To investigate blood lead levels in schoolchildren in two areas of Egypt to understand the current lead pollution exposure and its risk factors, aiming to improve prevention politicies.
Subjects and methodThis was a cross-sectional study in children (n=400) aged 6–12 years recruited from two areas in Egypt (industrial and urban). Blood lead levels were measured using an atomic absorption method. Detailed questionnaires on sources of lead exposure and history of school performance and any behavioral changes were obtained.
ResultsThe mean blood lead level in the urban area of Egypt (Dokki) was 5.45±3.90μg/dL, while that in the industrial area (Helwan) was 10.37±7.94μg/dL, with a statistically significant difference between both areas (p<0.05). In Dokki, 20% of the studied group had blood lead levels≥10μg/dL, versus 42% of those in Helwan. A significant association was found between children with abnormal behavior and those with pallor with blood lead level≥10μg/dL, when compared with those with blood lead level<10μg/dL (p<0.05). Those living in Helwan area, those with bad health habits, and those living in housing with increased exposure were at a statistically significantly higher risk of having blood lead level≥10μg/dL.
ConclusionLead remains a public health problem in Egypt. High blood lead levels were significantly associated with bad health habits and housing with increased exposure, as well as abnormal behavior and pallor.
Investigar os níveis de chumbo no sangue (NCSs) em crianças em idade escolar em duas áreas do Egito para entender a atual exposição à poluição por chumbo e seus fatores de risco, para melhorar as políticas de prevenção.
Indivíduos e métodoEsse foi um estudo transversal em crianças (400) com idades entre 6–12 anos recrutadas de duas áreas no Egito (industrial e urbana). Os NCSs foram medidos por um método de absorção atômica. Foram obtidos questionários detalhados sobre as fontes de exposição ao chumbo e o histórico de desempenho escolar e quaisquer alterações comportamentais.
ResultadosO NCS na área urbana do Egito (Dokki) foi 5,45±3,90μg/dL, ao passo que na área industrial (Helwan) foi 10,37±7,94μg/dL, com uma diferença significativa entre ambas as áreas (p<0,05). Na área de Dokki, 20% do grupo estudado apresentaram NCSs ≥10μg/dL, ao passo que na área de Helwan foi 42%. Foi encontrada uma associação significativa entre as crianças com comportamento anormal e aquelas com palidez com NCS≥10μg/dL, em comparação àquelas com NCS<10μg/dL (p<0,05). Aquelas que moram na área de Helwan aquelas com hábitos de saúde ruins e aquelas que moram em moradias com maior exposição estiveram significativamente em alto risco de apresentar NCS≥10μg/dL.
ConclusãoO chumbo ainda é um problema de saúde pública no Egito. Altos NCSs foram significativamente associados a hábitos de saúde ruins e moradia com maior exposição, bem como, comportamento anormal e palidez.
Blood lead level (BLL) is a major health hazard, especially in children, which warrants its frequent monitoring in order to avoid lead exposure as much as possible.1 Lead has been used in many products such as paint, pipes, and ceramics, and remains a public hazard. The main sources of lead and its pollution are mining operations, battery recycling plants, and smelting,2,3 as well as old lead-based peeled or chipped paint, especially during renovations of old houses,4,5 contact with contaminated dust or soil,6 lead in plumbing, automobile exhaust, by-products of both mining and metal working, and various consumer products.7,8 E-scrap recycling is an emerging area of concern as a source of occupational exposures among workers and a source of take-home exposures.9
Lead is not known to serve any physiological function, but it exists in almost all biological systems. It is absorbed via different routes; however, ingestion of contaminated dietary constituents accounts for the majority of lead toxicity in children.10 Children are more likely to be exposed than adults due to a high rate of inhalation and more intestinal absorption. Intense, high-dose exposure to lead causes acute symptomatic poisoning, characterized by colic, anemia, and depression of the central nervous system that may result in coma, convulsions, and death. Low BLLs are now known to affect multiple organs in the absence of apparent symptoms. Toxicity with low lead levels in utero and during childhood constitutes brain and nervous system damage. Exposure to low BLL (less than 10μg/dL) affects also the immune, reproductive and cardiovascular systems. Recent research indicates that, at blood levels of 5μg/dL or lower, neurobehavioural damage is liable to occur. It appears that brain injury can happen at any blood level threshold.11–13
The goals of the present study were to evaluate the extent of lead exposure in schoolchildren, whose age ranged from 6 to 12 years, in industrial and urban areas in Egypt and to investigate the possible influencing factors in order to clarify the current problem of lead exposure and to improve methods of prevention and control.
Subjects and methodsTwo hundred primary school children were randomly chosen in an industrial area in Egypt (Helwan); the same number of primary school children was selected in an urban area in Egypt (Dokki). The study was approved by the Medical Ethical Committee of National Research Centre, Dokki, Cairo, Egypt. Parental consents were obtained. Parents who opted to join the study answered detailed questionnaires on sources of water supply, housing (old houses with defective paints/use of lead-glazed ceramics), living with adults whose job involve battery repair, recycling, or processing, dietary habits (e.g., using newspapers to wrap the children's food), exposure to air pollution (location of the house close to main roads), and outdoor playing in dust areas. History of school performance, behavioral changes (such as anxiety and aggressiveness), and any clinical problems were investigated. Teachers and other school staff were asked to assess each child's performance and behaviors.
A thorough clinical examination was performed, including weight, height, blood pressure, cardiac, abdominal, and full neurological examinations. A psychological assessment was also performed through an interview with a specialist. Complete blood count and BLL were assessed.
Blood samples were collected according to the instructions of the Centers of Disease Control and Prevention (CDC). All sample collection tubes and materials were pre-screened for lead contamination. Blood samples were analyzed for lead level with the atomic absorption method described by Miller et al.,14 using heating graphite atomization (HGA 600 perkin Elmer). BLLs were considered as “elevated” or “level of concern” if equal to or greater than 10μg/dL, in accordance with the US Centers for Disease Control and Prevention criteria.15
Statistical analysisStatistical analysis was performed using SPSS version 16 for Windows (SPSS Inc., version 16 for Windows, IL, USA). Continuous data (BLLs) were expressed as mean±standard deviation and were compared using Student's t-test. Categorical data were expressed as frequencies and percentages, and were analyzed with the two-tailed chi-squared test. Multiple logistic regression analysis was used to analyze the predictors of BLL≥10μg/dL and to modify the association between the clinical data and high BLL by adding the covariates. p-Values<0.05 were considered to be statistically significant.
ResultsA total of 400 children were enrolled in the present study; their age ranged from 6 to 12 years, with a mean of 9.40±2.33. From the total, 205 children were males and 195 were females. Mean BLL was 10.37±7.94μg/dL in Helwan and 5.45±3.90μg/dL in Dokki, with a significant difference between both areas (p<0.05). No significant difference was found between the genders in both areas regarding BLL (p>0.05). In Dokki, 20% of the studied group had BLLs≥10μg/dL, while in Helwan this value was 42%. The percentage of children with BLL≥5μg/dL was 44% in Dokki, versus 64% in Helwan.
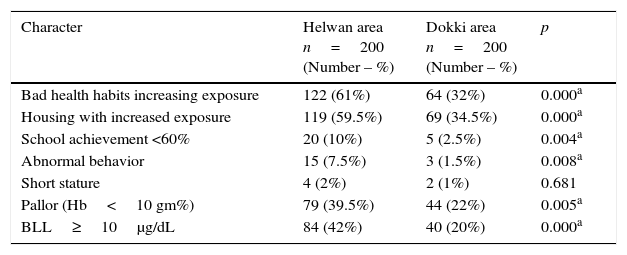
The social and clinical data of the study population are presented in Table 1, showing that children in Helwan had significantly worse results in health habits, housing with increased exposure, school achievement <60%, abnormal behavior, and pallor than children in Dokki (p<0.05).
Clinical and social data of the study population (n=400).
| Character | Helwan area n=200 (Number – %) | Dokki area n=200 (Number – %) | p |
|---|---|---|---|
| Bad health habits increasing exposure | 122 (61%) | 64 (32%) | 0.000a |
| Housing with increased exposure | 119 (59.5%) | 69 (34.5%) | 0.000a |
| School achievement <60% | 20 (10%) | 5 (2.5%) | 0.004a |
| Abnormal behavior | 15 (7.5%) | 3 (1.5%) | 0.008a |
| Short stature | 4 (2%) | 2 (1%) | 0.681 |
| Pallor (Hb<10 gm%) | 79 (39.5%) | 44 (22%) | 0.005a |
| BLL≥10μg/dL | 84 (42%) | 40 (20%) | 0.000a |
Hb, hemoglobin; BLL, blood lead level.
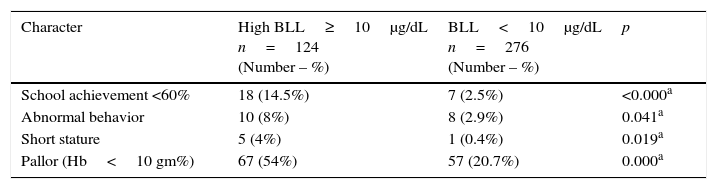
The association between clinical data of all children and high BLL is presented in Table 2. A significant association was observed between BLL≥10μg/dL and children with school achievement <60%, children with abnormal behavior, children with short stature, and pallor, when compared with those with BLL<10μg/dL (p<0.05).
Association of clinical data of children and high blood lead levels (≥10μg/dL).
| Character | High BLL≥10μg/dL n=124 (Number – %) | BLL<10μg/dL n=276 (Number – %) | p |
|---|---|---|---|
| School achievement <60% | 18 (14.5%) | 7 (2.5%) | <0.000a |
| Abnormal behavior | 10 (8%) | 8 (2.9%) | 0.041a |
| Short stature | 5 (4%) | 1 (0.4%) | 0.019a |
| Pallor (Hb<10 gm%) | 67 (54%) | 57 (20.7%) | 0.000a |
Hb, hemoglobin; BLL, blood lead level.
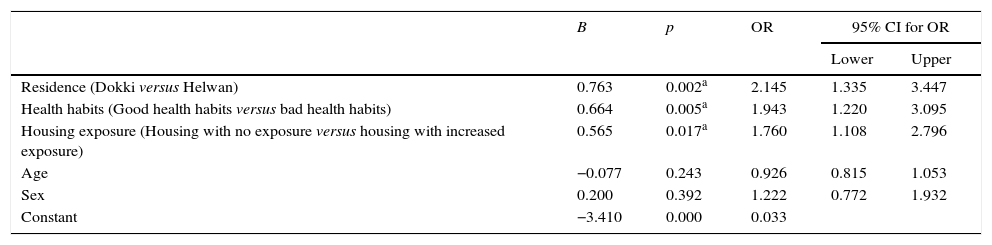
Multiple logistic regression analysis was done to study the predictors of high blood lead level (≥10μg/dL; Table 3). It was found that those living in Helwan, those with bad health habits, and those living in housing with increased exposure were at a significantly higher risk of having BLL≥10μg/dL (OR=2.16, 95% CI 1.35–3.47; OR=1.947, 95% CI 1.22–3.10; OR=1.72, 95% CI 1.09–1.09, respectively, p<0.05 in all).
Predictors of high blood lead level (≥10μg/dL; multiple logistic regression analysis).
| B | p | OR | 95% CI for OR | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Residence (Dokki versus Helwan) | 0.763 | 0.002a | 2.145 | 1.335 | 3.447 |
| Health habits (Good health habits versus bad health habits) | 0.664 | 0.005a | 1.943 | 1.220 | 3.095 |
| Housing exposure (Housing with no exposure versus housing with increased exposure) | 0.565 | 0.017a | 1.760 | 1.108 | 2.796 |
| Age | −0.077 | 0.243 | 0.926 | 0.815 | 1.053 |
| Sex | 0.200 | 0.392 | 1.222 | 0.772 | 1.932 |
| Constant | −3.410 | 0.000 | 0.033 | ||
OR, odds ratio; CI, confidence interval.
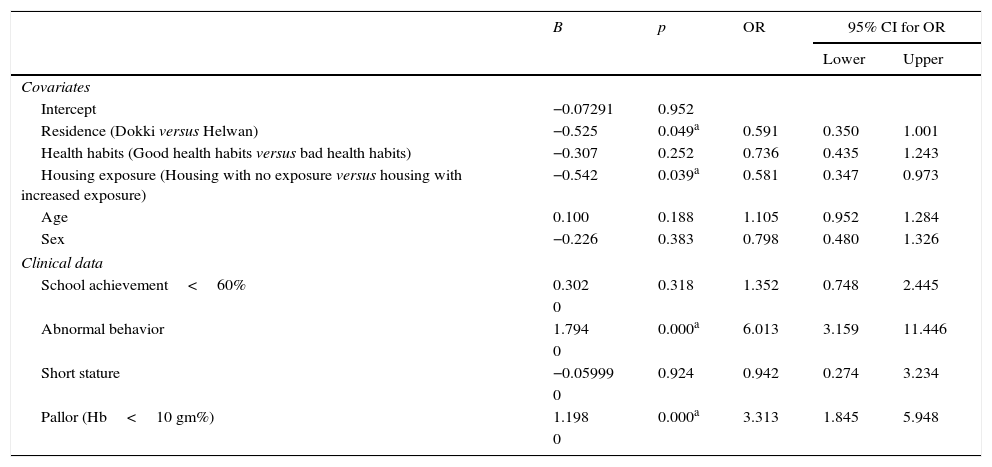
Table 4 shows the association between the clinical data and high BLL after modification by the predictors of high BLL as covariates. Abnormal behavior and pallor were associated with high BLL≥10μg/dL (p<0.05), while school achievement <60% and short stature were not associated with high BLL (p>0.05) after modification by the covariates.
Association of clinical data and high blood lead level (≥10μg/dL) after modification by covariates (multiple logistic regression analysis).
| B | p | OR | 95% CI for OR | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Covariates | |||||
| Intercept | −0.07291 | 0.952 | |||
| Residence (Dokki versus Helwan) | −0.525 | 0.049a | 0.591 | 0.350 | 1.001 |
| Health habits (Good health habits versus bad health habits) | −0.307 | 0.252 | 0.736 | 0.435 | 1.243 |
| Housing exposure (Housing with no exposure versus housing with increased exposure) | −0.542 | 0.039a | 0.581 | 0.347 | 0.973 |
| Age | 0.100 | 0.188 | 1.105 | 0.952 | 1.284 |
| Sex | −0.226 | 0.383 | 0.798 | 0.480 | 1.326 |
| Clinical data | |||||
| School achievement<60% | 0.302 | 0.318 | 1.352 | 0.748 | 2.445 |
| 0 | |||||
| Abnormal behavior | 1.794 | 0.000a | 6.013 | 3.159 | 11.446 |
| 0 | |||||
| Short stature | −0.05999 | 0.924 | 0.942 | 0.274 | 3.234 |
| 0 | |||||
| Pallor (Hb<10 gm%) | 1.198 | 0.000a | 3.313 | 1.845 | 5.948 |
| 0 | |||||
OR, odds ratio; CI, confidence interval; Hb, hemoglobin.
Childhood lead poisoning is a major problem that can be prevented worldwide. In the current study, mean BLL in an urban area of Egypt (Dokki) was 5.45±3.90μg/dL, while that in an industrial area (Helwan) was 10.37±7.94μg/dL. In Dokki, 20% of the studied group had BLLs≥10μg/dL, versus 42% in Helwan. The percentage of children with BLL≥5μg/dL was 44% in Dokki, versus 64% in Helwan. In Singapore, from 1995 to 1997, the mean reported BLL was 6.6μg/dL in 269 children.16 Children aged 12–19 years were assessed in 1999 in the United States, and a mean BLL of 1.1μg/dL was found17; from 2007 to 2010, the geometric means at age 1–2 years and 3–5 years were found to be 1.5μg/dL and 1.2μg/dL, respectively.18
A cross-sectional survey for BLLs included 3831 children recruited at hospitals in France. The geometric mean BLL was 1.49μg/dL; 0.09% of the children had BLL exceeding 10μg/dL, and in 1.5%, BLL exceeded 5μg/dL.19
In 2011, a study of blood lead concentrations of 226 schoolchildren was conducted in Alpuyeca, Morelos, Mexico. The mean BLL was 7.23μg/dL; BPb>5μg/dL and >10μg/dL were observed in 64% and 18% of the children, respectively. In almost 50% of the households, the use of lead-glazed ceramics was reported.20
Ecuadorian infants and young children (n=130) aged 0.33–5.8 years had their BLL assessed; the mean BLL was 29.4μg/dL (SD: 24.3; range: 3.0–128.2; median: 21.7; geometric mean: 20.7μg/dL).21 To measure current lead exposure in Japanese children, blood samples were collected from 229 children aged 9–10 years in Asahikawa, Japan; The geometric mean was 0.96μg/dL.22
Children in six communities near to the now-closed Kabwe mines and smelters in Zambia were studied for BLL; the mean was 4.83μg/dL. The lowest BLL measured was 1.36μg/dL. The highest BLL detected using the testing system was 6.5μg/dL.23
BLL was also assessed in the Bagega community in Nigeria; the median was 71μg/dL (range: 8–332μg/dL). Elevated BLL (≥10μg/dL) was found in 99.5% of the studied group.24 In Wuhan, China, children less than 18 years were investigated throughout the entire year of 2012. For all subjects, the geometric mean was 4.48μg/dL. Elevated BLLs (≥10μg/dL and ≥5μg/dL) were found in 2% and in 44%, respectively.25 In South Africa, a cross-sectional analytical studies including 160 young schoolchildren was performed. The mean BLL was 7.4μg/dL (range: 2.2–22.4μg/dL). BLLs≥5μg/dL were observed in 74% of the children, while 16% had BLL≥10μg/dl.26
BLLs were assessed in children living in Riyadh, Saudi Arabia. The mean (±SD) BLL was 5.2±1.7, ranging from 1.7 to 10.6μg/dL; in 17.8% of children, BLL was greater than 10μg/dL.27
A study conducted in three schools in Nablus, Palestine, assessed BLL in 178 children (140 boys, 38 girls, age range 6–8 years). The mean BLL was 3.2±2.4μg/dL, and levels above 10μg/dL were observed in 4.5% of children.28
In the present study, high BLLs were found in Helwan area, which is an old industrial area with many lead-related industries and factories. Other predictors of high BLL were bad health habits (using newspapers to wrap children's food; no hand washing before meals and after playing outside; contact with contaminated dust, soil and toys; ingestion of preserved eggs; and consumption of fried food) and housing with increased exposure (old lead-based peeled or chipped paint, renovations, lead in plumbing, and use of lead glazed ceramics).
No significant differences were detected in the current study between males and females in both Dokki and Helwan regarding BLLs, which can be explained by the similar behavior and outdoor activities of both genders. This result is in agreement with those by Allen Counter et al.21 Cao et al.29 found that the mean BLLs in boys (23.57mg/L) were higher than that in girls (21.2mg/L), which was explained by the greater distinct behavior and outdoor activities found with growing boys, leading to contact with environmental lead pollution.
In a multivariable analysis by Spanier et al.,5 mean BLLs of children whose housing underwent interior renovation was 12% higher than that of children whose housing units were not renovated (p<0.01). Renovation, repairs, and painting rule, routine interior housing renovation were associated with a modest increase in children's BLLs, which are in agreement with the present study.
In the present study, school achievement <60%, short stature, and pallor (Hb<10g/dL) were associated with high BLLs (≥10μg/dL). Liu et al.30 conducted a prospective cohort study that assessed children in four elementary schools. They detected a significant association between BLL and increased scores for teacher-reported behavioral problems (emotional reactivity, anxiety/depression, and pervasive developmental problems), which is in agreement with the present results. Boys experience the deleterious cognitive effects of lead more than girls do.31
A cross-sectional assessment in children and adolescents aged 0–17 years from six communities in the Corrientes river basin was conducted. Children and adolescents with BLLs>5μg/dL had twice the risk of stunting when compared with those with lower BLLs.32 Fleisch et al.33 concluded that, in peripubertal boys, higher BLLs were associated with lower serum IGF-1, which is attributed to the inhibition of the hypothalamic–pituitary-growth axis by lead exposure, leading to growth delay. The results of the previous two studies are in agreement with the present results, which indicated a significant association between high BLLs and stunting.
The present study had some limitations, which included its small sample size; its cross-sectional design, which may not allow the follow-up of the association between clinical data and BLLs; and the questionnaire used to collect information on risk factors, which may have caused recall bias. The measurement of BLL through the gold standard method (graphite atomic absorption method) added strength to the present study.
In conclusion, BLL was measured and associated to exposure risk factors in two areas (one urban and one industrial) in Egypt. BLLs were significantly higher in the industrial area than in the urban area. High BLLs were significantly associated with bad health habits and housing with increased exposure, as well as with school achievement <60%, short stature, and pallor (Hb<10g/dL).
In Egypt, definitive plans and regulations should be carried out to improve the prevention and control of childhood lead poisoning. Regulatory policies are needed to reduce lead release from different industries. BLL screening and testing should be improved. In industrial areas, screening programs for BLL should be implemented. Finally, the prevention and treatment of childhood lead poisoning should be improved in Egypt.
This study examined the relationship between BLL and its risk factors in Egyptian children. The use of a representative sample of the general Egyptian children strengthened the present findings. However, there were some limitations to this study. First, it had a cross-sectional design, and lead exposure throughout the entire childhood development could not be assessed. Second, other hazardous environmental pollutants (e.g., mercury and cadmium) were not evaluated. Finally, the sample size was relatively small.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: AbuShady MM, Fathy HA, Fathy GA, Fatah Sa, Ali A, Abbas MA. Blood lead levels in a group of children: the potential risk factors and health problems. J Pediatr (Rio J). 2017;93:619–24.