To determine the prevalence of congenital hypothyroidism in children with filter-paper blood-spot TSH (b-TSH) between 5 and 10μIU/mL in the neonatal screening.
MethodsThis was a retrospective study including children screened from 2003 to 2010, with b-TSH levels between 5 and 10μIU/mL, who were followed-up during the first two years of life when there was no serum TSH normalization. The diagnosis of congenital hypothyroidism was defined as serum TSH ≥10μIU/mL and start of levothyroxine treatment up to 2 years of age.
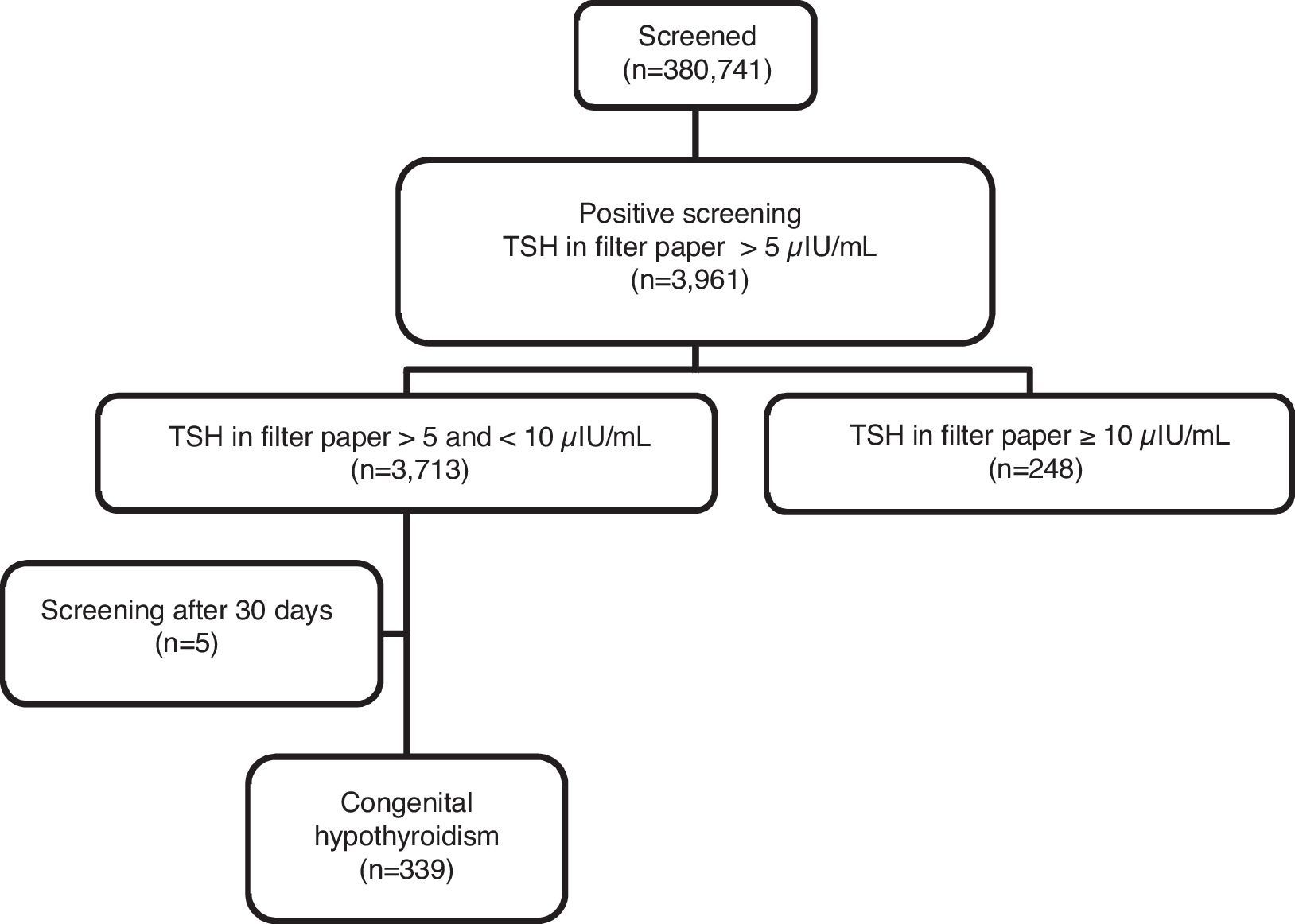
ResultsOf the 380,741 live births, 3713 (1.04%) had filter paper TSH levels between 5 and 10μIU/mL and, of these, 339 (9.13%) had congenital hypothyroidism. Of these, 76.11% of the cases were diagnosed in the first three months of life and 7.96% between 1 and 2 years of age.
ConclusionThe study showed that 9.13% of the children with b-TSH levels between 5 and 10μIU/mL developed hypothyroidism and that in approximately one-quarter of them, the diagnosis was confirmed only after the third month of life. Based on these findings, the authors suggest the use of a 5μIU/mL cutoff for b-TSH levels and long-term follow-up of infants whose serum TSH has not normalized to rule out congenital hypothyroidism.
Determinar a prevalência de hipotireoidismo congênito em crianças com TSH em papel filtro (TSH-f) entre 5 e 10μUI/mL na triagem neonatal.
MétodosEstudo retrospectivo incluindo crianças triadas de 2003 a 2010, com TSH-f entre 5 e 10μUI/mL, que foram acompanhadas nos dois primeiros anos de vida quando não houve normalização do TSH sérico. O diagnóstico de hipotireoidismo congênito foi definido como TSH sérico igual ou superior a 10μUI/mL e início de tratamento com levotiroxina até os dois anos de idade.
ResultadosDos 380.741 nascidos vivos triados, 3.713 (1,04%) apresentaram TSH-f entre 5 e 10μUI/mL e, destes, 339 (9,13%) tinham hipotireoidismo congênito. Destes, 76,11% dos casos foram diagnosticados nos primeiros três meses de vida e 7,96% entre um e dois anos de idade.
ConclusãoO estudo mostra que 9,13% das crianças com TSH-f entre 5 e 10μUI/mL desenvolveram hipotireoidismo e que em cerca de um quarto delas o diagnóstico só se confirmou após o terceiro mês de vida. Com base nestes achados, sugere-se a utilização do ponto de corte de TSH-f de 5μUI/mL e o acompanhamento em longo prazo dos lactentes cujo TSH sérico não tenha se normalizado para descartar o hipotireoidismo congênito.
Congenital hypothyroidism (CH), the most frequent congenital endocrine disorder and one of the main causes of preventable intellectual disability with early diagnosis and adequate treatment, was one of the first diseases screened in neonatal screening (NS) programs.1–4
The incidence of CH before the creation of NS programs was estimated at 1:6500,5 but soon after the start of NS programs, it increased to approximately 1:3000 to 1:4000 live births (LB).6 In recent years, there has been a further increase in CH incidence in several parts of the world, ranging from 1:1030 to 1:2679 LB.7–10 This fact is probably associated with an increase in the survival of preterm newborns,4,7 environmental11 and ethnic factors,7 as well as the reduction in the cutoff values of thyroid-stimulating hormone (TSH) on filter-paper blood-spot (b-TSH) in NS programs.4,12
Initially, higher b-TSH cutoff values were adopted to avoid recalls and excessive costs, with the justification that mild forms of CH would have no consequences for the neurological development.4 However, some authors suggested that there were insufficient studies to support this hypothesis,4 and the European Consensus on Congenital Hypothyroidism of 2014 highlighted, as the primary objective of NS, the detection of all cases of primary CH.1
In recent years, several screening services have chosen to lower the cutoff value of b-TSH to reduce the number of cases of undiagnosed CH.4,13–17
With this reduction, several programs reported an increase in the number of children diagnosed with CH through NS programs,4,13–17 although there has also been an increase in the number of children with suspected CH and, therefore, a higher rate of recall.4,15,16,18
Since 2001, the Neonatal Screening Program of Universidade Estadual de Campinas (UNICAMP) has used a cutoff of 5μIU/mL for b-TSH and has performed clinical and laboratory follow-up for at least the first two years of life of all children whose serum TSH levels do not normalize.
Considering this context, the aim of the present study was to determine the prevalence of CH in children with b-TSH levels between 5 and 10μIU/mL in the NS.
MethodsNeonatal screening strategy for congenital hypothyroidismUNICAMP'S Neonatal Screening Reference Service (Serviço de Referência em Triagem Neonatal [SRTN]) is currently responsible for neonatal screening, diagnosis, and comprehensive care of children in the VII (Campinas) and XIV (São João da Boa Vista) Regional Health Departments, regions that have approximately 5000 LB per month.
Since its creation, UNICAMP SRTN has worked with the same team for the treatment and follow-up of the cases, and the laboratory and imaging investigations have always been performed at the services of UNICAMP hospital complex.
UNICAMP SRTN uses the cutoff value for b-TSH of 5μIU/mL in a dried whole-blood sample on filter paper, equivalent to 11μIU/mL in serum, and the analyses are performed by time-resolved fluorometry (AutoDELFIA–Perkin Elmer Life Sciences, MA, USA).
UNICAMP SRTN uses the following protocol for the diagnosis and treatment of CH: children with b-TSH >5μIU/mL or ≤0.01μIU/mL are called in for serum TSH and free thyroxine (T4L) measurement. If serum TSH and T4L levels are normal, the children are discharged from the service. Children with serum TSH values higher than the reference values for age, but lower than 10μIU/mL, are monitored through clinical and laboratory examinations in UNICAMP SRTN for the first two years of life or until test normalization.
Those with serum TSH ≥10μIU/mL are referred for follow-up at the Congenital Hypothyroidism Outpatient Clinic of Hospital de Clínicas of UNICAMP, where they are evaluated individually; and those who persist with TSH >10μIU/mL or have T4L levels <0.9ng/dL are diagnosed as having CH and start treatment with sodium levothyroxine (L-T4).
Serum TSH and T4L collections are performed according to the technical standards of the Clinical Pathology Laboratory of UNICAMP; the analyses are performed by electrochemiluminescence, using TSH and T4L reference values of 0.41–4.5μIU/mL and 0.9–1.8ng/dL, respectively.
Study designA retrospective study was carried out using the UNICAMP SRTN database to obtain the number of LB screened from April 2003 to September 2009. Children with b-TSH values between 5 and 10μIU/mL in samples collected in the first month of life were selected.
Primary CH was considered when the child had serum TSH ≥10μIU/mL at any time during the first two years of life, regardless of T4L concentration, and received treatment with L-T4.
The following information was obtained from the medical files of children with CH and b-TSH levels between 5 and 10μIU/mL: b-TSH value; age at NS collection; gender; initial, confirmatory TSH and T4L levels, as well as levels at the start of treatment; and age at the start of treatment.
The following definitions of serum TSH and T4L values were considered for the study: initial test (the first test collected after b-TSH); confirmatory test (the first test for which serum TSH was ≥10μIU/mL for each child); and test at the start of treatment (the last test collected prior to medication introduction).
Statistical analysisData were processed using the software SPSS, (SPSS Inc. version 16.0, Chicago, IL, USA). The results of the qualitative variables are shown as absolute and relative frequencies.
For the TSH and T4L measurements, the median, minimum, and maximum values, range (total range), and interquartile range (IQR) were determined. When assessing TSH concentrations, the use of medians was chosen to avoid upper limit problems (>100μIU/mL) for some results.
The results of the quantitative variables were expressed as mean±standard deviation or median (IQR).
The prevalence of CH was determined based on the data of the study group with the respective 95% confidence interval (CI). The Mann–Whitney test was used for comparisons in relation to gender. A p-value <0.05 was considered significant in all analyses.
Ethical aspectsThis project was approved by the Research Ethics Committee of Faculdade de Ciências Médicas of UNICAMP.
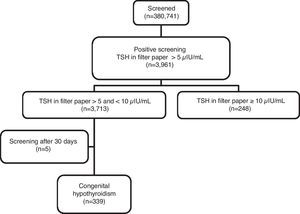
ResultsIn the period from April 2003 to September 2009, 380,741 LB were screened for CH through UNICAMP SRTN. A total of 3,961 newborns were enrolled to complement the investigation, resulting in a recall frequency of 1.04% (95% CI: 1.01–1.07). Of these, 248 (6.26%) had b-TSH ≥10μIU/mL and 3,713 (93.74%) had b-TSH between 5 and 10μIU/mL (Fig. 1). Using the cutoff value of 10μIU/mL, the recall rate would have been 0.07% (95% CI: 0.06–0.08).
After clinical and laboratory follow-up of the 3713 children with b-TSH levels between 5 and 10μIU/mL, 339 (206 males and 133 females) were diagnosed with CH and started hormone replacement therapy with L-T4, which corresponds to a CH prevalence of 9.13% (95% CI: 8.2–10.1; Fig. 1).
The mean and median ages at b-TSH collection were 5±4 days of life and 3 (4) days of life, respectively, and at the initial serum collection, 24±14 days of life and 20 (13) days of life. The confirmatory examination was collected at a mean age of 77±121 days of life and a median of 28 (48) days of life.
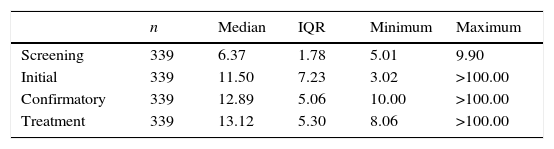
As shown in Table 1, despite borderline values of b-TSH in the NS (5.01–9.90μUI/mL), TSH values at baseline showed a large variation in the studied group (3.02 to >100μIU/mL). The initial TSH of 113 children was <10μIU/mL, with a median of 7.00 (2.75) μIU/mL. Confirmatory TSH in this group ranged from 10.00 to >100.00μIU/mL, with a median of 11.50 (2.14) μIU/mL. The remaining 226 cases had an initial TSH≥10μIU/mL, of which 190 initiated treatment immediately; the remaining 36 patients, all with initial TSH<30μIU/mL and normal or high T4L levels, were maintained without medication at the first moment, with later introduction of hormonal replacement due to the persistence of TSH elevation.
TSHa in neonatal screening and initial, confirmatory, and after start of treatment exams.
| n | Median | IQR | Minimum | Maximum | |
|---|---|---|---|---|---|
| Screening | 339 | 6.37 | 1.78 | 5.01 | 9.90 |
| Initial | 339 | 11.50 | 7.23 | 3.02 | >100.00 |
| Confirmatory | 339 | 12.89 | 5.06 | 10.00 | >100.00 |
| Treatment | 339 | 13.12 | 5.30 | 8.06 | >100.00 |
n, number of cases; IQR, interquartile range.
Confirmatory serum TSH levels and levels at the start of treatment also showed significant variation (Table 1), and 63 (18.58%) of the children had TSH levels>20μIU/mL at the start of the treatment.
No differences were observed in relation to gender regarding the values of b-TSH (p=0.073) and initial (p=0.777) and confirmatory (p=0.376) TSH levels, as well as levels at the start of treatment (p=0.843).
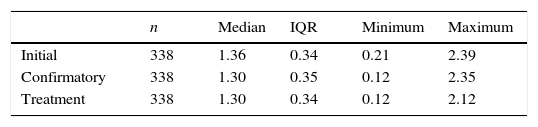
It is noteworthy that 15 of the 339 children presented T4L levels below the reference values in the first evaluation, with 14 of them showing TSH values >15μIU/mL and only one, with a TSH value of 6.06μIU/mL at the first collection, showed elevation of TSH levels above 10μIU/mL in subsequent collections. Regarding the last laboratory evaluation performed prior to the start of treatment, 17 of the 339 had T4L levels below the reference value, whereas TSH ranged from 10μIU/mL to >100μIU/mL; in 13 of these 17 patients, the values were >20μIU/mL (Table 2).
T4La in the initial, confirmatory, and after start of treatment exams.
| n | Median | IQR | Minimum | Maximum | |
|---|---|---|---|---|---|
| Initial | 338 | 1.36 | 0.34 | 0.21 | 2.39 |
| Confirmatory | 338 | 1.30 | 0.35 | 0.12 | 2.35 |
| Treatment | 338 | 1.30 | 0.34 | 0.12 | 2.12 |
n, number of cases; IQR, interquartile range.
No differences were observed regarding gender in the initial (p=0.318) and confirmatory (p=0.706) T4L values, as well as in values at the start of treatment (p=0.542).
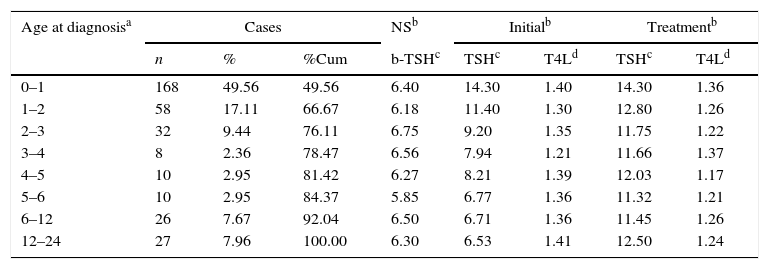
Diagnosis was confirmed between 2 and 695 days of life, with a mean of 94±143 days of life and a median of 32 (68), and 76.11% of the children with CH had the diagnosis confirmed before the age of three months. However, 23.89% of the cases that would later receive the diagnosis of CH had TSH values above the upper limit of normal, but with no indication of treatment, and remained on follow-up with periodic measurements of TSH and T4L, being defined as having hypothyroidism between 3 and 24 months of age. Among these children with CH, 7.96% had a diagnosis confirmed between 1 and 2 years of age (Table 3).
Thyroid function according to age range at diagnosis.
| Age at diagnosisa | Cases | NSb | Initialb | Treatmentb | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | %Cum | b-TSHc | TSHc | T4Ld | TSHc | T4Ld | |
| 0–1 | 168 | 49.56 | 49.56 | 6.40 | 14.30 | 1.40 | 14.30 | 1.36 |
| 1–2 | 58 | 17.11 | 66.67 | 6.18 | 11.40 | 1.30 | 12.80 | 1.26 |
| 2–3 | 32 | 9.44 | 76.11 | 6.75 | 9.20 | 1.35 | 11.75 | 1.22 |
| 3–4 | 8 | 2.36 | 78.47 | 6.56 | 7.94 | 1.21 | 11.66 | 1.37 |
| 4–5 | 10 | 2.95 | 81.42 | 6.27 | 8.21 | 1.39 | 12.03 | 1.17 |
| 5–6 | 10 | 2.95 | 84.37 | 5.85 | 6.77 | 1.36 | 11.32 | 1.21 |
| 6–12 | 26 | 7.67 | 92.04 | 6.50 | 6.71 | 1.36 | 11.45 | 1.26 |
| 12–24 | 27 | 7.96 | 100.00 | 6.30 | 6.53 | 1.41 | 12.50 | 1.24 |
n, number of cases; NS, neonatal screening; % Cum, cumulative relative frequency.
This is a study with a significant sample, based on the experience of a service, among few in Brazil,14,19,20 which uses a lower b-TSH cutoff value than most NS programs.
The use of a lower b-TSH cutoff value in the analyzed sample resulted in a 14.8-fold increase in the recall rate, which would be of 0.07% using the cutoff of 10μIU/mL, whereas it was 1.04% with a cutoff of 5μIU/mL. Another SRTN from Brazil showed a similar recall rate of 1.08%, using a cutoff value of 4.5μIU/mL,14 while Korada et al.,13 using a cutoff value of 6mIU/L, reported a recall rate of 0.23%. These data suggest that there is no direct association between the cutoff values used in NS for CH and recall rates, as concluded by Loeber21 in the analysis of 37 European countries’ NS in 2004.
The use of lower cutoff points, with a consequent increase in the number of recalled children, leads to higher costs with NS, in addition to generating anxiety for parents and relatives of healthy children.18 During the study period, children with b-TSH between 5 and 10μIU/mL were submitted to at least one venous blood collection to evaluate thyroid function and, after follow-up, approximately 90% of them were defined as false-positive cases.
However, during the evaluation of these six years of NS with an b-TSH cutoff value of 5μIU/mL, 339 children who would not have been detected with a cutoff point of 10μIU/mL were diagnosed with CH.
Other NS programs also detected additional cases of CH with a lower cutoff value, as observed in the study by Barone et al.,14 in which 63% of the 475 identified cases of CH had b-TSH between 4.5 and 9.5mIU/L, and in the study by Korada et al.,13 which confirmed two cases of CH among the 67 full-term infants with b-TSH between 6 and 10mIU/L. One of the SRTNs in the state of São Paulo, which reduced the cutoff value for b-TSH between 2005 and 2008, also detected six cases of CH among 1181 children with initial b-TSH levels between 5 and 10μIU/mL.20 Recently, Jones et al.17 observed, among the 304 diagnosed CH cases, 26 children with b-TSH between 8 and 10mIU/L.
Therefore, in this study, as well as in others,4,13–17 it has been demonstrated that children with CH might not be detected with the higher cutoff points that are usually used, and these additional diagnosed cases justify, in the present authors’ opinion and in that of other authors, the reduction in the cutoff value of b-TSH.4,13
Analysis of the thyroid function in this group detected children with serum TSH levels >20μIU/mL or T4L levels below the reference value, for whom immediate treatment is recommended.1
It is not yet clear in the literature whether patients with mild elevations in TSH levels are at risk for cognitive impairment.4,5,8,22 It has been suggested that cases of neurological disorders that result in decreased school performance are related to mild forms of CH, not detected by NS due to elevated b-TSH cutoff values.4,23 A recent study showed that children with b-TSH between the 75th and 99.95th percentiles are more likely to have poor school performance, when compared to those with b-TSH below the 75th percentile.24 Thus, many have argued that until there is evidence of absence of risk of intellectual disability without the use of L-T4, the treatment of these cases is preferable.4,25
Although most of the group was diagnosed with CH and started treatment early, many children who remained on follow-up due to non-normalization of TSH values developed hypothyroidism between 3 months and 2 years of age.
One factor possibly involved in the late confirmation of CH is breastfeeding, since breast milk may be an exogenous source of T4, sometimes even masking clinical signs of CH.26,27 There is a chance that, given the usual recommendation of exclusive breastfeeding until the sixth month, some children with mild CH may have shown a more marked elevation in TSH levels after weaning or reduced breastfeeding. Another possibility is that they are due to thyroid hormone synthesis defect that often take some time for hypothyroidism to be established, as previously reported.28
In brief, the results of this study demonstrated that the use of the b-TSH cutoff value of 5μIU/mL in NS allowed the detection of additional CH cases and many of these children only had the diagnosis confirmed later. Considering that among the different countries and even within the same country there is no homogeneity in diagnostic and follow-up criteria, more studies are needed to provide evidence for an ideal cutoff value for b-TSH and to establish more specific diagnostic and treatment protocols.1,4,29 Based on the findings of the present study, the authors suggest the use of a cutoff value of 5μIU/mL and long-term follow-up for these children, aiming to detect all CH cases.
Conflicts of interestThe authors declare no conflicts of interest.
To patients and their families who were part of the study. To the employees of the Outpatient Clinic of Pediatrics of Hospital das Clínicas da UNICAMP and the Neonatal Screening Reference Service of UNICAMP, especially to biologist Carmen Sílvia Gabetta, Head of the Laboratory of the Neonatal Screening Reference Service of UNICAMP.
Please cite this article as: Christensen-Adad FC, Mendes-dos-Santos CT, Goto MM, Sewaybricker LE, D'Souza-Li LF, Guerra-Junior G, et al. Neonatal screening: 9% of children with filter paper thyroid-stimulating hormone levels between 5 and 10μIU/mL have congenital hypothyroidism. J Pediatr (Rio J). 2017;93:649–54.