The population-based cancer registries (PBCR) and the Information System on Live Births in Brazil (Sistema de Informações sobre Nascidos Vivos [SINASC]) have information that enables the test for risk factors associated with leukemia at an early age. The aim of this study was to identify maternal and birth characteristics associated with early-age acute leukemia (EAL) in Brazil.
MethodsA case-cohort study was performed using secondary dataset information of PBCR and SINASC. The risk association variables were grouped into (i) characteristics of the child at birth and (ii) characteristics of maternal exposure during pregnancy. The case–control ratio was 1:4. Linkage was performed using R software; odds ratio (OR) and 95% confidence interval (CI) were calculated by logistic regression models.
ResultsEAL was associated with maternal occupational exposure to chemicals (agricultural, chemical, and petrochemical industry; adjOR: 2.18, 95% CI: 1.16–4.10) and with birth defects (adjOR: 3.62, 95% CI: 1.19–11.00).
ConclusionsThe results of this study, with the identification of EAL risk factors in population-based case-cohort study, strengthen the knowledge and improve databases, contributing to investigations on risk factors associated with childhood leukemia worldwide.
Os registros de câncer de base populacional (RCBP) e o Sistema Nacional de Nascidos Vivos (SINASC) possuem informações que possibilitam testar hipóteses sobre fatores de riscos associados às leucemias. O objetivo principal deste projeto é identificar quais as características ao nascimento das crianças que estariam associadas ao risco de desenvolver Leucemia Aguda (LA) na primeira infância.
MétodosForam utilizadas informações de 12 RCBP e do Sistema de Informação de Nascidos Vivos das mesmas localidades. Foram elegíveis 272 casos e 1.088 controles no período de 1996 a 2010. As associações de riscos de LA foram agrupadas em, (i) características da criança ao nascer, e (ii) características de exposição materna durante a gestação da criança. A relação de casos e controles foi de 1:4. As análises para padronização, estruturação do banco de dados e análises estatísticas foram realizadas através dos aplicativos Excel, R-Studio e SPSS 21.
ResultadosHouve associação entre anomalias congênitas (RC 3,62, IC95% 1,19-11,00) e exposição ocupacional materna a produtos químicos (OR 2,18, p 0,002) com o risco do desenvolvimento de LA.
ConclusãoA utilização de banco de dados secundários populacionais para a identificação de fatores de risco para LA fortaleceu o intercâmbio de conhecimentos e melhoria das bases de dados, e contribuiu para investigações sobre as associações de riscos nas leucemias agudas em contexto mundial.
Worldwide, leukemia is the most common malignancy diagnosed in children under the age of 5 years. The incidence rate of acute lymphoblastic leukemia (ALL) has a sharp peak between 2 and 4 years of age at diagnosis, and tends to affect more boys than girls.1 The etiology of childhood leukemia remains a challenge, although the premise that early-age leukemia (EAL) arises from somatic clonal cells originating during fetal life encourages the research toward factors associated with environmental exposures.1
The concept of causality has been established based on evidences that need further testing. This is particularly true in public health and social sciences. For childhood leukemia, a causal study-model should take into consideration that leukemias have diverse, age-dependent, biological subtypes, and above all a multistep pathogenesis. Some perinatal characteristics, such as birth weight, birth order, mode of delivery, maternal age, and maternal occupational exposure, have been associated with childhood leukemia in case–control studies.2–6 Since classic case–control studies can be inefficient in the case of rare diseases such as EAL, a methodological alternative is to combine a case–control study into a cohort. This study aimed to investigate maternal and birth characteristics associated with EAL risk factors using a case-cohort model. The variables were assessed through information gathered from secondary population-based registries.
Materials and methodsStudy designA population-based case-cohort study was performed. Cases and controls were obtained from 12 population-based cancer registries (PBCR) and from the Information System on Live Births in Brazil (Sistema de Informações sobre Nascidos Vivos [SINASC]) from the same cities as the PBCR. This strategy allowed all individuals of the population base to have the same probability of being selected to form the control group, regardless of when the data was collected.
DataInitially, 372 total cases of leukemia were identified from 12 PBCR cities: Aracaju, Belém, Belo Horizonte, Cuiabá, Curitiba, Fortaleza, João Pessoa, Manaus, Natal, Porto Alegre, Recife, and Vitória, with information available overtime (2000–2009). The inclusion criteria were: children born after the year 2000, aged between 0 and 5 years with a confirmed diagnosis of acute leukemia (acute lymphoblastic leukemia [ALL], acute myeloid leukemia [AML], and non specified leukemia – [NSL]) made between 2000 and 2009.
Controls were selected from the SINASC, in the same cities of the cases, who had also been born after the year 2000 (n=5,623,179). Four controls per case were chosen, using systematic random sampling from the SINASC data by birth order, year, and sex. The SINASC database was accessed for the selection of eligible controls, as well as to obtain the pregnancy and perinatal information of cases and controls. Multiple pregnancies were excluded from the study (n=104,757; 1.9%).
The characteristics at childbirth analyzed were gender, skin color, maternal age, maternal education, maternal occupation during the child's pregnancy, child birth order, mode of delivery, gestational age (weeks) at birth, five-minute APGAR score, birth weight, fetal growth, and birth defects (ICD-10).
Maternal age was evaluated as a continuous variable with units of five-year intervals, and a categorical variable with two levels (<30 and ≥30). Maternal occupational exposure was classified into a categorical variable with three levels: (1) not working, defined as stay-at-home mothers and students; (2) chemical, defined as workers in the agricultural, chemical, and petrochemical industry and, (3) others, not otherwise specified (NOS). Birth order was calculated from the number of previous pregnancies, counting both living and dead children plus one. The five-minute Apgar scores were categorized into two levels: ≤8 and >8. Birth weight was evaluated as a continuous variable with units of 500g and 1000g, and a categorical variable with two levels (≤3000g and>3000g). Sex, birth weight, and gestational age were used to classify weight into gestational age categories. Large for gestational age (LGA) was defined as having a birth weight above the specific 90th percentile for sex and gestational age; small for gestational age (SGA) was defined as having a birth weight below the 10th percentile; and appropriate for gestational age (AGA) was defined as having a weight between the 10th and 90th percentiles. A large, published birth cohort of birth weight percentiles in the Brazilian population was used as a reference for these categories.7
Statistical analysisA record linkage-based case-cohort study with PBCR and SINASC databases was performed. As these two databases do not have a unique identifier, it was necessary to adopt probabilistic data linkage using the variables present in both databases and to use different combinations to compare and block exclusion variables. The full details of this methodology have been described elsewhere.8 The Record Linkage package was used on R software (R Foundation for Statistical Computing, Viena, Austria). The probabilistic algorithm was used to identify records related to unique individuals in both databases combined. The mother's name was the key variable, and the Soundex (Brazil) algorithm (http://CRAN.R-project.org/package=soundexBR) was used for phonetic comparison; the Levenshtein distance was employed to compare strings. Using this record linkage methodology, 272 cases were identified, representing 73% of the EAL cases in the PBCR. One hundred cases were excluded, but there were no statistical differences regarding sex, skin color, and leukemia subtype when compared with 272 EAL.
Unconditional logistic regression analysis was performed in SPSS (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, version 21.0. NY, USA) to calculate odds ratios (OR) and 95% confidence intervals (CI) for evaluating the association between gender, maternal age, maternal education, maternal occupation during the child's pregnancy, birth order, gestational age (weeks), mode of delivery, five-minute Apgar score, birth weight, fetal growth, birth anomalies, and EAL. Separated analyses were carried out for ALL, AML, and NSL. Any univariate finding with a p-value of <0.20 was treated as a potential confounder and used to adjust other findings.
EthicsThe study was approved by the Research Ethics Committee of the José Alencar Gomes da Silva National Cancer Institute (Instituto Nacional do Câncer [INCA]), ref. No.: 10856213.0.0000.5274.
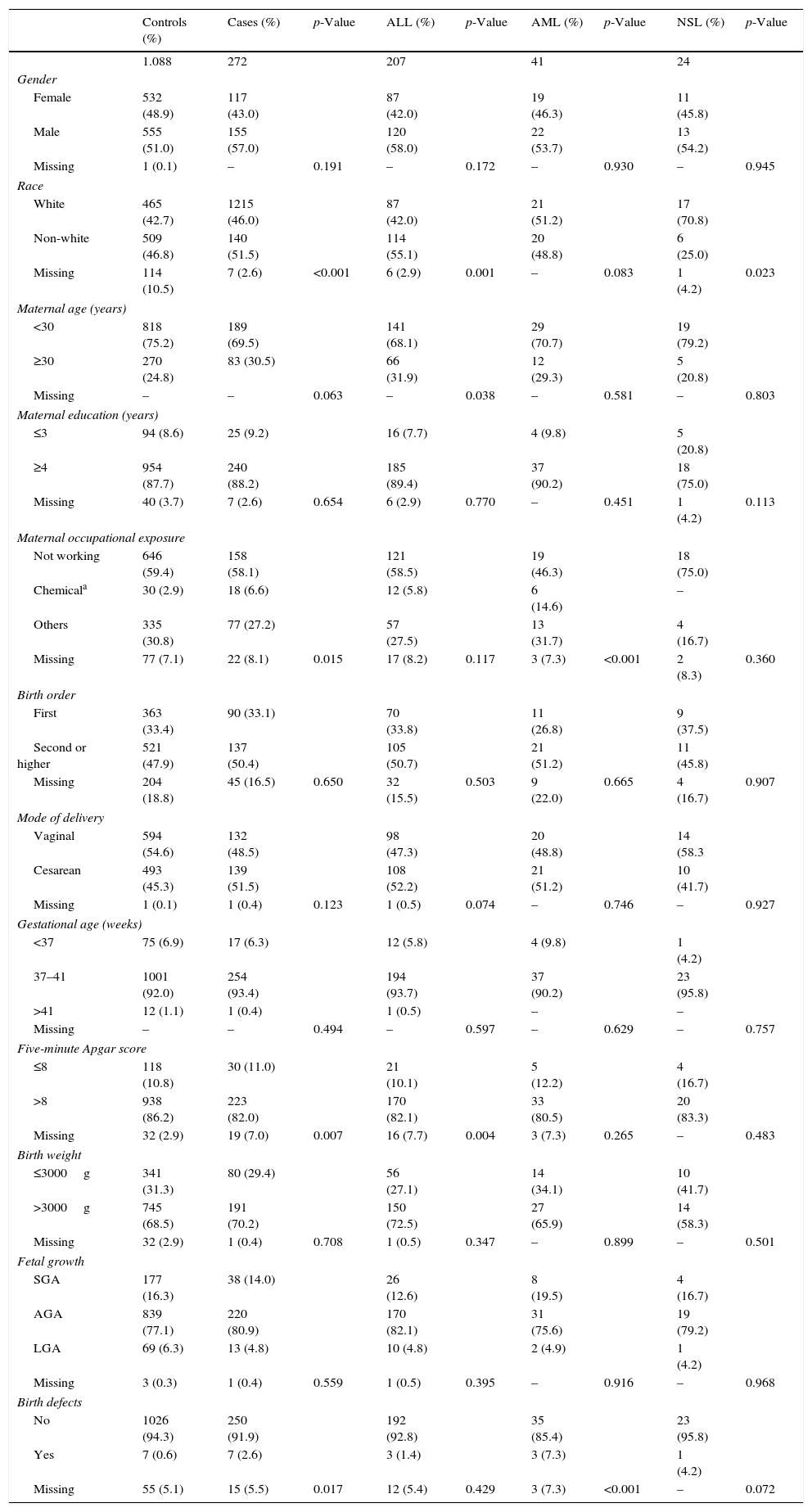
ResultsThe frequencies of maternal and perinatal variables among cases and controls, including socio-demographic and gestational characteristics, are shown in Table 1. There were 207 cases of ALL, 41 AML, and 24 AL-NSL, and 1088 controls, according to the distribution of 12 PBCR selected.
Frequency of maternal and perinatal characteristics for cases and controls, Brazil, 2000–2009.
| Controls (%) | Cases (%) | p-Value | ALL (%) | p-Value | AML (%) | p-Value | NSL (%) | p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| 1.088 | 272 | 207 | 41 | 24 | |||||
| Gender | |||||||||
| Female | 532 (48.9) | 117 (43.0) | 87 (42.0) | 19 (46.3) | 11 (45.8) | ||||
| Male | 555 (51.0) | 155 (57.0) | 120 (58.0) | 22 (53.7) | 13 (54.2) | ||||
| Missing | 1 (0.1) | – | 0.191 | – | 0.172 | – | 0.930 | – | 0.945 |
| Race | |||||||||
| White | 465 (42.7) | 1215 (46.0) | 87 (42.0) | 21 (51.2) | 17 (70.8) | ||||
| Non-white | 509 (46.8) | 140 (51.5) | 114 (55.1) | 20 (48.8) | 6 (25.0) | ||||
| Missing | 114 (10.5) | 7 (2.6) | <0.001 | 6 (2.9) | 0.001 | – | 0.083 | 1 (4.2) | 0.023 |
| Maternal age (years) | |||||||||
| <30 | 818 (75.2) | 189 (69.5) | 141 (68.1) | 29 (70.7) | 19 (79.2) | ||||
| ≥30 | 270 (24.8) | 83 (30.5) | 66 (31.9) | 12 (29.3) | 5 (20.8) | ||||
| Missing | – | – | 0.063 | – | 0.038 | – | 0.581 | – | 0.803 |
| Maternal education (years) | |||||||||
| ≤3 | 94 (8.6) | 25 (9.2) | 16 (7.7) | 4 (9.8) | 5 (20.8) | ||||
| ≥4 | 954 (87.7) | 240 (88.2) | 185 (89.4) | 37 (90.2) | 18 (75.0) | ||||
| Missing | 40 (3.7) | 7 (2.6) | 0.654 | 6 (2.9) | 0.770 | – | 0.451 | 1 (4.2) | 0.113 |
| Maternal occupational exposure | |||||||||
| Not working | 646 (59.4) | 158 (58.1) | 121 (58.5) | 19 (46.3) | 18 (75.0) | ||||
| Chemicala | 30 (2.9) | 18 (6.6) | 12 (5.8) | 6 (14.6) | – | ||||
| Others | 335 (30.8) | 77 (27.2) | 57 (27.5) | 13 (31.7) | 4 (16.7) | ||||
| Missing | 77 (7.1) | 22 (8.1) | 0.015 | 17 (8.2) | 0.117 | 3 (7.3) | <0.001 | 2 (8.3) | 0.360 |
| Birth order | |||||||||
| First | 363 (33.4) | 90 (33.1) | 70 (33.8) | 11 (26.8) | 9 (37.5) | ||||
| Second or higher | 521 (47.9) | 137 (50.4) | 105 (50.7) | 21 (51.2) | 11 (45.8) | ||||
| Missing | 204 (18.8) | 45 (16.5) | 0.650 | 32 (15.5) | 0.503 | 9 (22.0) | 0.665 | 4 (16.7) | 0.907 |
| Mode of delivery | |||||||||
| Vaginal | 594 (54.6) | 132 (48.5) | 98 (47.3) | 20 (48.8) | 14 (58.3 | ||||
| Cesarean | 493 (45.3) | 139 (51.5) | 108 (52.2) | 21 (51.2) | 10 (41.7) | ||||
| Missing | 1 (0.1) | 1 (0.4) | 0.123 | 1 (0.5) | 0.074 | – | 0.746 | – | 0.927 |
| Gestational age (weeks) | |||||||||
| <37 | 75 (6.9) | 17 (6.3) | 12 (5.8) | 4 (9.8) | 1 (4.2) | ||||
| 37–41 | 1001 (92.0) | 254 (93.4) | 194 (93.7) | 37 (90.2) | 23 (95.8) | ||||
| >41 | 12 (1.1) | 1 (0.4) | 1 (0.5) | – | – | ||||
| Missing | – | – | 0.494 | – | 0.597 | – | 0.629 | – | 0.757 |
| Five-minute Apgar score | |||||||||
| ≤8 | 118 (10.8) | 30 (11.0) | 21 (10.1) | 5 (12.2) | 4 (16.7) | ||||
| >8 | 938 (86.2) | 223 (82.0) | 170 (82.1) | 33 (80.5) | 20 (83.3) | ||||
| Missing | 32 (2.9) | 19 (7.0) | 0.007 | 16 (7.7) | 0.004 | 3 (7.3) | 0.265 | – | 0.483 |
| Birth weight | |||||||||
| ≤3000g | 341 (31.3) | 80 (29.4) | 56 (27.1) | 14 (34.1) | 10 (41.7) | ||||
| >3000g | 745 (68.5) | 191 (70.2) | 150 (72.5) | 27 (65.9) | 14 (58.3) | ||||
| Missing | 32 (2.9) | 1 (0.4) | 0.708 | 1 (0.5) | 0.347 | – | 0.899 | – | 0.501 |
| Fetal growth | |||||||||
| SGA | 177 (16.3) | 38 (14.0) | 26 (12.6) | 8 (19.5) | 4 (16.7) | ||||
| AGA | 839 (77.1) | 220 (80.9) | 170 (82.1) | 31 (75.6) | 19 (79.2) | ||||
| LGA | 69 (6.3) | 13 (4.8) | 10 (4.8) | 2 (4.9) | 1 (4.2) | ||||
| Missing | 3 (0.3) | 1 (0.4) | 0.559 | 1 (0.5) | 0.395 | – | 0.916 | – | 0.968 |
| Birth defects | |||||||||
| No | 1026 (94.3) | 250 (91.9) | 192 (92.8) | 35 (85.4) | 23 (95.8) | ||||
| Yes | 7 (0.6) | 7 (2.6) | 3 (1.4) | 3 (7.3) | 1 (4.2) | ||||
| Missing | 55 (5.1) | 15 (5.5) | 0.017 | 12 (5.4) | 0.429 | 3 (7.3) | <0.001 | – | 0.072 |
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; NSL, non-specified leukemia; SGA, small for gestational age; AGA, appropriate for gestational age; LGA, large for gestational age.
Birth defects were predominant in AML subtypes (p=0.07). Seven cases had birth defects (Q35.9 – cleft palate, unspecified, Q66.1 – talipes calcaneovarus, Q79.3 – gastroschisis, Q89.9 – congenital malformation, unspecified, and Q90.9 – Down syndrome, unspecified) representing 2.6% of EALs. The birth anomalies in the control group (0.6%) were: Q17.9 – congenital malformation of ear, unspecified, Q27.0 – congenital absence and hypoplasia of umbilical artery, Q37.9 – unspecified cleft palate with unilateral cleft lip, Q54.9 – hypospadias, unspecified, Q89.8 – other specified congenital malformations, and Q90.9 – Down syndrome, unspecified.
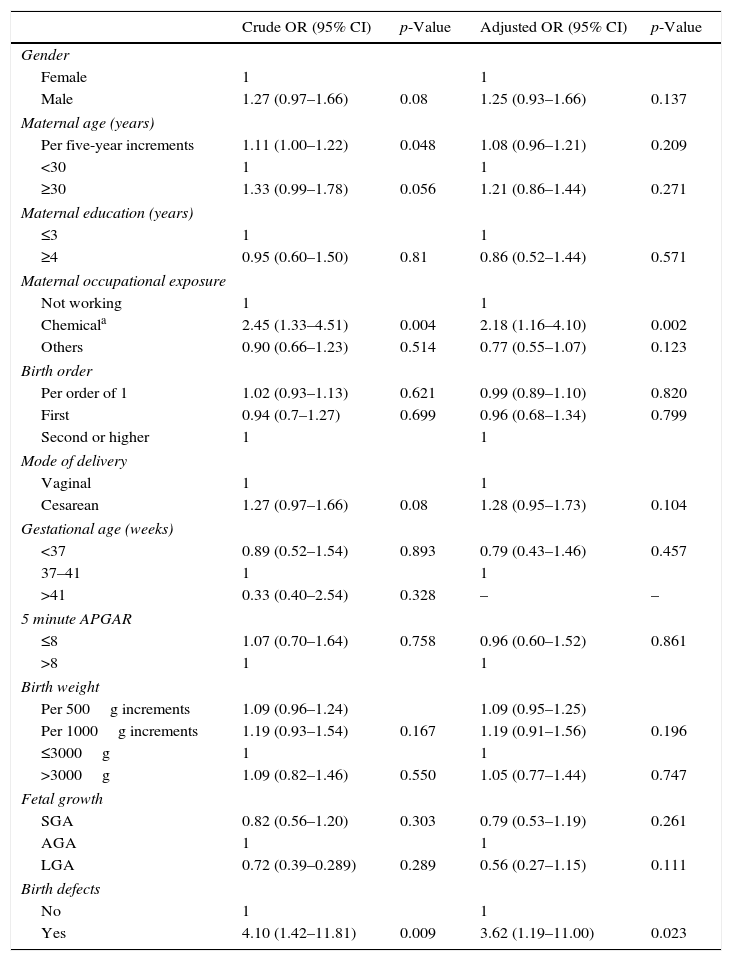
The association between maternal and perinatal variables and EAL are shown in Table 2, through crude and adjusted analyses. There were increased and significant risk associations between EAL and chemical maternal occupational exposures (ORcrude=2.45; 95% CI: 1.33–4.51); and birth defects (ORcrude=4.10; 95% CI: 1.42–11.81). After the adjusted analysis, maternal occupational exposure to chemicals and birth defects remained strongly associated with EAL (OR[adj]=2.18; 95% CI: 1.16–4.10; OR[adj]=3.62; 95% CI: 1.19–11.00, respectively). Maternal age was tested as a continuous variable with five-year increments (ORcrude=1.11; 95% CI: 1.00–1.22); cesarean (OR crude=1.28; 95% CI: 0.95–1.73) and birth weight presented statistically marginally significant association effects. The occurrence of missing variables excluded the post-term cases (>41 gestation weeks) variable from the fit analysis, when the adjOR was calculated.
Association between maternal and perinatal characteristics and early-age acute leukemia, Brazil, 2000–2009.
| Crude OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | |
|---|---|---|---|---|
| Gender | ||||
| Female | 1 | 1 | ||
| Male | 1.27 (0.97–1.66) | 0.08 | 1.25 (0.93–1.66) | 0.137 |
| Maternal age (years) | ||||
| Per five-year increments | 1.11 (1.00–1.22) | 0.048 | 1.08 (0.96–1.21) | 0.209 |
| <30 | 1 | 1 | ||
| ≥30 | 1.33 (0.99–1.78) | 0.056 | 1.21 (0.86–1.44) | 0.271 |
| Maternal education (years) | ||||
| ≤3 | 1 | 1 | ||
| ≥4 | 0.95 (0.60–1.50) | 0.81 | 0.86 (0.52–1.44) | 0.571 |
| Maternal occupational exposure | ||||
| Not working | 1 | 1 | ||
| Chemicala | 2.45 (1.33–4.51) | 0.004 | 2.18 (1.16–4.10) | 0.002 |
| Others | 0.90 (0.66–1.23) | 0.514 | 0.77 (0.55–1.07) | 0.123 |
| Birth order | ||||
| Per order of 1 | 1.02 (0.93–1.13) | 0.621 | 0.99 (0.89–1.10) | 0.820 |
| First | 0.94 (0.7–1.27) | 0.699 | 0.96 (0.68–1.34) | 0.799 |
| Second or higher | 1 | 1 | ||
| Mode of delivery | ||||
| Vaginal | 1 | 1 | ||
| Cesarean | 1.27 (0.97–1.66) | 0.08 | 1.28 (0.95–1.73) | 0.104 |
| Gestational age (weeks) | ||||
| <37 | 0.89 (0.52–1.54) | 0.893 | 0.79 (0.43–1.46) | 0.457 |
| 37–41 | 1 | 1 | ||
| >41 | 0.33 (0.40–2.54) | 0.328 | – | – |
| 5 minute APGAR | ||||
| ≤8 | 1.07 (0.70–1.64) | 0.758 | 0.96 (0.60–1.52) | 0.861 |
| >8 | 1 | 1 | ||
| Birth weight | ||||
| Per 500g increments | 1.09 (0.96–1.24) | 1.09 (0.95–1.25) | ||
| Per 1000g increments | 1.19 (0.93–1.54) | 0.167 | 1.19 (0.91–1.56) | 0.196 |
| ≤3000g | 1 | 1 | ||
| >3000g | 1.09 (0.82–1.46) | 0.550 | 1.05 (0.77–1.44) | 0.747 |
| Fetal growth | ||||
| SGA | 0.82 (0.56–1.20) | 0.303 | 0.79 (0.53–1.19) | 0.261 |
| AGA | 1 | 1 | ||
| LGA | 0.72 (0.39–0.289) | 0.289 | 0.56 (0.27–1.15) | 0.111 |
| Birth defects | ||||
| No | 1 | 1 | ||
| Yes | 4.10 (1.42–11.81) | 0.009 | 3.62 (1.19–11.00) | 0.023 |
OR, odds ratio; CI, confidence interval; SGA, small for gestational age; AGA, appropriate for gestational age; LGA, large for gestational age.
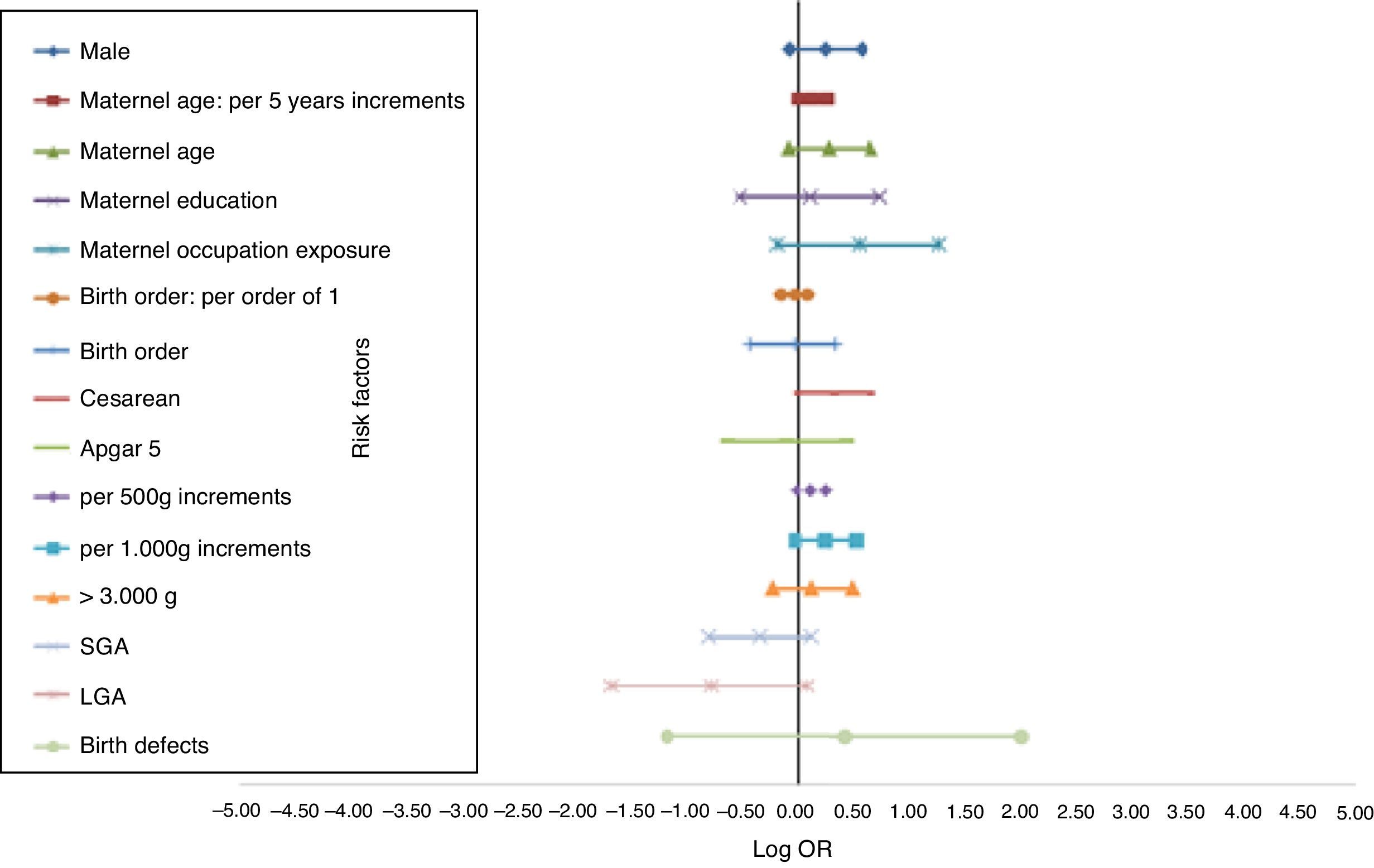
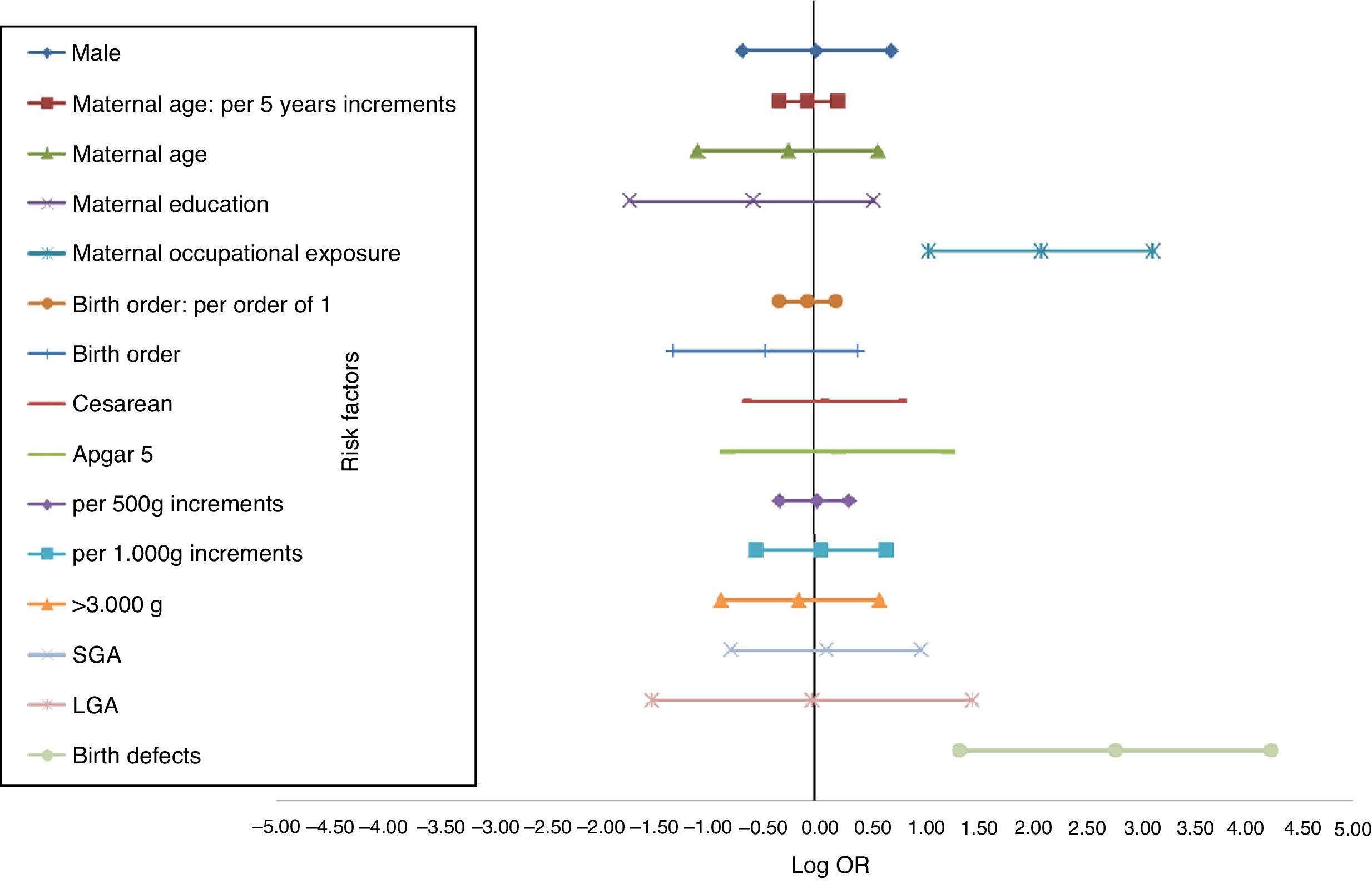
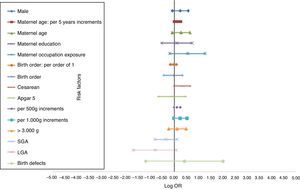
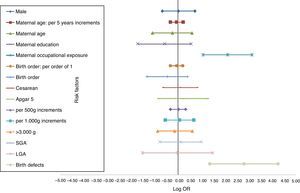
The distinct magnitude of risk associations between maternal and perinatal characteristics and ALL are described in Fig. 1, whereas Fig. 2 shows the risk associations with AML. Independent, marginally significant, risk factors for ALL were: gender (OR[adj]=1.29; 95% CI: 0.93–1.78); continuous maternal age (per five-year increments; OR[adj]=1.78; 95% CI: 1.00–1.30); maternal age≥30 years (OR[adj]=1.33; 95% CI: 0.92–1.93); delivery by cesarean section (OR[adj]=1.35; 95% CI: 0.97–1.89); continuous birth weight (per 500g; OR[adj]=1.13; 95% CI: 0.99–1.29); and continuous birth weight (per 1000g; OR[adj]=1.28; 95% CI: 0.98–1.68). For AML, only two independent risk factors were associated: chemical maternal occupational exposure (OR [adj]=8.24; 95% CI: 2.91–23.39) and the presence of birth defects (OR[adj]=16.39; 95% CI: 3.86– 69.55).
Childhood leukemia is the most common pediatric malignancy, and the peak incidence is between 2 and 5 years of age in most populations, suggesting in utero development. Maternal and perinatal characteristics are described as possible risk factors.1 A record linkage case-cohort study was conducted in which data collected from two population-based studies were explored. One of the datasets used, the SINASC, was created in 1990; since the year 2000, it has been recognized as a good quality birth registry, with accurate information.9 Similarly, the Brazilian PBCR used in this study has been consider by the International Agency for Research on Cancer (IARC) to be of good quality; it was classified as a group B defined coverage (approximately 50%) with the parameters suggested for incidence rate analysis.10 Therefore, the present study, with record linkage population-based case-cohort approach, provided reliable results concerning the risk factors associated with EAL in Brazil. Perinatal, maternal occupation, and mother-child characteristics in relation to EAL were explored herein.
Some studies have shown increased risk of childhood leukemia associated with maternal occupational exposures during pregnancy, especially for women working in the front line of agricultural activities or exposed to pesticides or solvents.11,12 In this population-based case-cohort study, an increased associated risk rate was observed among mothers self-identified as workers in the agricultural, chemical, and petrochemical industries; an eight-fold risk of AML was observed. These results corroborate those of a previous hospital-based case–control study, which found a strong association of maternal exposure to pesticides and infant leukemia in Brazil.12
Conception at advanced maternal age has been described as a childhood leukemia risk factor, although with an inconsistent result in some studies.13,14 In the present analysis, a statistically marginally significant association with EAL was found with maternal age evaluated as continuous variable. However, a significant risk was observed for ALL (OR=1.7). A possible explanation for the association of advanced maternal age and ALL could be the gametes’ long-term exposure to environmental agents.15 Delivery by cesarean section was also associated with ALL (HR=1.35). Delivery by cesarean section had been previously found to be associated with ALL in Hispanics in an international pooled analysis of case–control studies.16 Along with this epidemiological evidence, maternal education was also found to be associated with increased risk for ALL development when mothers had an education level higher than high school (HR=1.21; 95% CI: 1.0–1.47) in developed countries.17 A recent study analyzed ALL and maternal education inn Egyptian children, observing an association between high educational level and ALL risk (OR=2.05; 95% CI: 1.46–2.88).18 Using maternal education as a proxy variable for socioeconomic indicator level (SES-i), no association was observed between EAL and SES-i in the entire Brazilian PBCR settings. However, based this information, given as an implicit comparison, the results observed in this study must be analyzed prudently for the aforementioned reasons. The SES-i based on maternal education is not perfect; however, the SINASC dataset has good completeness and strong positive association of lower maternal education with poor SES-i (http://www.ibge.gov.br). Maternal education in cases and controls might reflect the social environment experience by all participants in this analysis.
Other variables, such as gestational age, Apgar score at birth, birth order, and birth weight were not associated with EAL. Children with low five-minute Apgar score had some unfavorable results of delivery, and this score is a predictor of childhood cancer, mainly solid tumors, but not childhood leukemia.19–22
Among all birth characteristics, birth weight has been well-described as a risk factor for ALL in early ages and for some pediatric embryonal tumors.2–5 Although birth weight was not associated with EAL in the present investigation, in a previous Brazilian case–control designed study, the odds ratio between i-ALL and birth weight was 1.30, after adjusting for confounders such as sex, maternal age, family income, and pesticide exposure during pregnancy. In that study, the hospital-based controls were in the stratum of 4000g or more, comparatively to those born weigh in between 2500 and 2999g.23 To understand the potential biological mechanisms that may explain the link between high birth weight and risk of ALL, it is important to differentiate high absolute weight and high relative weight at birth. Birth weight adjusted for gestational age was first examined for the risk of ALL in two English databases, and no significant associations were observed (absolute birth weight and weight adjusted for gestational age).24 Recently, the Childhood Leukemia International Consortium (CLIC), using a pooled analysis of case–control studies, demonstrated that accelerated fetal growth is associated with an increased risk of ALL.4 This evidence raises speculation about the function of insulin-like receptor gene family, especially the insulin-like growth factor-1 (IGF-1). The ratio of IGF-I concentration measured in umbilical cord blood and positively associated with birth weight, placental weight, and weight index were assessed; high circulating levels of IGF-I were reported in infants with high birth weight.25,26
Birth defects are a strong associated risk factor for childhood cancer. Down syndrome is a well-known risk factor for infant leukemia, especially for AML.27,28 In the present study, a high risk for AML (three birth anomalies among 41 cases) was observed. The three birth anomalies were Down syndrome, cleft palate, and unspecified congenital malformation (one each).
Some considerations should be mentioned regarding the limitations of the present results. Firstly, the loss of 27% of cases not found when linking the two databases (PBCR/SINASC) may have compromised the interpretation of results, although the effect would be loss of statistical power, but no change in the risk. It has been described that 35% of Brazilians do not live in the city of birth.9,29 The most likely explanation for the loss of matched cases–controls is that migration is very common in Brazil, mainly because parents look for opportunities in large cities, consequently with loss of outcomes in city of birth. Another limitation regarded specific maternal occupational exposure data, whose completeness in SINASC is considered low. The definition of categories in SINASC and PBCR concerning occupation follows the updated resolution of the International Standard Classification of Occupations (ISCO-08). Occupation is defined as a set of activities characterized by a high degree of similarity in ISCO-8, although not related to specific exposures.11 Because of the small number in each category assessment, the variables agricultural, chemical, and petrochemical industries were combined in a single group as occupational exposures to potential carcinogenic substances. A causal study model should take into account the diverse cell subtypes of childhood leukemia, and the low completeness of these variables in PBCR represents another limitation of this analysis.
Finally, another pitfall of this study lies in the description of the birth defect of SINASC and PBCR, which needs improvements, due to the high number of other specified congenital malformations (Q89.9). It has already been described that specific birth defects are underreported in hospital registries.28,29 However, the authors believe this lack of definition has not led to differential error direction in the present results of absolute risk factors for EAL in Brazil.
The strengths of this study should be also highlighted. Firstly, the case-cohort design assumes that all variables were obtained at the beginning of the study (at base-line), thus, data were obtained before any disease developed. Secondly, data from two good quality population-based datasets were used, which increases the likelihood that these findings represent actual risk factors. Despite all the aforementioned limitations, this was the first study in Brazil to consult two secondary databases in order to establish an association between risk factors and the development of leukemia in children at an early age. Finally, the authors believe that the present findings provides evidence that maternal exposures during pregnancy and birth characteristics should be studied in greater depth as cause-effect in utero leukemogenesis.
Conflicts of interestThe authors declare no conflicts of interest.
The authors are grateful to all the coordinators of the PBCRs and the SINASC in Brazil who contributed to this work, including those in Aracaju/SE, Belém/PA, Belo Horizonte/MG, Cuiabá/MT, Curitiba/PR, Fortaleza/CE, João Pessoa/PB, Manaus/AM, Natal/RN, Porto Alegre/RS, Recife/PE, and Vitória/ES.
Please cite this article as: Reis RS, Silva NP, Santos MO, Oliveira JF, Thuler LC, de Camargo B, et al. Mother and child characteristics at birth and early age leukemia: a case-cohort population-based study. J Pediatr (Rio J). 2017;93:610–8.
Study conducted at Instituto Nacional do Câncer (INCA), Centro de Pesquisa, Rio de Janeiro, RJ, Brazil.