To review the pathophysiology and evaluation methods of linear growth and bone mineral density in children and adolescents diagnosed with inflammatory bowel disease.
Source of dataNarrative review carried out in the PubMed and Scopus databases through an active search of the terms: inflammatory bowel disease, growth, failure to thrive, bone health, bone mineral density, and children and adolescents, related to the last ten years, searching in the title, abstract, or keyword fields.
Synthesis of findingsInflammatory bowel diseases of childhood onset may present as part of the clinical picture of delayed linear growth in addition to low bone mineral density. The presence of a chronic inflammatory process with elevated serum levels of inflammatory cytokines negatively interferes with the growth rate and bone metabolism regulation, in addition to increasing energy expenditure, compromising nutrient absorption, and favoring intestinal protein losses. Another important factor is the chronic use of glucocorticoids, which decreases the secretion of growth hormone and the gonadotrophin pulses, causing pubertal and growth spurt delay. In addition to these effects, they inhibit the replication of osteoblastic lineage cells and stimulate osteoclastogenesis.
ConclusionInsufficient growth and low bone mineral density in pediatric patients with inflammatory bowel disease are complex problems that result from multiple factors including chronic inflammation, malnutrition, decreased physical activity, late puberty, genetic susceptibility, and immunosuppressive therapies, such as glucocorticoids.
Revisar a fisiopatologia e os métodos de avaliação do crescimento linear e densidade mineral óssea em crianças e adolescentes com diagnóstico de doença inflamatória intestinal.
Fontes dos dadosRevisão narrativa a partir de pesquisa nas bases de dados PubMed e Scopus por meio de busca ativa dos termos inflammatory bowel disease, growth, failure to thrive, bone health, bone mineral density, children e adolescents nos últimos dez anos e com busca nos campos título, resumo ou palavra-chave.
Resumo dos achadosAs doenças inflamatórias intestinais com início na infância podem apresentar como parte do quadro clínico atraso do crescimento linear além de baixa densidade mineral óssea. A presença de processo inflamatório crônico com elevados níveis séricos das citocinas inflamatórias interfere negativamente na velocidade do crescimento e na regulação do metabolismo ósseo, além de aumentar o gasto energético, comprometer a absorção de nutrientes e favorecer perdas proteicas intestinais. Outro fator importante é o uso crônico de glicocorticoides, que diminuem a secreção de hormônio do crescimento e dos pulsos das gonadotrofinas e ocasionam atraso puberal e no estirão do crescimento. Além desses efeitos, inibem a reprodução das células da linhagem osteoblástica e estimulam a osteoclastogênese.
ConclusãoA insuficiência do crescimento e a baixa densidade mineral óssea em pacientes pediátricos com doença inflamatória intestinal são problemas complexos e que decorrem de múltiplos fatores, inclusive inflamação crônica, desnutrição, diminuição da atividade física, puberdade tardia, suscetibilidade genética a terapias imunossupressoras, como os glicocorticoides.
Inflammatory bowel diseases (IBD) are chronic diseases with a multifactorial etiology due to the interaction of genetic, environmental, immunological, and intestinal microbiota factors.1 They are characterized by chronic inflammatory reaction in the gastrointestinal tract (GIT) and are represented by three entities: Crohn's disease (CD), ulcerative colitis (UC) and indeterminate colitis (IC).2
The presence of an inflammatory process results in high energy expenditure for the child's body, and compromises nutrient absorption.3,4 The care of these children involves the therapeutic management of IBD with inflammation control and care to ensure the growth and development characteristic of this age group.5 Although the implementation of IBD control treatment is associated with an improvement in the linear growth pattern, a considerable proportion of children remains with short stature.6,7
A reduction in bone mineral density (BMD) is also observed in patients with IBD. The insufficient gain of bone mass at puberty can have consequences throughout life and predispose to osteoporosis in adulthood.8
Linear growth failure and low bone mass are complex and multifactorial phenomena, resulting from the interaction of inadequate dietary intake, excessive gastrointestinal losses (protein-losing enteropathy), malabsorption, increased nutritional requirements (increased energy expenditure due to the chronic inflammatory process), and body response to inflammatory mediators4 (Table 1).
It is of utmost importance to understand the mechanisms associated with linear growth failure and BMD reduction in this group of patients, aiming to prevent or minimize damage. Therefore, this literature review aimed to address aspects of the pathophysiology of linear growth failure in childhood IBD, emphasizing the effect of inflammation on bone metabolism, and methods for assessing linear growth and bone health in the pediatric population.
Immunopathogenesis of IBDThe pathogenesis of IBD has yet to be fully elucidated, although it is known that the immune system dysfunction of the GIT mucosa plays an important role.6 A dysregulated immune response to intestinal bacterial antigens in an individual with a genetic predisposition is one of the explanations for the development of chronic inflammation. The increased incidence of IBD is attributed to environmental factors associated with the modern human lifestyle.9
Genomic association studies have identified more than 200 loci related to genetic susceptibility to IBD. These genes encode proteins involved in the innate and adaptive immune response, autophagy, and integrity of the intestinal mucosa. Among these, polymorphisms in the NOD2 gene show the greatest association with genetic predisposition to IBD.10
Regarding the genetic factors associated with IBD, most genes related to CD susceptibility were not associated with growth delay. Wine et al., in a study that evaluated children with CD and NOD2 genotype, which is associated with ileus involvement, did not find a correlation with growth delay or failure.11 Levine et al. evaluated the effects of tumor necrosis factor (TNF) promoter polymorphisms on growth delay in children with CD. Patients with loss-of-function polymorphisms (i.e., lower TNF production) had higher mean height Z-scores of and were less likely to develop growth delay.12
Russell et al. evaluated 299 Scottish children (200 with CD, 74 with UC, and 25 with IC) and found that patients with the OCTN1/OCTN2 haplotype of susceptibility to IBD5 locus disease were more likely to be shorter.13
With increased intestinal permeability due to breach of the intestinal mucosal barrier, there is great exposure of the immune system to antigens, mainly bacteria. The first line of defense against these antigens is the innate immune system, mediated primarily by neutrophils, macrophages, and dendritic cells, and by the production of proinflammatory cytokines such as IL-1β, IL-6, IL-18, TNF, IL-12, IL-23, IFN-α, and IFN-β.14
As with the innate immunity, the adaptive immune response is also activated in IBD, resulting in persistence of the chronic inflammatory state. Humoral immunity, effector T lymphocytes (TH), regulatory T lymphocytes (Treg), NK-cells and innate lymphoid cells of the intestinal mucosa also participate in it. Historically, CD was characterized by a predominance of TH1 profile and consequent increase of IL-2, IL-12, and IFN-γ Conversely, UC was characterized by an atypical TH2 profile, since it shows low levels of IL-4 and increased levels of IL-5 and IL-13. However, with the discovery of TH17 cells, it was observed that both diseases also present the TH17 profile and its main interleukins, such as IL-17A, IL-21 and IL-23.14
Recently, microRNAs (small, single-stranded RNAs, non-protein coding) that play an important role in gene regulation were associated with the immunopathogenesis of IBD.14 The dysregulation of these microRNAs may result in excess inflammation.15
Bone linear growthLinear bone growth in children and adolescents is essentially driven by the process of chondrogenesis in the growth plate. The growth plate has a single cell type, the chondrocyte, distributed in three layers: resting, proliferative, and hypertrophic zones. The chondrogenesis process is characterized by the proliferation and hypertrophy of the chondrocytes associated with the extracellular matrix secretion. Then, the hypertrophic zone is invaded by blood vessels, osteoclasts, and osteoblasts, and newly formed cartilage is remodeled into bone tissue, increasing bone size longitudinally.16
The main factors regulating bone linear growth are food intake and hormones, and both malnutrition and obesity can lead to changes in linear growth. Thyroid hormones, growth hormone (GH), insulin-like growth factor-1 (IGF1), androgen, and estrogen positively regulate linear growth. The glucocorticoids, whether endogenous or exogenous, negatively regulate linear growth. The GH/IGF1 axis, among the hormones described above, plays an essential role in growth regulation after birth. The GH receptor is widely expressed in the body, including the liver and the growth plate. Once bound to its receptor, GH induces the production of IGF1. Thus, GH has a direct action on the resting zone, stimulating chondrocyte differentiation and an indirect action via IGF1, which promotes the hypertrophy of these cells. IGF1 is produced both in the liver and in the growth plate itself, and its expression is increased by GH.16
Proinflammatory cytokines, especially TNF, IL-1β, and IL-6, play an important role in linear growth control in children and adolescents with IBD, both by acting on the GH/IGF1 axis and by their direct actions on the growth plate. The association between growth retardation and low IGF1 levels has already been established in pediatric patients with IBD. In these patients, a state of resistance to GH is characterized, since its production is usually normal.17
TNF decreases the expression of GH receptors in the liver, and consequently decreases IGF1 production.18 Infliximab therapy has shown to increase IGF1 levels in both animal models of colitis and in pediatric patients with CD.17 In a synergistic action with IL-1β, both inhibit chondrocyte proliferation and growth plate hypertrophy, as well as accelerate apoptosis of these cells.19 Moreover, TNF and IL-1β directly inhibit the production of sex steroids in the gonads; these hormones are also responsible for bone growth.18
In animal models of transgenic mice with TNF overexpression, growth retardation was demonstrated in these animals when compared with wild mice.20 Moreover, in an experiment using rat fetal bone cultured with TNF, lower bone growth was observed in this group when compared with the control group. Subsequently, treatment with etanercept (a soluble TNF receptor) resulted in an improvement in the longitudinal growth of these bones in culture. Using this same experiment model, a similar result was found when rat fetal bones were cultured with IL-1β. They showed lower bone growth rates when compared with the control group and improvement in growth when treated with anakinra (a recombinant IL-1β receptor antagonist).19
A transgenic mouse animal model with IL-6 overexpression demonstrated reduction in growth between 50% and 70% when compared with wild mice.21 Growth retardation in these mice was associated with a reduction in IGF1 and IGF-binding protein type 3 (IGFBP3), with normal GH production.21,22 According to Benedetti et al., the reduction in serum IGF1 concentration is due to the increased proteolysis of IGFBP3, which consequently decreases the half-life of IGF1. This finding was also observed in children with systemic juvenile idiopathic arthritis, in whom high levels of IL-6 showed a negative correlation with IGF1 and IGFBP3.21,22
In the growth plate, IL-6 increases osteoclastogenesis and reduces osteoblast activity. This imbalance favors growth plate cartilage thinning, with lower bone growth.17
Bone metabolism and IBDBone mass depends on bone remodeling homeostasis, i.e., on the balanced dynamics between osteoblast and osteoclast activity, which is firmly controlled by the immune system.23 Bone formation involves the proliferation and migration of osteoprogenitor cells and osteoblast differentiation. However, if this balance occurs in favor of osteoclast activity, bone resorption will occur.24
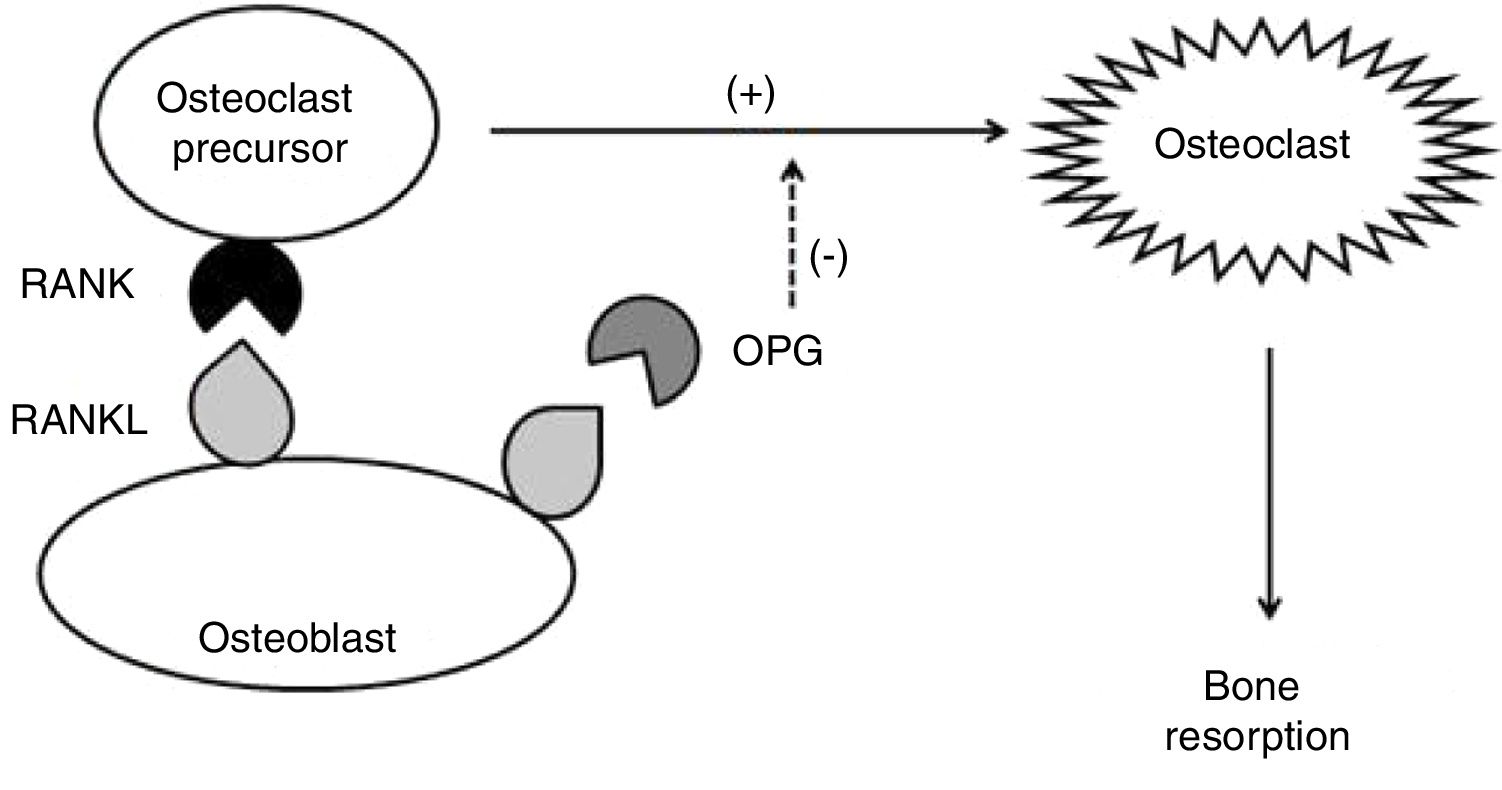
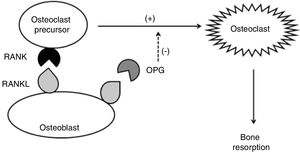
Some cytokines are involved in this process: IL-1β, IL-6, and TNF, which stimulate osteoclast activity in an autocrine manner; receptor activator of nuclear factor kappa-B ligand (RANKL – from the TNF family), which binds to RANK transmembrane receptors (receptor activator of nuclear factor kappa-beta) of osteoclasts, inducing osteoclastogenesis; and osteoprotegerin (OPG), which negatively regulates osteoclast formation and activation, disrupting the RANK–RANKL binding by binding to RANKL. OPG is produced primarily by osteoblasts and their precursors but can also be expressed by B cells and dendritic cells (Fig. 1). In contrast, transforming growth factor beta (TGF-β), produced by osteoblasts and bone marrow cells, is the most abundant of the growth factors stored in the bone; it stimulates bone formation, but also inhibits the differentiation, formation, and activity of mature osteoclasts.25
Under physiological conditions, the formation of osteoclasts is dictated by the interaction of RANKL with its receptor, RANK. While high levels of RANKL are potentially osteoclastogenic, low levels of RANKL can actually increase bone formation. The excessive increase in RANKL, which is typically the crucial event that rules bone resorption, is downregulated by OPG. That is, the binding of RANKL to OPG limits osteoclastogenesis and, consequently, bone resorption.25
However, a number of inflammatory cytokines may contribute to bone loss. RANKL expression is induced by IL-1β, IL-6, IL-11, IL-17, and TNF, which not only increase RANKL expression but also induce the release of the soluble RANKL form and suppress OPG production. These mechanisms occur in many autoimmune and inflammatory diseases.24,26
Serum levels of many proinflammatory interleukins (including IL-1β, IL-6, and TNF) are increased in IBD, favoring the bone resorption pathway. The key to the regulatory mechanism of osteoclastic differentiation and activity would therefore be found in the association of RANKL and OPG. The increase in OPG levels may represent a continuous homeostatic response, attempting to reverse osteoclastogenesis and thus maintain normal bone mass.27
Changes in the RANKL/OPG ratio may be responsible for bone loss in patients with IBD, leading to osteopenia and osteoporosis.27 In a case–control study that measured OPG and RANKL levels in patients with IBD, it was observed that OPG levels were 2.4-fold higher in CD and 1.9-fold higher in UC, while RANK levels were not significantly different in patients with IBD when compared with healthy controls. However, a significant negative correlation was found between plasma levels of OPG and BMD, suggesting that bone loss in IBD is associated with changes in the RANK/OPG system.28
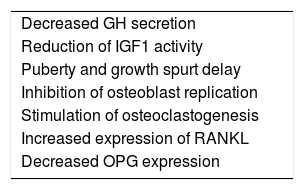
Glucocorticoids and linear growthAmong the glucocorticoids (GC) actions on hormones and cytokines responsible for linear bone growth, the following are noteworthy: decrease in GH secretion through a negative feedback mechanism, reduction in IGF1 activity without a decrease in IGF1 serum levels, and decrease in the gonadotrophin pulses, leading to pubertal and growth spurt delay.29 In addition to these effects, GCs act directly on bone cells by inhibiting the replication of osteoblast lineage cells and inducing the apoptosis of mature osteoblasts and osteocytes. Additionally, they stimulate osteoclastogenesis, increasing RANKL expression and decreasing OPG expression in osteoblastic cells30 (Table 2).
Although GCs can suppress bone growth, decreased height as early as at the time of IBD diagnosis shows that this result is a consequence of the disease, and not just an adverse effect of treatment. Several studies failed to observe an independent effect of GC on growth, especially with short-duration or low-dose treatment, reinforcing the hypothesis that IBD severity is much more important as a risk factor for growth delay.31 Another relevant point is that daily and cumulative doses of GC and treatment duration are very important factors in the pathogenesis of bone mass reduction in patients.32
Methods of linear growth and bone metabolism evaluationAmong the methods used, the most important are height-for-age (H/A) and growth velocity (GV).33,34
H/A expresses the child's linear growth. This is the index that best indicates the cumulative effect of adverse situations on the child's growth.33 In the WHO growth curves, a cutoff point of 2 or more SD below the mean is used to identify children with short stature for age.33
However, studies that define linear growth failure based on stature alone may underestimate the growth of some children, since patients with relatively normal heights may have grown little over a period of time. This is the case of children with IBD, who show decreased GV during periods of disease activity and tend to improve it when the disease goes into remission.34
GV is calculated as the difference between two height measurements in a given time interval. It is usually expressed in centimeters per year (cm/year). The minimum time interval for the measurement of the two recommended measures is six months. After the calculation, the value is plotted on the GV chart for age (as it constitutes the difference of measurements at two moments, the point on the chart should be included in the mean interval between the two ages of measurement).35
In 1966, Tanner et al. published the first GV charts for boys and girls, which are still currently used worldwide for GV assessment. Children and adolescents should ideally grow at the same percentile for GV, around the 50th percentile and not below the 25th or above the 75th percentiles.36,37
Identifying individuals with low bone mass early in life may be an important strategy to take preventive and therapeutic measures aimed at optimizing bone mass gain and promoting healthy skeletal growth. Double-energy X-ray absorptiometry or bone densitometry (DXA), the most widely used method for assessing BMD, is considered the “gold standard”.38
The advantages of DXA include the fact that it is widely available in Brazil; it is a fast, accurate test that uses low levels of ionizing radiation. However, the interpretation of densitometric measurements is much more complex in the growing individual than in adults.39 For this reason, in 2013, the International Society for Clinical Densitometry (ISCD) revised a specific guideline for performing and interpreting densitometry in children and adolescents. Low BMD is defined when the Z-score is ≤−2 SD for age.40
Biochemical markers of bone turnover may also be used to evaluate bone formation and resorption rates that reflect osteoblast and osteoclast activity, respectively. The RANK/RANKL/OPG system is probably the main one involved in the development of osteopenia/osteoporosis in IBD and other inflammatory diseases.27
Thus, the OPG/RANKL ratio may be useful for determining the rate of bone resorption in several disease conditions, especially in inflammatory diseases.41,42
Interventions to optimize growth and bone massThe treatment of growth failure is carried out mainly through the control of inflammatory activity, both in the acute phase and during the maintenance of disease remission.43
Exclusive enteral therapy with a polymeric formula, in addition to re-establishing the nutritional status of the patient with an adequate supply of nutrients, is used to induce disease remission.44 Additionally, GV is significantly improved when remission induction is used with exclusive enteral diet, in comparison to corticosteroid treatment, with the advantage of not having side effects.45
Due to its side effects, the use of corticosteroids should be carried out for short periods and preferably associated with aminosalicylates or immunosuppressive therapy with thiopurines, for the maintenance of persistent remission.46
Anti-TNF immunobiological therapy, i.e., infliximab or adalimumab, is especially indicated for patients who do not respond to thiopurine treatment and require corticosteroid therapy for inflammation control more frequently.47 Studies have shown good results in linear growth recovery in adolescents and children, in addition to a beneficial effect on bone metabolism.42,48
Growth hormone replacement therapy remains controversial, and little is known about the possible beneficial effects of GH use on linear growth in patients with IBD. It can be considered in selected cases that present pubertal delay, but its prolonged use did not show better results than the disease activity control.47,49
In those patients with low BMD, the recommended first-line therapies for pediatric patients include calcium and vitamin D supplementation and the implementation of regular exercises. Monitoring should be carried out through bone densitometry.5,50 It is necessary to reinforce family care and relations, physical activity, and the importance of self-care for the treatment success.
Final considerationsThe deleterious effects on linear growth and bone mass constitute a major concern for professionals who treat patients with inflammatory bowel diseases in the pediatric age group, given the clinical evidence of patients with severe growth delay that occurs regardless of the adequate nutrition and control of inflammation.
In the context of patients living in developing countries, it is worth emphasizing the possibility of another condition that may be overlapping with IBD: environmental enteric dysfunction (environmental enteropathy), which may impair linear growth (stunting) through pathogenic mechanisms that overlap with those described in IBD.51
More studies are necessary to consolidate the knowledge of the mechanisms that lead to growth failure and low BMD, as well as interventions aimed at this process to guarantee the linear growth of children and adolescents with IBD.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Ferreira PV, Cavalcanti AS, Silva GA. Linear growth and bone metabolism in pediatric patients with inflammatory bowel disease. J Pediatr (Rio J). 2019;75:S59–S65.