glycoproteinAGA was quantified by immunoturbidimetry using Roche reagents (Roche™, USA), calibrators and controls, in an automated Cobas Mira system (Roche™, USA). The presence of inflammation was defined as a value >0.9g/L.
Retinol, β-carotene, and α-tocopherolThe quantification of serum retinol, β-carotene, and α-tocopherol levels followed the technical procedure described by Erhardt et al.15 The cutoff points developed by the WHO for retinol levels were used, considering: retinol deficiency when <0.70μmol/L. For α-tocopherol, ≥12μmol/L16 was used as reference, while β-carotene values were considered adequate when >0.9μmol/L.17
25-Hydroxy vitamin D25(OH)D levels were measured using the high-performance liquid chromatography (HPLC) method. 25(OH)D insufficiency was defined as levels <72.5nmol/L.18
Data analysisThe data were typed in duplicate and verified through VALIDATE, a module of Epi-Info, version 6.0 (WHO/CDC – Atlanta, GE, USA) to verify consistency during the typing process. Statistical analysis of the data was performed with SPSS version 13.0 (SPSS Inc. – Chicago, IL, USA).
Continuous variables were tested for normality using the Kolmogorov-Smirnov test to evaluate the symmetry of the distribution curve of the variables. Data from variables with normal distribution were expressed as mean and standard deviation. The variables with non-Gaussian distribution were presented as median and interquartile range (IQR). In the description of the proportions, the binomial distribution was approximated to the normal distribution by the 95% confidence interval (95% CI).
In the univariate analysis, the strength of the association was assessed by the prevalence ratio and respective confidence interval, and Pearson's chi-squared test. Student's t-test or its non-parametric equivalent, Mann Whitney U-test, was used for the comparison between continuous data from independent samples. All statistical tests considered a significance level of 5% to reject the null hypothesis.
Ethical aspectsThe study was approved by the Ethics Committee for Human Research of Lauro Wanderley University Hospital of Universidade Federal da Paraíba (CEP/HULW Registry number 723/10), based on the ethical principles for research involving human beings, as stated, at that time, in Resolution 196/96 of the National Health Council. Adolescents and their parents/guardians were previously informed of the research objectives, as well as the methods to be used. After giving their consent, the parents/guardians signed an informed consent.
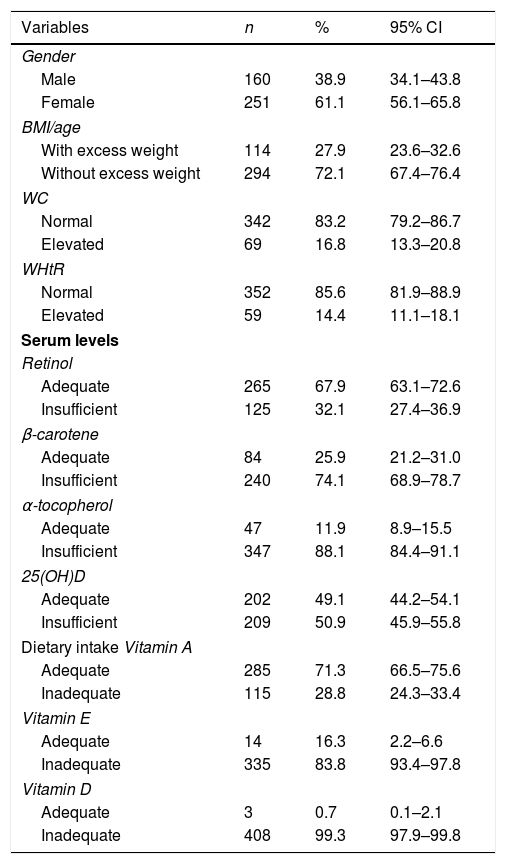
ResultsA total of 411 adolescents with a median age of 15 years (IQR: 14–16 years) participated in the study. According to the BMI/age, it was observed that 27.9% had excess weight, with 17.4% (n=71) being overweight and 10.5% (n=43) obese. When abdominal adiposity was evaluated, 16.8% and 14.4% had elevated WC and WHtR, respectively. Regarding serum levels of fat-soluble vitamins, most had low levels of retinol, α-tocopherol, β-carotene, and 25(OH)D. Inadequate values were also observed when evaluating the dietary intake of vitamins A, D, and E, as shown in Table 1.
Characteristics of school adolescents in Recife, Northeastern Brazil, 2013.
| Variables | n | % | 95% CI |
|---|---|---|---|
| Gender | |||
| Male | 160 | 38.9 | 34.1–43.8 |
| Female | 251 | 61.1 | 56.1–65.8 |
| BMI/age | |||
| With excess weight | 114 | 27.9 | 23.6–32.6 |
| Without excess weight | 294 | 72.1 | 67.4–76.4 |
| WC | |||
| Normal | 342 | 83.2 | 79.2–86.7 |
| Elevated | 69 | 16.8 | 13.3–20.8 |
| WHtR | |||
| Normal | 352 | 85.6 | 81.9–88.9 |
| Elevated | 59 | 14.4 | 11.1–18.1 |
| Serum levels | |||
| Retinol | |||
| Adequate | 265 | 67.9 | 63.1–72.6 |
| Insufficient | 125 | 32.1 | 27.4–36.9 |
| β-carotene | |||
| Adequate | 84 | 25.9 | 21.2–31.0 |
| Insufficient | 240 | 74.1 | 68.9–78.7 |
| α-tocopherol | |||
| Adequate | 47 | 11.9 | 8.9–15.5 |
| Insufficient | 347 | 88.1 | 84.4–91.1 |
| 25(OH)D | |||
| Adequate | 202 | 49.1 | 44.2–54.1 |
| Insufficient | 209 | 50.9 | 45.9–55.8 |
| Dietary intake Vitamin A | |||
| Adequate | 285 | 71.3 | 66.5–75.6 |
| Inadequate | 115 | 28.8 | 24.3–33.4 |
| Vitamin E | |||
| Adequate | 14 | 16.3 | 2.2–6.6 |
| Inadequate | 335 | 83.8 | 93.4–97.8 |
| Vitamin D | |||
| Adequate | 3 | 0.7 | 0.1–2.1 |
| Inadequate | 408 | 99.3 | 97.9–99.8 |
BMI, body mass index; WC, waist circumference; WHtR, waist-to-height ratio; CI, confidence interval; 25(OH)D, 25-hydroxy vitamin D, adequate serum retinol: >0.7μmol/L, adequate β-carotene: >0.9μmol/L; adequate α-tocopherol: >12μmol/L; adequate 25 (OH) D: >72.4 nmol/L. Estimated average requirement (EAR) of vitamin A for boys: >630μg/day (9–13 years), >485mg/day (14–18 years), vitamin A for girls: >625mg/day (9–13 years), >500μg/day (14–18 years); vitamin E >12mg/day; vitamin D >10μg/day.
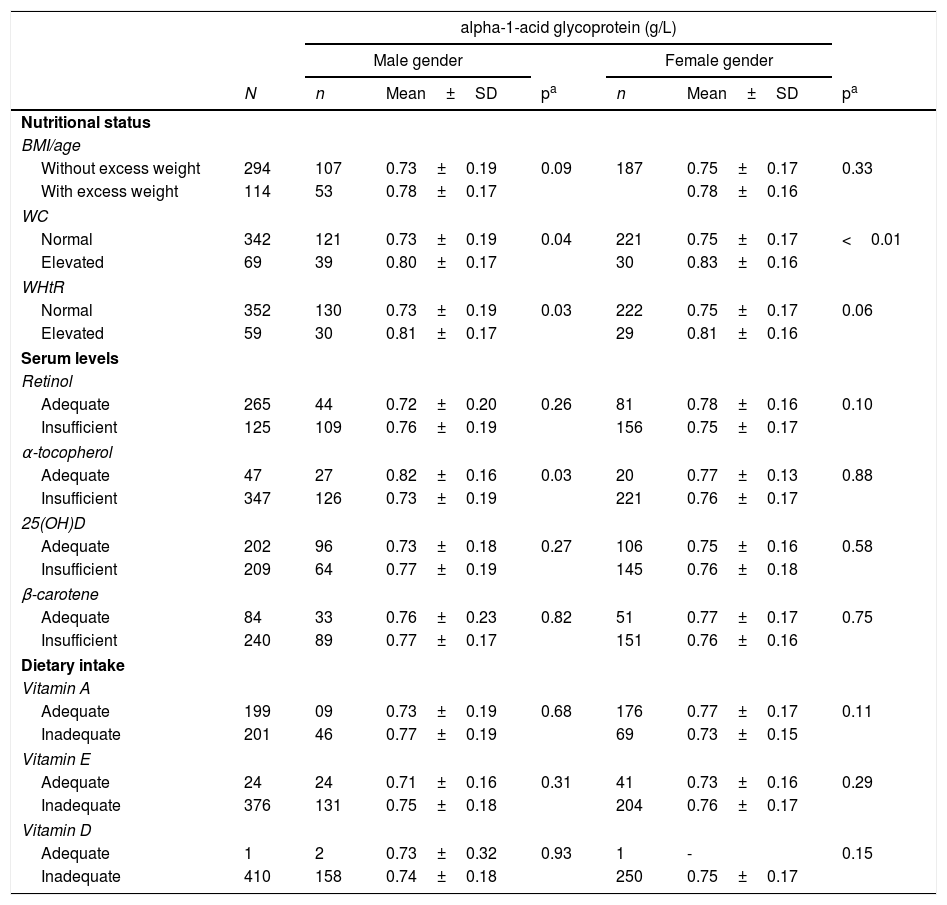
Regarding the inflammatory process, AGA did not show alterations in serum levels with the increase in total adiposity, but it was increased when male adolescents were classified as having abdominal obesity using the WC (p=0.04) and WHtR (p=0.03), whereas girls had their levels increased only with elevated WC (p<0.01; Table 2). Regarding vitamin concentrations, AGA showed a reduction in its levels in the presence of insufficient amounts of serum α-tocopherol in male adolescents (p=0.03). Regarding dietary intake, AGA did not show a significant difference with variations in the intake of fat-soluble vitamins.
Serum levels of alpha-1-acid glycoprotein according to nutritional status, serum concentrations and dietary intake of fat-soluble vitamins in adolescent students from Recife, Northeast Brazil, 2013.
| alpha-1-acid glycoprotein (g/L) | |||||||
|---|---|---|---|---|---|---|---|
| Male gender | Female gender | ||||||
| N | n | Mean±SD | pa | n | Mean±SD | pa | |
| Nutritional status | |||||||
| BMI/age | |||||||
| Without excess weight | 294 | 107 | 0.73±0.19 | 0.09 | 187 | 0.75±0.17 | 0.33 |
| With excess weight | 114 | 53 | 0.78±0.17 | 0.78±0.16 | |||
| WC | |||||||
| Normal | 342 | 121 | 0.73±0.19 | 0.04 | 221 | 0.75±0.17 | <0.01 |
| Elevated | 69 | 39 | 0.80±0.17 | 30 | 0.83±0.16 | ||
| WHtR | |||||||
| Normal | 352 | 130 | 0.73±0.19 | 0.03 | 222 | 0.75±0.17 | 0.06 |
| Elevated | 59 | 30 | 0.81±0.17 | 29 | 0.81±0.16 | ||
| Serum levels | |||||||
| Retinol | |||||||
| Adequate | 265 | 44 | 0.72±0.20 | 0.26 | 81 | 0.78±0.16 | 0.10 |
| Insufficient | 125 | 109 | 0.76±0.19 | 156 | 0.75±0.17 | ||
| α-tocopherol | |||||||
| Adequate | 47 | 27 | 0.82±0.16 | 0.03 | 20 | 0.77±0.13 | 0.88 |
| Insufficient | 347 | 126 | 0.73±0.19 | 221 | 0.76±0.17 | ||
| 25(OH)D | |||||||
| Adequate | 202 | 96 | 0.73±0.18 | 0.27 | 106 | 0.75±0.16 | 0.58 |
| Insufficient | 209 | 64 | 0.77±0.19 | 145 | 0.76±0.18 | ||
| β-carotene | |||||||
| Adequate | 84 | 33 | 0.76±0.23 | 0.82 | 51 | 0.77±0.17 | 0.75 |
| Insufficient | 240 | 89 | 0.77±0.17 | 151 | 0.76±0.16 | ||
| Dietary intake | |||||||
| Vitamin A | |||||||
| Adequate | 199 | 09 | 0.73±0.19 | 0.68 | 176 | 0.77±0.17 | 0.11 |
| Inadequate | 201 | 46 | 0.77±0.19 | 69 | 0.73±0.15 | ||
| Vitamin E | |||||||
| Adequate | 24 | 24 | 0.71±0.16 | 0.31 | 41 | 0.73±0.16 | 0.29 |
| Inadequate | 376 | 131 | 0.75±0.18 | 204 | 0.76±0.17 | ||
| Vitamin D | |||||||
| Adequate | 1 | 2 | 0.73±0.32 | 0.93 | 1 | - | 0.15 |
| Inadequate | 410 | 158 | 0.74±0.18 | 250 | 0.75±0.17 | ||
Student's t-test.BMI, body mass index; WC, waist circumference; WHtR, waist-to-height ratio; adequate serum retinol: >0.7μmol/L, adequate β-carotene: >0.9μmol/L; adequate α-tocopherol: >12μmol/L; adequate 25(OH)D: >72.4 nmol/L. Estimated average requirement (EAR) of vitamin A for boys: >630μg/day (9–13 years), >485mg/day (14–18 years), vitamin A for girls: >625mg/day (9–13 years), >500μg/day (14–18 years); vitamin E >12mg/day; vitamin D >10μg/day.
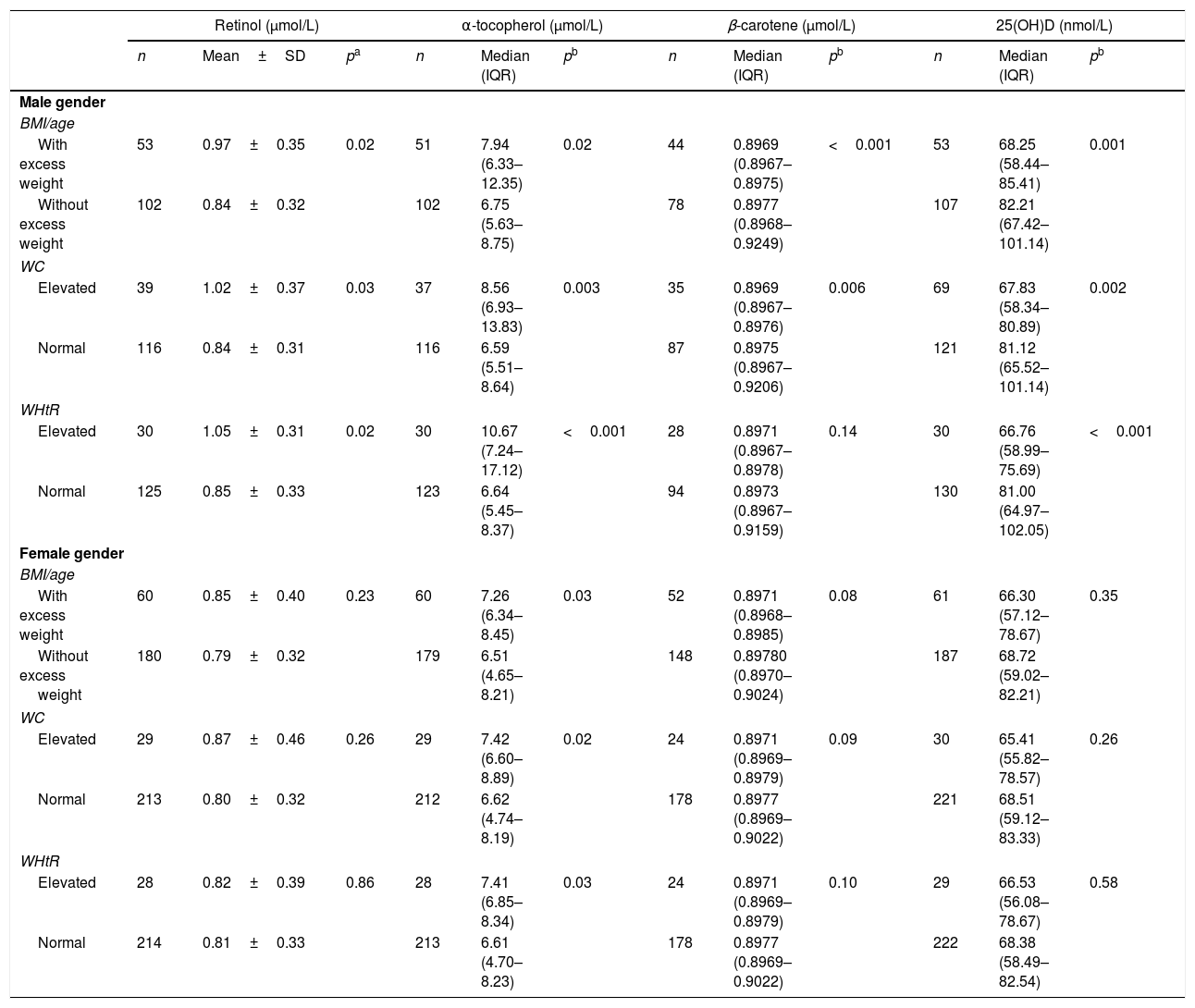
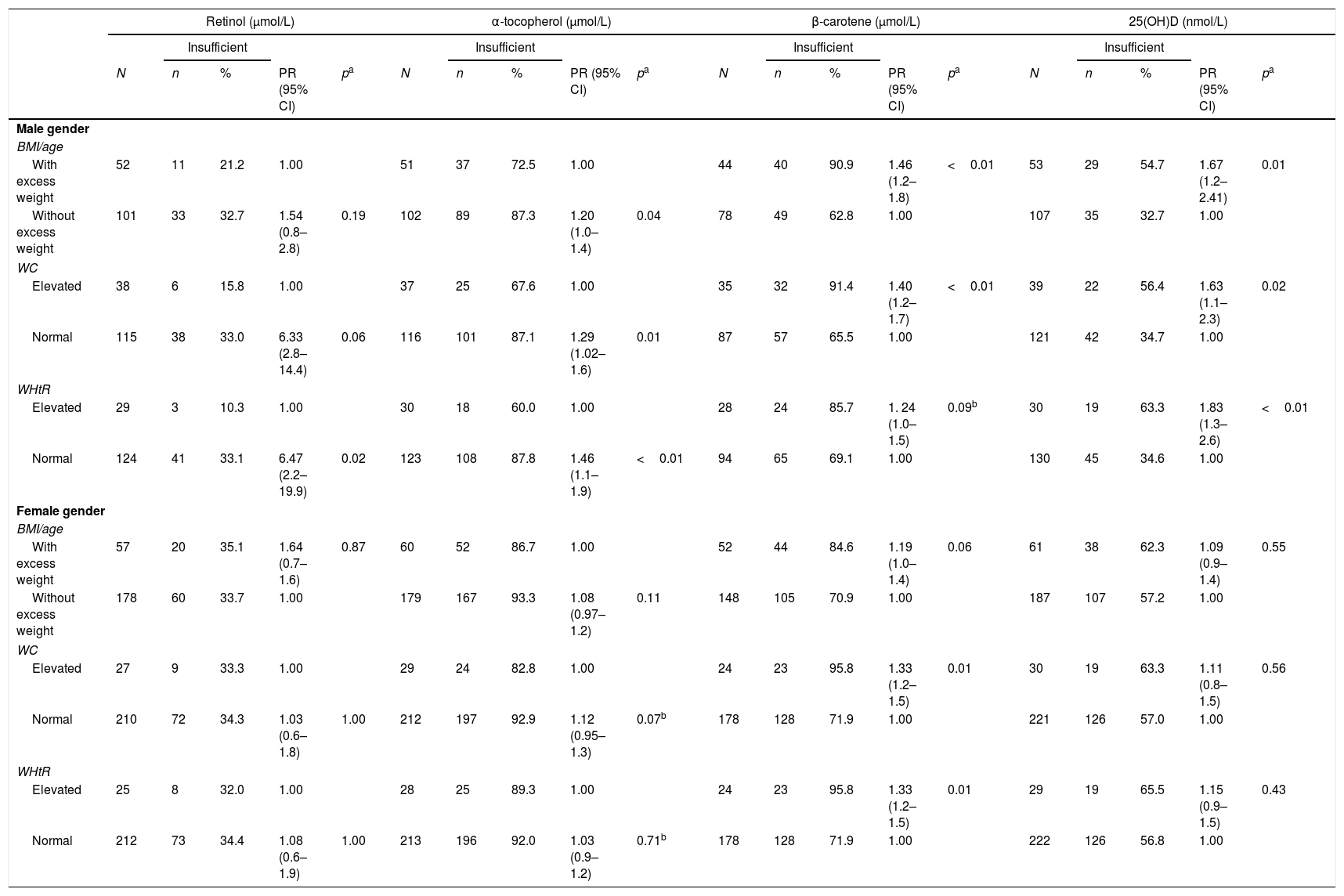
When evaluating the fat-soluble vitamin levels according to the nutritional status, boys showed an increase in retinol and α-tocopherol levels with increased adiposity, as well as a decrease in β-carotene and 25(OH)D levels. As for girls, only α-tocopherol showed significantly higher levels with higher adiposity, as shown in Table 3. When evaluating the association between adiposity type and vitamin levels, boys showed a higher risk of 25(OH)D and β-carotene deficiency in total and abdominal adiposity; however, when adiposity was absent, they showed a risk of retinol and α-tocopherol deficiency (Table 4). Girls, however, only showed the risk of β-carotene insufficiency when WC (PR=1.33) and WHtR (PR=1.33) were elevated.
Serum values of retinol, α-tocopherol, β-carotene and 25 hydroxy-vitamin D, according to body mass index, waist circumference and waist-to-height ratio in adolescent students from Recife, Northeastern Brazil, 2013.
| Retinol (μmol/L) | α-tocopherol (μmol/L) | β-carotene (μmol/L) | 25(OH)D (nmol/L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean±SD | pa | n | Median (IQR) | pb | n | Median (IQR) | pb | n | Median (IQR) | pb | |
| Male gender | ||||||||||||
| BMI/age | ||||||||||||
| With excess weight | 53 | 0.97±0.35 | 0.02 | 51 | 7.94 (6.33–12.35) | 0.02 | 44 | 0.8969 (0.8967–0.8975) | <0.001 | 53 | 68.25 (58.44–85.41) | 0.001 |
| Without excess weight | 102 | 0.84±0.32 | 102 | 6.75 (5.63–8.75) | 78 | 0.8977 (0.8968–0.9249) | 107 | 82.21 (67.42–101.14) | ||||
| WC | ||||||||||||
| Elevated | 39 | 1.02±0.37 | 0.03 | 37 | 8.56 (6.93–13.83) | 0.003 | 35 | 0.8969 (0.8967–0.8976) | 0.006 | 69 | 67.83 (58.34–80.89) | 0.002 |
| Normal | 116 | 0.84±0.31 | 116 | 6.59 (5.51–8.64) | 87 | 0.8975 (0.8967–0.9206) | 121 | 81.12 (65.52–101.14) | ||||
| WHtR | ||||||||||||
| Elevated | 30 | 1.05±0.31 | 0.02 | 30 | 10.67 (7.24–17.12) | <0.001 | 28 | 0.8971 (0.8967–0.8978) | 0.14 | 30 | 66.76 (58.99–75.69) | <0.001 |
| Normal | 125 | 0.85±0.33 | 123 | 6.64 (5.45–8.37) | 94 | 0.8973 (0.8967–0.9159) | 130 | 81.00 (64.97–102.05) | ||||
| Female gender | ||||||||||||
| BMI/age | ||||||||||||
| With excess weight | 60 | 0.85±0.40 | 0.23 | 60 | 7.26 (6.34–8.45) | 0.03 | 52 | 0.8971 (0.8968–0.8985) | 0.08 | 61 | 66.30 (57.12–78.67) | 0.35 |
| Without excess weight | 180 | 0.79±0.32 | 179 | 6.51 (4.65–8.21) | 148 | 0.89780 (0.8970–0.9024) | 187 | 68.72 (59.02–82.21) | ||||
| WC | ||||||||||||
| Elevated | 29 | 0.87±0.46 | 0.26 | 29 | 7.42 (6.60–8.89) | 0.02 | 24 | 0.8971 (0.8969–0.8979) | 0.09 | 30 | 65.41 (55.82–78.57) | 0.26 |
| Normal | 213 | 0.80±0.32 | 212 | 6.62 (4.74–8.19) | 178 | 0.8977 (0.8969–0.9022) | 221 | 68.51 (59.12–83.33) | ||||
| WHtR | ||||||||||||
| Elevated | 28 | 0.82±0.39 | 0.86 | 28 | 7.41 (6.85–8.34) | 0.03 | 24 | 0.8971 (0.8969–0.8979) | 0.10 | 29 | 66.53 (56.08–78.67) | 0.58 |
| Normal | 214 | 0.81±0.33 | 213 | 6.61 (4.70–8.23) | 178 | 0.8977 (0.8969–0.9022) | 222 | 68.38 (58.49–82.54) | ||||
BMI, body mass index; WC, waist circumference; WHtR, waist-to-height ratio; SD, standard deviation; IQR, interquartile range.
Factors associated with inadequate serum levels of retinol, α-tocopherol, β-carotene, and 25-hydroxy-vitamin D in adolescent students from Recife, Northeastern Brazil, 2013.
| Retinol (μmol/L) | α-tocopherol (μmol/L) | β-carotene (μmol/L) | 25(OH)D (nmol/L) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Insufficient | Insufficient | Insufficient | Insufficient | |||||||||||||||||
| N | n | % | PR (95% CI) | pa | N | n | % | PR (95% CI) | pa | N | n | % | PR (95% CI) | pa | N | n | % | PR (95% CI) | pa | |
| Male gender | ||||||||||||||||||||
| BMI/age | ||||||||||||||||||||
| With excess weight | 52 | 11 | 21.2 | 1.00 | 51 | 37 | 72.5 | 1.00 | 44 | 40 | 90.9 | 1.46 (1.2–1.8) | <0.01 | 53 | 29 | 54.7 | 1.67 (1.2–2.41) | 0.01 | ||
| Without excess weight | 101 | 33 | 32.7 | 1.54 (0.8–2.8) | 0.19 | 102 | 89 | 87.3 | 1.20 (1.0–1.4) | 0.04 | 78 | 49 | 62.8 | 1.00 | 107 | 35 | 32.7 | 1.00 | ||
| WC | ||||||||||||||||||||
| Elevated | 38 | 6 | 15.8 | 1.00 | 37 | 25 | 67.6 | 1.00 | 35 | 32 | 91.4 | 1.40 (1.2–1.7) | <0.01 | 39 | 22 | 56.4 | 1.63 (1.1–2.3) | 0.02 | ||
| Normal | 115 | 38 | 33.0 | 6.33 (2.8–14.4) | 0.06 | 116 | 101 | 87.1 | 1.29 (1.02–1.6) | 0.01 | 87 | 57 | 65.5 | 1.00 | 121 | 42 | 34.7 | 1.00 | ||
| WHtR | ||||||||||||||||||||
| Elevated | 29 | 3 | 10.3 | 1.00 | 30 | 18 | 60.0 | 1.00 | 28 | 24 | 85.7 | 1. 24 (1.0–1.5) | 0.09b | 30 | 19 | 63.3 | 1.83 (1.3–2.6) | <0.01 | ||
| Normal | 124 | 41 | 33.1 | 6.47 (2.2–19.9) | 0.02 | 123 | 108 | 87.8 | 1.46 (1.1–1.9) | <0.01 | 94 | 65 | 69.1 | 1.00 | 130 | 45 | 34.6 | 1.00 | ||
| Female gender | ||||||||||||||||||||
| BMI/age | ||||||||||||||||||||
| With excess weight | 57 | 20 | 35.1 | 1.64 (0.7–1.6) | 0.87 | 60 | 52 | 86.7 | 1.00 | 52 | 44 | 84.6 | 1.19 (1.0–1.4) | 0.06 | 61 | 38 | 62.3 | 1.09 (0.9–1.4) | 0.55 | |
| Without excess weight | 178 | 60 | 33.7 | 1.00 | 179 | 167 | 93.3 | 1.08 (0.97–1.2) | 0.11 | 148 | 105 | 70.9 | 1.00 | 187 | 107 | 57.2 | 1.00 | |||
| WC | ||||||||||||||||||||
| Elevated | 27 | 9 | 33.3 | 1.00 | 29 | 24 | 82.8 | 1.00 | 24 | 23 | 95.8 | 1.33 (1.2–1.5) | 0.01 | 30 | 19 | 63.3 | 1.11 (0.8–1.5) | 0.56 | ||
| Normal | 210 | 72 | 34.3 | 1.03 (0.6–1.8) | 1.00 | 212 | 197 | 92.9 | 1.12 (0.95–1.3) | 0.07b | 178 | 128 | 71.9 | 1.00 | 221 | 126 | 57.0 | 1.00 | ||
| WHtR | ||||||||||||||||||||
| Elevated | 25 | 8 | 32.0 | 1.00 | 28 | 25 | 89.3 | 1.00 | 24 | 23 | 95.8 | 1.33 (1.2–1.5) | 0.01 | 29 | 19 | 65.5 | 1.15 (0.9–1.5) | 0.43 | ||
| Normal | 212 | 73 | 34.4 | 1.08 (0.6–1.9) | 1.00 | 213 | 196 | 92.0 | 1.03 (0.9–1.2) | 0.71b | 178 | 128 | 71.9 | 1.00 | 222 | 126 | 56.8 | 1.00 | ||
BMI, body mass index; WC, waist circumference; WHtR, waist-to-height ratio; PR, prevalence ratio; CI, Confidence Interval; Adequate serum retinol >0.7μmol/L, adequate β-carotene: >0.9μmol/L; adequate α-tocopherol: >12μmol/L; adequate 25(OH)D: >72.4 nmol/L.
The high prevalence of excess weight among adolescents reflects the magnitude of the problem in this age group. Although body fat is related to some metabolic complications, several consequences of obesity are more strongly associated with the amount of abdominal fat19; this adipose deposition plays a key role and may induce health risks in overweight and obese individuals.20 It is postulated that obese individuals have persistent inflammation of the adipose tissue, and the proinflammatory cytokines, associated with the adipose tissue accumulation, would explain the development of obesity-related pathologies.21 In the evaluation between inflammation and adiposity, it was observed that AGA showed higher concentrations only in the presence of abdominal obesity in both genders, and no difference was observed in total adiposity. There are discordant findings in the literature, since there are reports of elevated AGA in adolescents classified as overweight and obese according to the BMI.22 Alfadda et al.23 observed that AGA mRNA expression would be correlated with TNF-α and adiponectin expression of subcutaneous adipose tissue, whereas in the visceral adipose tissue this expression is correlated with TNF-α, IL-6, and adiponectin, demonstrating greater AGA expression with abdominal fat deposition. This association could partly justify the present findings, since perhaps the greatest inducer for AGA elevation would be the inflammatory cytokines released by visceral adipose tissue. Additionally, when using only the BMI to classify obesity, a misdiagnosis may occur, because it is not sensitive in predicting the area of fat deposition or if the increase in body mass consists in greater amounts of muscle or fat.
The literature shows that, in the presence of inflammation, the levels of retinol,24 vitamin E,25 and vitamin D6 are significantly lower. Plasma concentrations of micronutrients are at least in part mediated by proinflammatory cytokines that suppress the hepatic production of many carrier proteins, increase capillary permeability, and promote the sequestration of some micronutrients to the liver and other organs.26
In the present study, it was not possible to evaluate the vitamins according to the presence of the inflammatory process, since none of the assessed adolescents had inflammation according to the cutoff point established for AGA. Nonetheless, when evaluating the mean concentration of AGA according to vitamin status, it was observed that male adolescents with the highest levels of AGA had adequate α-tocopherol values. According to previously mentioned reports, it would be expected to find an inverse association between inflammation and α-tocopherol. However, Ulatowski et al.27 reported that oxidative stress may increase the expression of the α-tocopherol transfer protein (α-TTP) gene, because as this vitamin is the main fat-soluble antioxidant, an increase in the distribution of vitamin E would prevent further oxidative damage.
When assessing vitamin levels in the presence of excess weight according to gender, boys with abdominal obesity showed an increase in retinol and α-tocopherol, while β-carotene and 25(OH)D were reduced. In girls, only α-tocopherol showed increased levels in the presence of central adiposity. These results demonstrate that increased abdominal adiposity could have a distinct influence on vitamin levels according to gender, possibly due to variations in hormone action mechanisms on the effects of obesity-mediated inflammation.
In an attempt to elucidate the association between excess adiposity, gender, inflammation, and endocrine signaling, Bloor and Symonds,28 through a literature review, described an increase in proinflammatory cytokines that occurs in obesity, with a subsequent activation of the hypothalamic-pituitary-adrenal (HPA) axis, which increases cortisol and contributes to increased deposition of visceral adipose tissue in males. Increased central adiposity also occurs in females; however, there is a gradual transition, with a decreased deposition of subcutaneous adipose tissue in the lower and upper limbs to the development of central adiposity, which gives females protection against the inflammatory process, as it prevents the visceral deposit from acting as an increased stress signaling factor in the HPA.
Therefore, boys would be more prone to triglyceride accumulation in adipocytes in the central region, leading to hypertrophy and increased production and secretion of adipokines in circulation, since adipokine gene expression is positively correlated with adipocyte size.29 As a consequence of the inflammation, there would be initially a greater mobilization of antioxidant vitamins in an attempt to fight oxidative stress, which could justify the increase in retinol and α-tocopherol levels. As for β-carotene, as in addition to having an antioxidant function, it would also be diverted to conversion into vitamin A in the liver, and it would probably have reduced levels in the presence of the inflammatory process. This increased demand for β-carotene could explain the greater risk of β-carotene insufficiency found in adolescents of both genders with abdominal obesity.
The 25(OH)D, on the other hand, may have its levels reduced by increased storage in adipose tissue.4 Moreover, plasma levels of 25(OH)D have been reported to be independently associated with CRP (C-reactive protein) and albumin,30 i.e., in the presence of systemic inflammation, acute-phase negative proteins such as albumin are reduced, which would decrease the transport of this vitamin into the bloodstream.
The approach of the studies carried out in adolescents found in the literature shows the absence of the association between fat-soluble vitamin levels and the inflammatory process. However, Duncan et al.,31 when evaluating the correlation coefficients of CRP in comparison to several micronutrients (including vitamins A, D, and E) in the plasma of adult individuals, found a poor correlation, indicating that the association is highly variable from patient to patient, suggesting that the interpretation of plasma micronutrient deficiency should only be performed when the presence of inflammation is ruled out.
Thus, the present study demonstrated that excess weight in adolescents may put these individuals at risk of metabolic inflammation when fat accumulation occurs in the abdominal region. Additionally, the increase in abdominal adiposity occurred with distinct alterations in the serum levels of fat-soluble vitamins according to gender.
Although there was no direct association between inflammation and the assessed vitamins, it is suggested that factors such as obesity and inflammation be considered in the biochemical interpretation of fat-soluble vitamins, to prevent the risk of data misinterpretation. However, further studies are required to better understand the mechanisms involved between the chronic inflammatory process present in obesity and the behavior of fat-soluble vitamins, their metabolic pathways, and their action mechanism in this process, considering the difference between genders.
As for the present study limitations, because it was a cross-sectional study, it was not possible to guarantee causal associations, and confounding variables may have affected the reported associations. Additionally, only one biochemical marker was used to monitor the presence of inflammation, i.e., AGA levels, which despite being considered an inflammatory marker of greater clinical validity, may not show greater diagnostic discriminatory power in specific inflammatory situations.
FundingThis study was funded by Conselho Nacional de Pesquisa (CNPq) (process No. 473387/2010-2) and the Ministry of Science and Technology (IMIP/MCT agreement; process No. 01.0265.00/2005).
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Paes-Silva RP, Gadelha PC, Lemos MC, Castro CM, Arruda IK, Diniz AS. Adiposity, inflammation and fat-soluble vitamins in adolescents. J Pediatr (Rio J). 2019;95:575–83.