This study aimed to critically review the literature available regarding the Zika virus outbreak in Brazil and its possible association with microcephaly cases.
SourcesExperts from Instituto do Cérebro do Rio Grande do Sul performed a critical (nonsystematic) literature review regarding different aspects of the Zika virus outbreak in Brazil, such as transmission, epidemiology, diagnostic criteria, and its possible association with the increase of microcephaly reports. The PubMed search using the key word “Zika virus” in February 2016 yielded 151 articles. The manuscripts were reviewed, as well as all publications/guidelines from the Brazilian Ministry of Health, World Health Organization and Centers for Disease Control and Prevention (CDC – United States).
Summary of findingsEpidemiological data suggest a temporal association between the increased number of microcephaly notifications in Brazil and outbreak of Zika virus, primarily in the Brazil's Northeast. It has been previously documented that many different viruses might cause congenital acquired microcephaly. Still there is no consensus on the best curve to measure cephalic circumference, specifically in preterm neonates. Conflicting opinions regarding the diagnosis of microcephaly (below 2 or 3 standard deviations) that should be used for the notifications were also found in the literature.
ConclusionThe development of diagnostic techniques that confirm a cause–effect association and studies regarding the physiopathology of the central nervous system impairment should be prioritized. It is also necessary to strictly define the criteria for the diagnosis of microcephaly to identify cases that should undergo an etiological investigation.
O objetivo deste estudo foi realizar uma revisão crítica da literatura sobre o surto de vírus Zika no Brasil e sua possível associação com casos de microcefalia.
Fonte de dadosEspecialistas em áreas afins do Instituto do Cérebro do Rio Grande do Sul realizaram uma revisão crítica (não sistemática) da literatura sobre o vírus Zika, suas formas de transmissão, a epidemia no Brasil, critérios diagnósticos e a possível associação com os casos crescentes de microcefalia. O uso da palavra chave “Zika virus” na base de dados do PubMed em fevereiro de 2016, retorna 151 publicações. Estes textos foram revisados assim como todas as publicações e recomendações do Ministério da Saúde, Organização Mundial da Saúde e Centro de Controle de Doenças (CDC – USA).
Síntese dos dadosOs dados epidemiológicos sugerem uma relação temporal entre aumento da notificação de casos de microcefalia e o surto de vírus Zika, principalmente no Nordeste do Brasil. Agentes virais comprovadamente podem ser causadores de microcefalia congênita adquirida. Não existe um consenso sobre a melhor curva de perímetro cefálico a ser utilizada, principalmente nos prematuros. Assim como também existem divergências sobre a definição de microcefalia (abaixo de 2 ou 3 desvios padrões) a ser padronizada nas notificações.
ConclusãoDeve-se priorizar o desenvolvimento de técnicas diagnósticas que confirmem uma relação causa–efeito e estudos sobre mecanismos da patogênese da infecção pelo Zika no sistema nervoso central. Também é necessário definir e universalizar os critérios diagnósticos para a identificação dos casos de microcefalia que devem ser submetidos à investigação etiológica.
The World Health Organization (WHO) has issued a warning establishing an international state of emergency due to the microcephaly incidence increase in endemic areas with Zika virus (ZikaV) proliferation.1
The disease, which arrived in Brazil possibly in 2014, has spread in the Northeast region and is migrating to the Americas. It is believed that it will quickly continue spreading, as its main vector, the Aedes aegypti mosquito, is undergoing a period of wide dissemination due to the high summer temperatures in the southern hemisphere.2–4
A possible association between intrauterine infection by ZikaV and early microcephaly was initially proposed, based on the observation of physicians in Northeastern Brazil, who detected a sudden increase in the incidence of births of microcephalic children after identification of the virus entry in Brazil. However, this cause–effect association still needs to be proven.5,6 The fact is that the ZikaV enters the central nervous system (CNS), breaking the protection of the blood–brain barrier, which has been previously demonstrated in animal experiments.7,8
The recent discovery of other forms of virus transmission, in addition to an infected insect bite, through sexual contact or secretions (saliva, urine), and the lack of vaccines or specific treatment has alarmed the population. Moreover, the lack of longer-duration biological markers that allow for diagnostic confirmation, geometrically increase the number of suspected cases and, consequently, the recording of false positive cases.9–11
Pediatricians are exposed to the demand for knowledge of this new disease, as they treat the neonate and initiate the investigation of the microcephaly status. As the scientific literature is still scarce and new findings and information have quickly appeared and been widely disseminated in the media, this article aimed to bring together a multidisciplinary group of experts in associated fields to critically review the scientific evidence, diagnostic protocols, differential diagnosis, and research perspectives that allow for the confirmation of the association between ZikaV and microcephaly.
MethodsThis was a non-systematic review article conducted by a panel of experts. This article was divided into topics considered significant for a better understanding of ZikaV outbreak in Brazil, such as the diagnostic difficulties of this infection and the differential diagnosis with other infections whose vector is also the Aedes aegypti mosquito; biological characteristics of ZikaV; possible associations between ZikaV infection and CNS development alterations; criteria for determining microcephaly; and etiological differential diagnosis of microcephalies. Additionally, the article discusses possible experimental models that would help to understand the physiopathogenesis of CNS lesions.
ResultsThe use of the key word “Zika virus” in the PubMed database in February 2016 yielded 151 publications. These texts were reviewed, as well as all publications and recommendations from the Brazilian Ministry of Health, World Health Organization, and Centers for Disease Control and Prevention (CDC – United States).
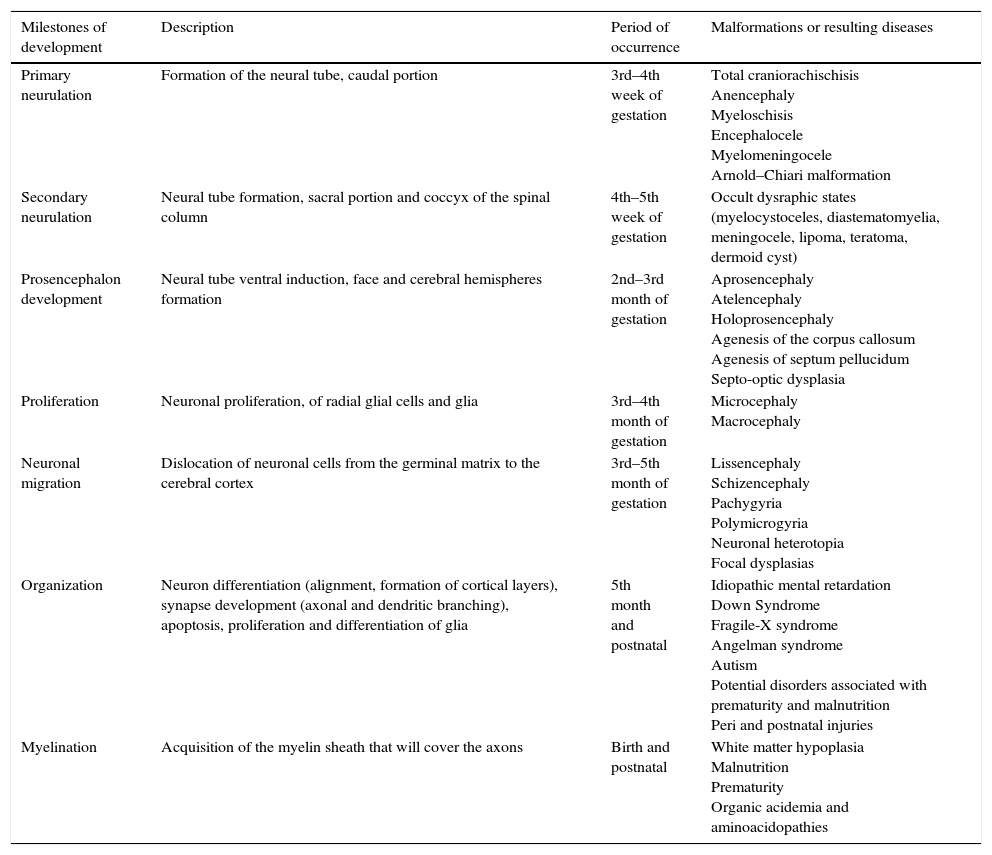
Stages of intrauterine development of the central nervous system (CNS) and associated disordersThe CNS development in humans starts at the gastrulation stage, approximately on the 14th embryonic day, when the thickening of the ectodermal membrane occurs, originating the neural plate. Subsequently, two major processes will occur sequentially: the formation of the neural tube and the development of the prosencephalon. Table 1 lists the developmental milestones, the time of their occurrence, and the defects arising from problems occurring at these stages.12
Milestones of central nervous system development.
| Milestones of development | Description | Period of occurrence | Malformations or resulting diseases |
|---|---|---|---|
| Primary neurulation | Formation of the neural tube, caudal portion | 3rd–4th week of gestation | Total craniorachischisis Anencephaly Myeloschisis Encephalocele Myelomeningocele Arnold–Chiari malformation |
| Secondary neurulation | Neural tube formation, sacral portion and coccyx of the spinal column | 4th–5th week of gestation | Occult dysraphic states (myelocystoceles, diastematomyelia, meningocele, lipoma, teratoma, dermoid cyst) |
| Prosencephalon development | Neural tube ventral induction, face and cerebral hemispheres formation | 2nd–3rd month of gestation | Aprosencephaly Atelencephaly Holoprosencephaly Agenesis of the corpus callosum Agenesis of septum pellucidum Septo-optic dysplasia |
| Proliferation | Neuronal proliferation, of radial glial cells and glia | 3rd–4th month of gestation | Microcephaly Macrocephaly |
| Neuronal migration | Dislocation of neuronal cells from the germinal matrix to the cerebral cortex | 3rd–5th month of gestation | Lissencephaly Schizencephaly Pachygyria Polymicrogyria Neuronal heterotopia Focal dysplasias |
| Organization | Neuron differentiation (alignment, formation of cortical layers), synapse development (axonal and dendritic branching), apoptosis, proliferation and differentiation of glia | 5th month and postnatal | Idiopathic mental retardation Down Syndrome Fragile-X syndrome Angelman syndrome Autism Potential disorders associated with prematurity and malnutrition Peri and postnatal injuries |
| Myelination | Acquisition of the myelin sheath that will cover the axons | Birth and postnatal | White matter hypoplasia Malnutrition Prematurity Organic acidemia and aminoacidopathies |
Modified from Volpe.12
The proliferation phase is quite complex and broad, starting around the second to the fourth month of pregnancy, with neuronal proliferation and generation of glial radial cells, extending from the fifth month until the end of the first year of life, when glial proliferation occurs. The initial process is characterized by the germinal matrix stem cells that divide symmetrically, forming the proliferative neuronal–glial units, which are distributed in the periventricular zone. After that, an asymmetric division starts, in which each stem cell originates another stem cell and a postmitotic neuronal cell. This asymmetric division determines the proliferative unit size.13
The postmitotic neurons migrate along the radial glia to form the different layers of the cerebral cortex. Approximately one-third of the neurons do not use the radial glia structure and migrate tangentially in the cortical direction.14 During the migration process, neurons pass through neurons that are already positioned in the cortex, leading to a lamination where the last arriving neurons in cortical area position themselves on the outermost surface of the cortex. This final, histologically distinct six-layer organization comprises an extraordinary diversity of neuronal subtypes, working as units for the formation of a powerful neural circuit. In recent years, remarkable progress has been made to understand the molecular events that control the development of the cerebral cortex, as well as the disorders associated with alterations in this process.15
The alterations in the cerebral cortex development are shown as a group of different malformations whose pathogenesis is not well defined yet. Among the cortical defects, a subset of conditions has been associated with alterations in cell migration and neurodifferentiation, among them the lissencephalies, polymicrogyria, and focal cortical dysplasias. Some researchers suggest that in addition to alterations in cell migration and neurodifferentiation, one must consider other factors in the pathogenesis of cortical malformations, such as cell proliferation, cell death, intracortical growth and development after cell migration, and the formation of axons and dendrites.16,17
The majority of pathologies associated with cell migration alterations have a variety of genes that may be associated with the diseased phenotype, but in some cases its appearance is sporadic. The high epileptogenicity of the cortex in these cases is noteworthy.18
Several environmental factors have been identified as causing cortical defects; maternal exposure during pregnancy to agents such as ethanol, some acids, anticonvulsants, mercury, radiation, and viral agents, among others, may result in cortical development disorders in the fetus.17
Microcephaly diagnosisThe observed association between fetal infection by ZikaV and the occurrence of microcephaly at birth has increased the importance of an accurate diagnosis of microcephaly. It is necessary to identify possible cases and at the same time, avoid expensive investigation and a state of alertness in the healthcare system. In practice, a head circumference at birth smaller than two standard deviations of the mean for gestational age has been used for clinical diagnosis of microcephaly. Reports on the prevalence of microcephaly using this criterion were approximately 0.5%, which is well below what would be expected for two standard deviations.19,20 This is probably due to a non-normal distribution of the measured head circumference. Severe microcephaly, i.e., smaller than three standard deviations of the mean, occurs in 1 per 1000 births.19
Although it is a relatively simple and reliable diagnostic method, the simple head circumference measurement might not reflect an abnormally small brain and does not offer data to demonstrate an abnormal skull shape. Thus, it is extremely important that the measurement and its interpretation are correct.
The head circumference measurement is carried out with a flexible and inelastic measuring tape. The skull diameter is measured at its largest circumference, using the tape around the head, by placing it on the forehead above the eyes, passing it above the ears and the most prominent portion of the back of the skull. In full-term babies, this perimeter must be greater than 32cm (small variation by gestational age and gender). The head circumference increases almost linearly up to 37–38 weeks of gestation.21
The choice of the curve and the criteria used to define microcephaly has been the subject of extensive discussion, especially regarding preterm infants.22,23 The Pan-American Health Organization suggests using both the Fenton curve and the curve of the InterGrowth study.24–26 However, the analysis made by Victora et al. shows how the choice of the curve can have an impact on the number of suspected cases, exponentially increasing the number of children to be investigated and exposed to CT radiation. These authors suggest that tools with greater specificity should be given priority, such as the InterGrowth curve in exchange for sensitivity.23
It is also important to perform an analysis of the proportion of head circumference in relation to other growth measures, such as weight and length, in order to define the suspected microcephaly etiology.19
In spite of being an objective measure, a study that assessed interobserver variation showed differences of more than 2cm in 5% of them.27 Therefore, the staff that performs the measurement should be trained and, when the measurement is borderline or abnormal, it should be repeated.
In the presence of abnormal skull shapes due to family, genetic characteristics, or molding caused by the birth process, the head circumference measurement can be misleading and not reflect a reduction in brain tissue. One suggestion to reduce microcephaly misdiagnosis would be to repeat the measurements one or more days after birth, especially in the presence of overriding sutures at birth with borderline measures. The abnormal shape should lead to further assessment for the presence of craniosynostosis and/or genetic diseases.
Etiological differential diagnosis of microcephaliesThe more adequate term is microcephalies, considering the different forms, etiologies, and clinical expression. The pathogenesis of microcephaly is heterogeneous, from genetic causes to environmental factors that can have an impact on neurodevelopment and, thus, influence brain growth. Therefore, any factor that can interfere with cell proliferation and/or differentiation, cell death, among others, can induce microcephaly. These factors may affect the brain development only or impair other parts of the body, determining dysmorphisms (syndromic microcephalies).22,28
To facilitate the clinical investigation and the differential diagnosis of microcephalies, they will initially be classified based on the time of diagnosis. Thus, microcephalies can be divided into two categories: congenital microcephaly, which is present from birth and postnatal microcephaly, which develops after the neonatal period and, in general, in the first two years of life.22,28–30 The microcephalies may be genetic or acquired, with the latter encompassing external/environmental factors that are potentially harmful to the brain.
In acquired congenital microcephalies, aggressive factors act during the intrauterine brain development. These factors include maternal infections (toxoplasmosis, cytomegalovirus, herpes virus, syphilis, rubella, human immunodeficiency virus [HIV], and now the possible association with ZikaV), exposure to drugs/toxic substances, especially the maternal consumption of alcohol (fetal alcohol syndrome), radiation, disruptive factors that disrupt normal brain development (e.g., hemorrhage, ischemia, hypoxic–ischemic syndrome, and traumatic brain injury), and nutritional deficiency (maternal malnutrition, placental failure, hypothyroidism, or maternal folate deficiency).22,28,31–33
The genetic congenital microcephalies may be associated with chromosomal abnormalities or certain genes, such as the autosomal recessive primary microcephaly (also termed microcephaly primary hereditary [MCPH], and historically known as microcephaly vera), in which at least 12 genes have been identified and encode proteins associated with the centrosome.34,35 Here the term primary microcephaly corresponds to those cases in which the reduction in brain volume is primarily due to the reduction of the neuronal population during neurogenesis.29,36 In general, the overall structure of the brain is maintained in MCPH and there are no other malformations or alterations in brain development patterns.
Postnatal microcephalies are those in which the child is born with normal head circumference, with a reduction in head growth velocity over time, reaching microcephaly levels. The postnatal-onset microcephalies are associated with genetic causes or external/environmental (acquired) factors.22
Examples of acquired postnatal microcephalies include those resulting from traumatic brain injury, cerebral ischemia/hemorrhage, encephalitis, and severe malnutrition, among others. Several postnatal microcephalies are determined by mutations that interfere with the regulation of gene expression during brain development.30
Among the genetic postnatal microcephalies, all associated with mutations (deletion, insertion, duplication, fusion, and amino acid point mutations), are those resulting from inborn errors of metabolism, neurodegenerative diseases, and several syndromes, such as Angelman syndrome, Pitt–Hopkins syndrome, Rubinstein–Taybi syndrome, Christianson syndrome, and MECP2-related disorders (Rett syndrome).30
Knowledge of the prevalence, clinical history, and detailed clinical and neurological assessment can lead to the diagnosis. The clinical history may reveal exposure to agent, accident, or episode harmful to the brain, and the clinical assessment may suggest some less common syndromes.
The ZikaVThe ZikaV, which belongs to the Flaviviridae family, is related to other flaviviruses of medical relevance transmitted by arthropod vectors, such as the causative agents of dengue fever, Chikungunya fever, yellow fever, and West Nile encephalitis. The ZikaV was isolated in 1947 from non-human primates (sentinel monkeys for yellow fever monitoring) in the Zika forest in Uganda, which was adopted as the name for this virus.37
There are at least two lineages of ZikaV, the African lineage (which some authors divide into West and East Africa) and the Asian lineage. Phylogenetic studies indicate that the virus currently spreading in the Americas derived from the Asian lineage.38 ZikaV transmission occurs primarily through a vector, the Aedes mosquitoes; in the sylvatic cycle, through species such as A. albopictus, among others, whereas in the urban cycle, the A. aegypti is the vector. The virus is transmitted by the hematophagous vector during feeding, by lodging in their salivary glands, where it multiplies without affecting the insect, remaining in it throughout its life. In addition to primates, it is possible that other mammals, such as zebras, elephants, and rodents, can also be natural reservoirs of ZikaV.
Flaviviruses are small (∼50nm) spherical particles, surrounded by a lipid envelope.39 The ZikaV is a single-strand RNA virus with 10.794 kilobase, with two non-coding regions and a long reading frame encoding a polyprotein. That is cleaved by host cell proteases to originate the capsid protein C, the precursor of membrane (prM) and envelope E protein, and seven non-structural proteins, called NS1 to NS7. The virions, or viral particles, contain 180 copies of protein E, unknown amounts of other proteins, and a single molecule of viral RNA. The surface of the virions is formed by E and M proteins. The E protein (∼53kDa) is glycosylated, being the main viral antigenic determinant, with membrane binding and fusion functions when the virus enters the infected cell.40 It has been postulated that virions are endocytosed and exposure to the acidic environment in lysosomes promotes the fusion between the virus envelope and the cell membranes. Upon release of viral RNA into the cytoplasm of infected cells, it is replicated and translated by the cellular machinery, leading to the formation of new viral particles.
Two complete genomes of the ZikaV, including the current variety circulating in the Americas are now available.38,41 Faye et al. studied 37 virus isolates from different sources (mosquitoes, patients, and animals, among others.) obtained in Africa, showing evidence of genetic recombination and how changes in the glycosylation pattern of protein E are likely adaptations to the vector A. dalzielii.40 Freire et al., when investigating 17 genomic sequences of the virus, suggested that changes in the use of codons for the NS1 protein in the Asian lineage of the ZikaV can be correlated with higher rates of replication and viral titer in humans.42
Intrauterine viral infections that result in injuries to the central nervous system are relatively rare; cytomegalovirus, herpes virus, and rubella are among those already known to cause fetal diseases. Among Flaviviruses, only a few cases of West Nile encephalitis virus in pregnant women were reported as the probable cause of neurological damage in fetuses.43
Epidemiology and surveillance protocol in BrazilThe first cases of human infection were reported in Nigeria and Tanzania from 1952 to 1954.37 The virus later spread to the Asian continent and the international community only started to recognize the epidemic potential of the ZikaV after 2005 and especially after the 2007 outbreak in Micronesia (Yap island in the Pacific Ocean) and the 2012/2013 outbreak in French Polynesia. Imported cases were reported after 2013 in Germany, Canada, Italy, Japan, the United States, and Australia. In 2014 the presence of the virus was described in the Easter Island (Chile – Pacific Ocean).44 On April 29, 2015, the circulation of this virus was detected for the first time in Brazil and Latin America (continent). Researchers from the Universidade Federal da Bahia (UFBA) reported the identification of ZikaV by RT-PCR in eight of the 25 tested samples (Camaçari/BA).3,45
In October 2015, the Brazilian government initiated joint investigations with the state of Pernambuco after observing the increasing number of cases of microcephaly, especially in that state, after confirmation of the alteration in the pattern of microcephaly cases. In November, it launched the first National Guideline on Microcephalies, which still included the diagnosis of microcephaly through head circumference (HC) measurement ≤33cm. In that same month, after confirmation of the presence of ZikaV in the amniotic fluid of pregnant women in the state of Paraiba by Fiocruz, the association of ZikaV infection with microcephaly was confirmed.46 In December, the government released the Microcephaly Protocol – Surveillance – Version 1, changing the diagnosis of microcephaly, which started to be considered when HC was ≤32cm, also including in this protocol pregnant women with rash, miscarriage, microcephalic fetus, and stillbirth.44 On December 14, the government released the Microcephaly Protocol – Health Care. It is worth mentioning that this change in head circumference measure for the diagnosis of microcephaly can influence data analysis.
In January 2016, the National Guideline for Early Stimulation of Babies with Microcephaly was released. Four cases related to ZikaV were also identified by the CDC in that month. The Brazilian government has made adjustments in the operational definitions of cases: (A) Definitions of cases for microcephaly surveillance: (i) Newborn with microcephaly, (ii) Miscarriage suggestive of congenital infection, (iii) Stillbirth with microcephaly and/or CNS malformations suggestive of congenital infection, (iv) Fetus with microcephaly and/or CNS alterations, suggestive of congenital infection; and (B) Definitions of cases for surveillance of ZikaV infection during pregnancy: (i) Pregnant woman with acute rash, suggestive of ZikaV infection.46
The suspected microcephaly cases must be reported immediately to the health authorities and registered at the Public Health Event Registration Form (Registro de Eventos de Saúde Pública [RESP] – Microcephalies), available at: www.resp.saude.gov.br. The notification of suspected cases of microcephaly in the RESP does not exclude the need to report the case to the Live Births Information System (Sistema de Informações sobre Nascidos Vivos [SINASC]).47,48
After the establishment of the association of ZikaV with microcephaly, it is necessary for care and prevention measures to be carried out and intensified, but it is essential to explain and sooth the population, and to analyze the actual number of cases.
On January 20, 2016, the Brazilian Ministry of Health issued an epidemiological report with some relevant and interesting changes, such as changing the name “Surveillance and Response Protocol to the Occurrence of Microcephaly related to ZikaV infection” to “Surveillance and Response Protocol to the Occurrence of Microcephaly,” regardless of the cause. This same report describes, in separate, the 224 cases of microcephaly confirmed through imaging assessment, with typical alteration in the six cases with positive sample for ZikaV.49
Another epidemiological report on February 2, 2016 was issued with a cumulative total (2015–2016) of 4783 reported cases of microcephalies and/or other CNS disorders, including possible cases related to ZikaV and other infections. It is important to note that Brazil does not have almost 5000 cases of ZikaV-related microcephaly, as many patients and also some health professionals may imagine. In fact, this number corresponds to the notification of suspected cases: the number of cases still under investigation is 3670, whereas the number of cases already assessed is around 1113. Of these assessed cases, microcephaly related to congenital infection was ruled out in 709 (63%). The remaining cases, 404 (37%), were considered confirmed for microcephaly and/or other CNS abnormalities, although the identification of the ZikaV presence in the amniotic fluid, placenta and fetal tissues occurred in only 17 cases to date.49
Another epidemiological report on February 12, 2016 was issued with a cumulative total (2015–2016) of 5079 reported cases of microcephaly and/or other CNS disorders, including possible cases related to ZikaV and other infections. Since the beginning of the investigations, 462 confirmed cases were reported in 175 municipalities of 13 Brazilian states. The northeast region has 98% of the municipalities with confirmed cases: Pernambuco has 167 cases, followed by Bahia (101), Rio Grande do Norte (70), Paraíba (54), Piauí (29), Alagoas (21), Ceará (11) Espírito Santo (3), Rio de Janeiro (t2), Pará (1), Goiás (1), Mato Grosso do Sul (1), and Rio Grande do Sul (1).50
The Brazilian Medical Genetics Society-Zika embryopathy Task Force indicates four case report limitations. First, a previous underreporting of actual cases of microcephalies in Brazil and the fact that the notification protocol itself may have favored the increase of reported cases. Second, mild cases of microcephaly might not have been notified because the head circumference measurement was not routinely performed. Third, the ZikaV infection was not confirmed in newborns and mothers by a laboratory, and a history of non-specific rash illness during pregnancy is subjective and can lead to a recall bias, resulting in misclassification of potential exposure to ZikaV. Fourth: the reporting of cases does not comment on other common findings in intrauterine infections, such as hepatosplenomegaly, skin rash, chorioretinitis, or some characteristics that have been reported in cases of suspected ZikaV infection, including hearing loss, pale macula, and swallowing difficulties.5
This analysis of the numbers should not be used to decrease preventive measures; however, it would be very interesting to advise pregnant women to adopt measures to reduce the presence of the Aedes aegypti, by eliminating breeding sites and protecting themselves against mosquito exposure, by keeping doors and windows closed or screened, wearing long pants and long-sleeved shirts, and using repellents allowed during pregnancy.
Multidisciplinary advice, such as the routine assessment of a child at the pediatrician, can be an opportunity for these women to receive recommendations on planning their next pregnancy and that they should consult with the gynecologist before stopping their contraceptive methods.
Clinical picture of ZikaV infections and differential diagnosis with dengue and Chikungunya feverThe dengue and Chikungunya viruses have similar clinical signs and symptoms, especially in the early stages of the disease.51 This also occurs with ZikaV. For this reason it is essential to differentiate between these three diseases.
ZikaApproximately 80% of infected individuals are asymptomatic.52,53 When present, symptoms can last from a few days to one week. Symptoms are usually mild, with characteristic sudden onset of fever, arthralgia, maculopapular rash, or nonpurulent conjunctivitis. According to data from previous outbreaks, severe forms are rare and that is the reason why hospitalizations and deaths are unusual.53,54 According to the CDC, the diagnosis of Zika should be suspected when two or more symptoms (including fever, rash, arthralgia, or conjunctivitis) are present during or within a period of up to two weeks after staying in an endemic area for the virus.55
During the 2007 Zika outbreak in Micronesia, the most frequent symptoms were skin rash, fever, arthralgia, and conjunctivitis; headache, myalgia, retro-orbital pain, edema, and vomiting were less common. Of the 49 confirmed cases, no hospitalizations, hemorrhagic manifestations, or deaths were reported.53
Dengue feverDengue should be suspected in all patients residing in endemic areas or who traveled to these regions in the past two weeks.56 The diagnosis should be considered in patients with fever (sudden onset, with a peak of 39–40°C), diffuse skin flushing, leukopenia, and thrombocytopenia.57,58 The symptoms last for approximately five to seven days. Headache, arthralgia, back pain, myalgia, bone pain, and gastrointestinal symptoms (anorexia, vomiting, and discomfort) are common. Retro-orbital pain, after light pressure is applied to the eyeball, is also usual.56,57 Signs suggestive of hemorrhage include petechiae, purpura, epistaxis, gingival and vaginal bleeding, melena, and hematemesis. In this situation, physicians must be alert for dengue hemorrhagic fever, which can result in circulatory collapse and, consequently, shock.56,57
ChikungunyaUnlike dengue and Zika, most people infected with the Chikungunya virus are symptomatic, with less than 15% being asymptomatic.59,60 The time of viremia is usually one week.61 The fever has a rapid onset, with high temperatures (generally >39°C), accompanied by myalgia, asthenia, headache, arthralgia, and rash. Soon after the onset of fever, there are complaints of severe myalgia and arthralgia, which will even prevent patient ambulation.62 Rash occurs in 20–80% of cases with maculopapular presentation and usually located on the trunk.63 Less common and unspecific symptoms include pruritus, lymphadenopathy, and digestive disorders.63 Patients requiring hospitalization are usually elderly and those with coexisting disorders (cardiac, respiratory, neurological, or diabetes). Another group with a high risk for severe infection is the neonatal group (especially mothers who carried the virus during pregnancy and exposure during birth) and young children.64
Laboratory diagnosisLaboratory diagnosis of ZikaV infections is mainly based on molecular biology and serologic tests.65,66 Amplification of the viral RNA through reverse transcription-polymerase chain reaction (RT-PCR) can be performed in several biological materials such as serum, cerebrospinal fluid, amniotic fluid, saliva, and urine.9,10,67 In patients with suspected infection by horizontal transmission, its positivity is associated with the acute phase of infection between three and seven days after the onset of symptoms, when low-level viremia usually occurs.3,65 When available, they are the diagnostic tests of choice after the acute phase. The interpretation of serological tests for ZikaV, such as ELISA IgM and IgG or plaque-reduction neutralization test (PRNT) must be performed carefully, as they show cross-reactivity with other arboviruses, especially in patients previously infected with other flaviviruses, such as dengue fever.65 Ideally, serology for dengue should be performed concomitantly and repeated after two weeks for comparison of titers; positivity in neutralizing antibody titers four times higher than dengue fever is considered confirmatory.53,67
In suspected cases, as there is no complete knowledge of the accuracy of several tests in the diagnosis of congenital infection, RT-PCR should be performed if available, ideally within the first two days in cord blood, cerebrospinal fluid, and placenta, associated to antibody measurements in cerebrospinal fluid and serum. The immunohistochemical test for ZikaV in the placenta and umbilical cord should also be performed whenever possible. The detection of ZikaV in any of the abovementioned materials through RT-PCR or immunohistochemistry establishes a diagnosis of congenital infection. The reagent serology is confirmatory when titers are higher than in dengue serology, as described above. In the event of fetal death, the diagnosis consists of performing RT-PCR and immunohistochemistry in fetal tissue.67
Neuroimaging and ZikaVAccording to the guidelines of the American Academy of Neurology on Microcephaly, neuroimaging studies are useful in identifying structural lesions in the assessment of children with microcephaly, helping in the investigation of genetic, acquired, or environmental etiologies; magnetic resonance imaging (MRI) is the most accurate method for the identification of specific patterns that can help diagnosis.22
Regarding congenital infections, the mechanism of infection and brain damage depends on the etiological agent, determining neuroradiological and pathological manifestations with distinct patterns. Viral agents, for instance, tend to produce a selective necrosis of specific cell types, whereas bacteria and fungi are less selective. Moreover, different patterns of calcifications seen at the imaging and pathological studies are typical of STORCH infections (syphilis, toxoplasmosis, rubella, CMV, HIV, and herpes simplex), and the time of the insult during fetal life is related to the teratogenic or encephaloclastic effects.68
The main objective of the imaging assessment of a possible intrauterine infection by ZikaV is the detection of neurological complications related to transplacental infection, such as microcephaly, cerebral calcifications, neuronal migration disorders, loss of brain tissue, and ventricular system dilation. The extent and severity of intracranial alterations are directly related to the gestational period when the fetus was infected by the virus; it is more severe and extensive during the first trimester of pregnancy and milder in the third trimester. The imaging assessment of the fetus or newborn has been indicated in cases of maternal ZikaV infection, either confirmed or inconclusive, as well as through laboratory tests or compatible clinical pictures.
During the prenatal period, obstetric ultrasonography is the exam of choice and is recommended for the investigation of possible structural abnormalities of the central nervous system and for monitoring fetal and brain growth every three to four weeks.69
In the postnatal period, transfontanellar ultrasonography is the initial method of investigation for the newborn with a head circumference ≤32cm. The presence of any abnormalities detected by the transfontanellar ultrasound should be investigated through more detailed examinations with higher diagnostic accuracy, such as computed tomography or magnetic resonance imaging.67
The brain abnormalities resulting from intrauterine infection by ZikaV have been described mainly by computed tomography in the postnatal period. The main imaging findings originate from a series of 35 cases of microcephaly, born to mothers who had contact with endemic areas. Of the total of 35 infants, 27 were submitted to computed tomography and subsequently reported to the CDC.5 The imaging findings in that cohort consisted of cerebral calcifications, predominantly periventricular, but also of calcifications in the brain parenchyma, thalamus, and basal ganglia. Neuronal migration anomalies were also detected, such as lissencephaly, pachygyria, and polymicrogyria, present in up to one-third of cases. Ventriculomegaly secondary to cortical/subcortical atrophy has also been often found.5 There are also case reports that used other imaging methods, such as obstetric ultrasound to carry out intrauterine investigation of pregnant women with a history of ZikaV infection, which showed gross cerebral calcifications, abnormalities in the cerebellar vermis and corpus callosum dysgenesis.70
Recently, the case report of a fetus with confirmed ZikaV infection submitted to autopsy and neuropathological study showed microcephaly, predominantly cortical and subcortical calcifications, simplified gyral pattern, neuronal migration disorders, loss of brain tissue, and asymmetric ventricular dilation. Although that study did not include more advanced neuroimaging studies, the described sonographic findings would certainly be better identified and demonstrated by fetal or postnatal computed tomography or magnetic resonance imaging.71
Strategies for studying the association between ZikaV and microcephalyGlobal understanding of normal neurodevelopment and its possible alterations depends on extensive knowledge of the brain formation and the pattern of connections between neurons and between brain regions, as well as the synaptic communications that constitute these adequate connections. The new methods of cell and organoid models allow for in vitro simulations of the processes involved in embryonic neurogenesis, the possible alterations or interventions that may be related to abnormal brain development. It is now possible to elucidate issues regarding the main gaps in knowledge on brain formation, generating unique results on embryonic neurogenesis and all the processes involved during cortical development, as well as the complete understanding about the action of agents that can potentially cause alterations in embryonic neurodevelopment.
Human neurons and/or glial cells are not readily available for experimental research. Studies of pathologies associated with the central nervous system have traditionally been limited to animal models or less relevant cell lines for the understanding of neuronal pathophysiology.72–74 The importance of using human cells similar to the disease to be investigated is evident by the number of drugs that show efficacy and safety when tested in animal models, but subsequently fail in clinical trials.74 Thus, it is necessary to use new models that accurately represent real human diseases and their physiological peculiarities to improve the success rate of new discoveries, mainly those related to neurodevelopment.73
The technology required to generate the so-called cell models is currently available, capable of reproducing somatic cells at the embryonic level, or even producing mini-brains from these reprogrammed cells. These models have high relevance for the study of diseases in humans, providing excellent conditions for the understanding of mechanisms and constituting an accomplished tool for high-yield experiments, also allowing for the construction of platforms for the screening of new drugs in several diseases.72
Cells obtained by cell reprogramming are called induced pluripotent stem cells (iPS) and are very similar to embryonic stem cells, with the same characteristics of self-renewal and differentiation potential in three lines of embryonic leaflets.75,76 The use of the iPS cell generation technology is based on two major action areas, the generation of specific cells for use in regenerative medicine, and/or modeling of disease and screening for new drugs. iPS cells can be generated from patients with a particular disease or from healthy individuals, and subsequently differentiated into adult cells. Based on diseases whose phenotype is expressed in adult cells, genome editing and correction can be performed, as well as screening for compounds capable of correcting cell disorder and evaluation of the possible toxicity of some drugs in these cells.73 From an iPS cell, in vitro small organoids with a miniature organization very similar to that of the human brain can be obtained, which are called mini-brains, being able to recapitulate a surprising number of brain development characteristics.
Viral infections capable of promoting changes in neurodevelopment can be investigated through the exposure of human iPS cells to viruses, allowing for a detailed study of the different stages of viral infection progression and the consequences of exposure during embryonic brain development. D’Aiuto et al., in 2014, reported a study on the susceptibility of human iPS cells to cytomegalovirus and its performance during the formation of an adult neuron. The study showed significant differences in alterations caused by cytomegalovirus to embryonic cells, neural precursor cells and adult neurons, suggesting that exposure to cytomegalovirus in the embryonic period is crucial to the development of brain malformations caused by the virus.77
In cases of microcephaly caused by genetic mutations, for instance, mini-brains were generated from cells (iPS) derived from skin fibroblasts from a patient with microcephaly. The generated mini-brains resembled the phenotype of microcephaly, being much smaller when compared with mini-brains generated from control patient cells, allowing for a detailed study of embryonic neurogenesis, especially the cortical formation of these brains.78,79
DiscussionThe association between by the ZikaV infection during pregnancy and the development of microcephaly has alarmed the population worldwide.5–8 Microcephaly is a disorder of the neuronal proliferation phase, which occurs early in pregnancy (third to fourth month)12 and, in some described cases, appears to coincide with the symptoms of the infection in the mother. As the criteria for the definition of microcephaly have not been used in a standardized manner and as there was clearly an underreporting of such cases in Brazil, it is difficult to establish with certainty whether there is a real increase in the incidence of this pathology.22–26 Additionally, the available neuroimaging studies show that other malformations of fetal cortical development are also present, such as neuronal migration disorders (third to fifth month of pregnancy) and diffuse calcifications (neuronal death), suggesting either a long term of the virus pathogenesis in the CNS, or the susceptibility of more phases of cortical development.5,12–14,70
Considering the broad dissemination of the Aedes aegypti in Brazil and that the methods used for vector population control are slow to show results, it is expected that cases of ZikaV infection will continue to increase, the acquisition of more knowledge about both the pathology and the etiological agent an urgent priority. Recent studies suggest that alterations in the molecular components of ZikaV, especially of the E protein present on the virus surface, could be correlated with an increased “aggressiveness” of the ZikaV, reinforcing its neurotropism and capacity to cause disease in humans.40,42 The situation requires multiple and multidisciplinary approaches to control the vector and ZikaV infection, including the education of the population. A well-informed pediatrician is a central part of this process.
It is a fact that the ZikaV can overcome the placental barrier and reach the amniotic fluid and fetal tissues.11 But it is necessary to reassure pregnant women, as it is not possible to affirm that this increase in microcephaly notifications is solely related to the virus, as many initially suspected cases were ruled out.23 Cases with laboratory confirmation are few in relation to the high number of notifications; nevertheless, prevention measures are still necessary and should be identified.
The availability of tests for the laboratory diagnosis of ZikaV infection, both in the acute phase and later, is still very restricted. The difficulty in confirming or ruling out infection also affects the understanding of the natural course of the disease and its association with microcephaly and also with Guillain–Barré syndrome.54 Because of the great worldwide concern regarding the teratogenic potential of ZikaV infection, there will be greater efforts for the development of more affordable testing with greater accuracy, such as serology with less risk of cross-reactivity.80
In conclusion, there is a clear temporal association between the increased reporting of cases of microcephaly and the ZikaV epidemic, mainly in the Northeast of Brazil. However, the development of diagnostic techniques to confirm a cause-and-effect association, the pathogenesis mechanisms of ZikaV infection in the central nervous system, and more adequately defined diagnostic criteria for the identification of cases of microcephaly that should be submitted to investigation are still required.
Conflicts of interestThe authors declare no conflicts of interest.
MLN, CRC, and JCC receive a research productivity grant from CNPq.
Please cite this article as: Nunes ML, Carlini CR, Marinowic D, Neto FK, Fiori HH, Scotta MC, et al. Microcephaly and Zika virus: a clinical and epidemiological analysis of the current outbreak in Brazil. J Pediatr (Rio J). 2016;92:230–40.
Study carried out at Instituto do Cérebro (InsCer) do Rio Grande do Sul and Faculdade de Medicina, Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS), Porto Alegre, RS, Brazil.