To assess the relationship between mouth breathing and growth disorders among children and teenagers.
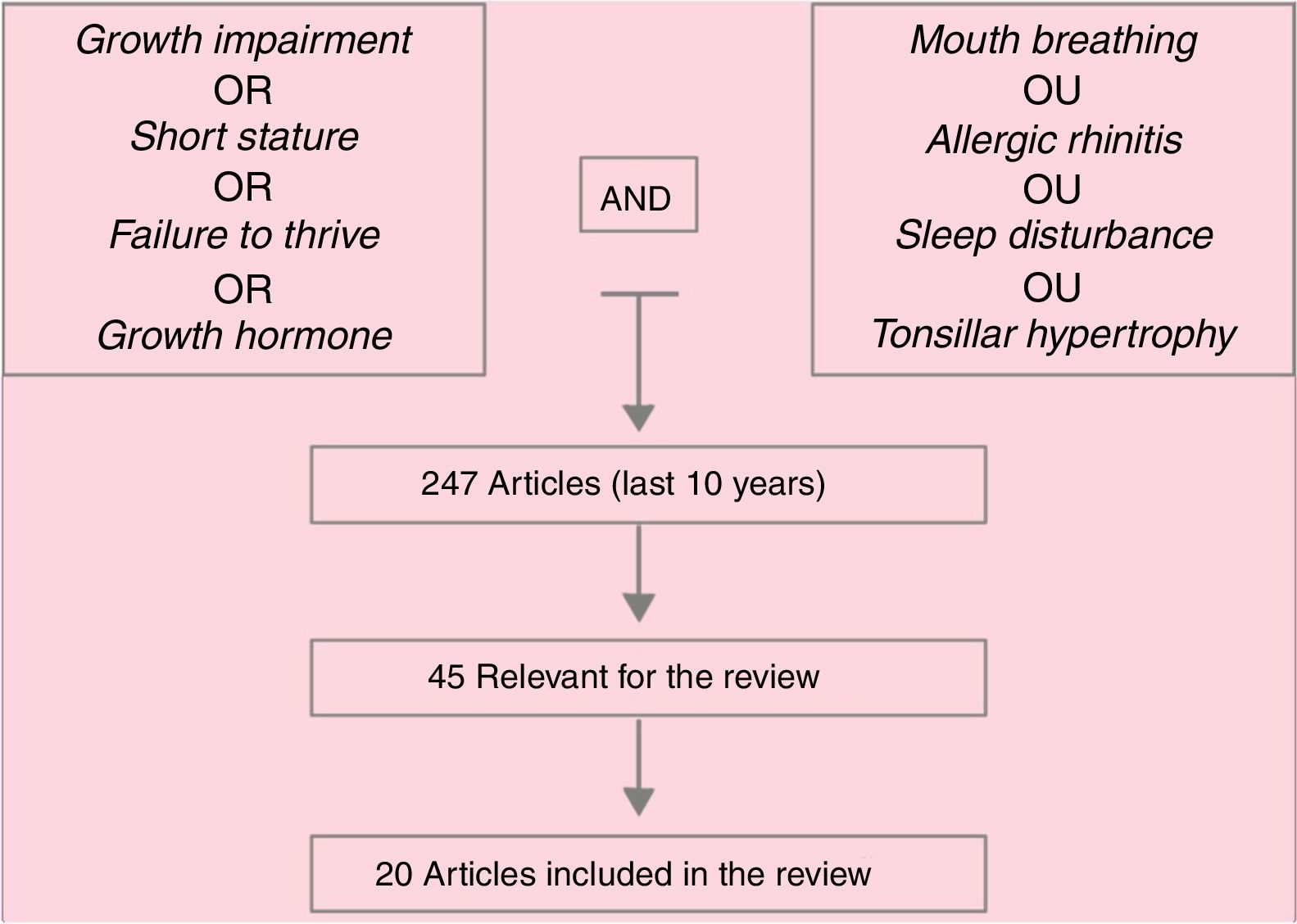
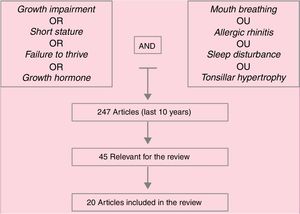
Data sourceSearch on MEDLINE database, over the last 10 years, by using the following terms: “mouth breathing”, “adenotonsilar hypertrophy”, “allergic rhinitis”, “sleep disturbance” AND “growth impairment”, “growth hormone”, “failure to thrive”, “short stature”, or “failure to thrive”.
Data summaryA total of 247 articles were identified and, after reading the headings, this number was reduced to 45 articles, whose abstracts were read and, of these, 20 were deemed important and were included in the review. In addition of these articles, references mentioned in them and specific books on mouth breathing deemed important were included. Hypertrophy of palatine and/or pharyngeal tonsils, whether associated with allergic rhinitis, as well as poorly controlled allergic rhinitis, are the main causes of mouth breathing in children. Respiratory sleep disorders are frequent among these patients. Several studies associate mouth breathing with reduced growth, as well as with reduced growth hormone release, which are reestablished after effective treatment of mouth breathing (clinical and/or surgical).
ConclusionsMouth breathing should be considered as a potential cause of growth retardation in children; pediatricians should assess these patients in a broad manner.
Avaliar a relação entre respiração oral e distúrbios do crescimento entre crianças e adolescentes.
Fonte de dadosBusca na base de dados do MEDLINE, nos últimos 10 anos, com o emprego dos seguintes termos: “mouth breathing” ou “adenotonsilar hypertrophy”, ou “allergic rhinitis” ou sleep disturbance” AND “growth impairment” ou “growth hormone” ou "failure to thrive” ou “short stature” ou ‘failure to thrive”.
Síntese dos dadosForam identificados 247 artigos, que após a leitura dos títulos foram reduzidos a 45, cujos resumos foram lidos e desses 20 foram considerados de importância e integraram a revisão. Além desses, referências por eles citadas e livros-texto específicos sobre respiração oral considerados importantes foram incluídos. A hipertrofia de tonsilas palatinas e/ou faríngeas, associada ou não à rinite alérgica, assim como a rinite alérgica mal controlada, é a principal causa de respiração oral na criança. Distúrbios respiratórios do sono são frequentes entre esses pacientes. Vários estudos associam a respiração oral à redução do crescimento, bem como à redução de liberação de hormônio do crescimento, que são restabelecidos após o tratamento efetivo da respiração oral (clínico e/ou cirúrgico).
ConclusõesA respiração oral deve ser cogitada como possível causa de retardo de crescimento em crianças e cabe ao pediatra a tarefa de investigar esses pacientes de forma mais abrangente.
A mouth breather is every individual who breaths through the mouth as a result of a pathological adaptation, whether in the presence of nasal and/or pharyngeal obstruction.1 The primary function of the nose is to bring the inhaled air to the lungs under ideal conditions for hematosis, i.e., heated, humidified, and free of microorganisms and pollutants present in room air.2
In children, nasal breathing is more important than in adults. At birth, nasal breathing is a mandatory condition due to the high position of the larynx in comparison with the oral cavity, which allows the newborn to be breastfed and breathe. The high location of the epiglottis, in this case, makes it difficult for air to enter the lower airways when the flow comes from the mouth, causing an intense respiratory discomfort in the presence of bilateral nasal obstruction.1,2
Additionally, nasal breathing in children aids the growth of central facial bones and the functional arrangement of all the muscles related to breathing and chewing.1,2 Under chronic nasal obstruction conditions, an underdevelopment of the palatal processes of maxilla may be observed, leading to the appearance of high arched hard palate. Additionally, mouth breathing requires several muscular and postural adaptations to adjust to a new form of breathing, chewing, and even swallowing food.2
Lowering of the mandible, hypotonic lip musculature, and changes in the phases of swallowing are common findings in these children and, if unidentified and treated early, can become irreversible. Furthermore, mouth breathing concomitant to nasal obstruction may predispose to airway collapse and, consequently, respiratory sleep disorders (RSD). Fig. 1 presents the article search method papers used in this review.
Prevalence of mouth breathersThe prevalence of chronic mouth breathing in children has been hardly studied and its distribution in the different age groups is unknown. Values between 3.4% and 56.8% have been documented based on the studied population (healthy, with dental, respiratory problems, or others), age group assessed, and diagnosis method used (questionnaire, physical examination).3–5
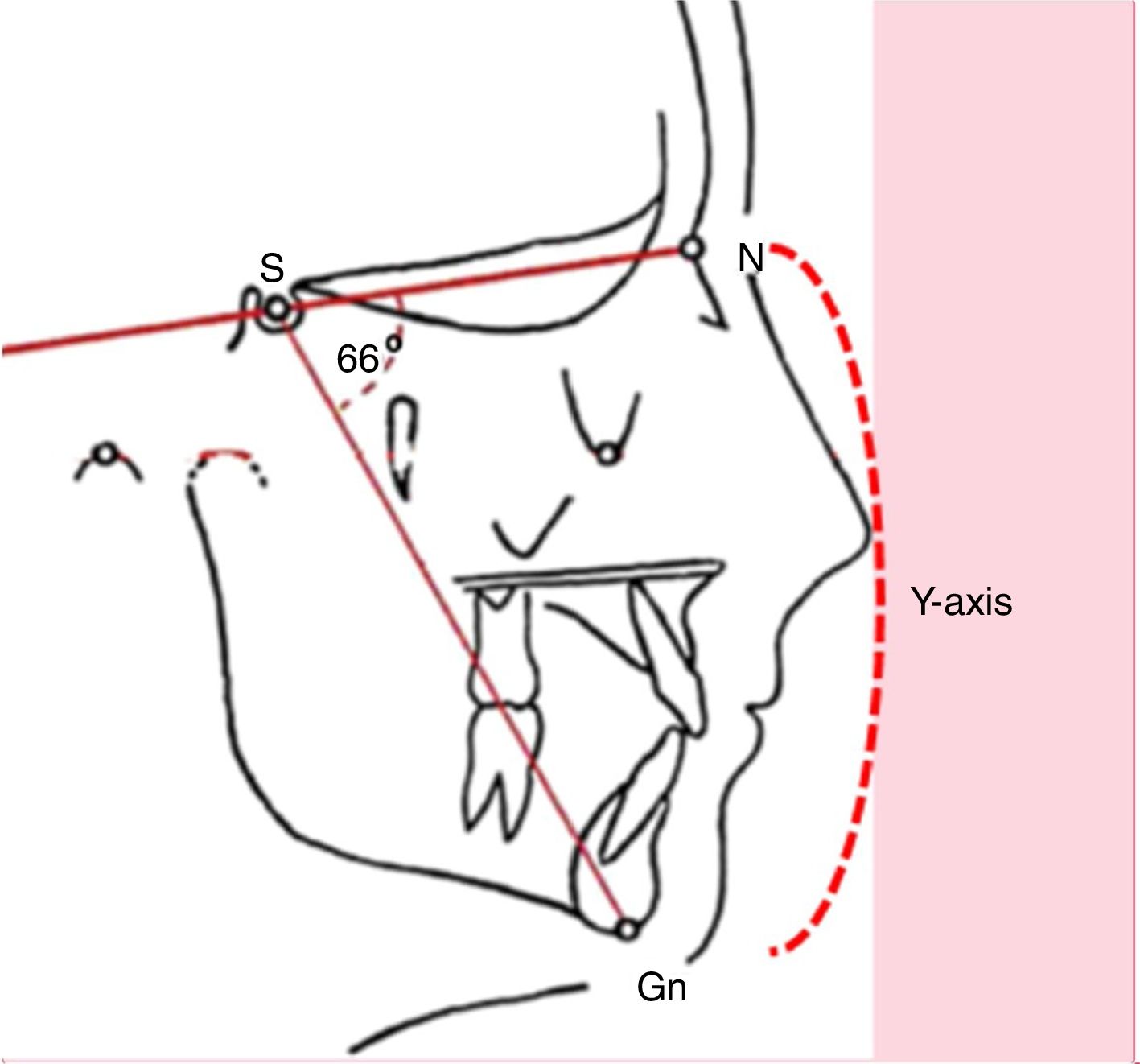
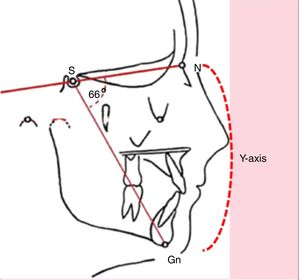
Clinical statusChronic mouth breathing may determine skeletal and myofunctional changes that hinder facial growth. Many children with mouth breathing present elongated facies, incomplete lip closure, shortened upper lip with accentuated concavity, everted lower lip, and presence of dark circles, characterizing adenoid facies.6–9 At cephalometric evaluation, an increased Y-axis is observed (Fig. 2).
The presence of snoring, whether associated with apnea or respiratory distress during sleep, is a frequent complaint among parents or caregivers, followed by restless sleep, frequent night awakenings,1 bruxism,10 and sometimes somnambulism.10–12 Mouth breathing is often associated with other harmful oral habits, such as thumb sucking, pacifier sucking, sucking and biting the lips, and nail biting, among others, impacting quality of life.13
Chewing, swallowing, and speech problems (linguodental phonemes) are also observed. Maxillary arch atresia and the presence of high arched palate are common findings associated with cross-bite, which can already be observed in preschool children.6-9,11
In addition, the presence of RSDs, such as snoring, increased airway resistance, or even apnea, is common among these patients; its frequency ranges from 3.3% (children at 5–6 years) to 42% (mouth breathers).5 Obstructive sleep apnea syndrome (OSAS) is the most severe form of RSD, and affected children may have neurocognitive impairment due to attention and concentration deficits, hyperactivity, morning tiredness, decreased conceptual, verbal and non-verbal reasoning skills, school disorders,14,15 or even delayed weight and height gain.1,2 Currently, cor pulmonale16 and systematic arterial hypertension17 are very rare complications associated with OSAS.
Etiology of mouth breathing in childrenAmong the several causes of mouth breathing in children, the most frequent are hypertrophy of pharyngeal (adenoids) and/or palatine tonsils (amygdala) and untreated (and, therefore, uncontrolled) allergic rhinitis. Nonetheless, other etiologies should also be considered: unilateral or bilateral choanal atresia, anatomical variations of the nasal conchae, nasal foreign body, septal deformities or nasal masses, and even rarer entities that can cause nasal congestion, such as cystic fibrosis, primary ciliary dyskinesia, and primary immunodeficiencies.
Hypertrophy of pharyngeal and/or palatine tonsils, albeit present in the early phase of life, usually becomes apparent around the age of 2 years, as it follows the development of the lymphoid system and can worsen with the advancement of age. Most of the times, the diagnosis is confirmed through a simple lateral radiography of the face and, in doubtful cases, through nasofibroscopy. Hypertrophy of the palatine tonsils can be confirmed through a simple oroscopy.
Rhinitis can be defined as a symptomatic inflammation of the nasal mucous membrane and is characterized by nasal obstruction, rhinorrhea (anterior and posterior), sneezing, and itchy nose. It is a very common problem since pre-school age and can affect up to 40% of children.18 Nasal congestion is the most frequent complaint in children with pre-school rhinitis.19
When not properly controlled, allergic rhinitis can evolve to chronic nasal obstruction and, consequently, mouth breathing. In addition to the characteristic clinical status, at physical examination these patients present ocular conjunctival hyperemia, dark circles, transverse nasal sulcus, and hypertrophied nasal conchae, hindering the free passage of air. Often, there is an association of allergic rhinitis with pharyngeal tonsil hypertrophy,20–22 considerably worsening the respiratory condition.23,24 When assessed by specific questionnaires, children with moderate to severe persistent allergic rhinitis present a higher frequency of sleep disorders than control children, especially in the domains of nocturnal respiratory disorders, daytime sleepiness, and parasomnias.25,26 Additionally, there is evidence of relationship between the severity of allergic rhinitis and the intensity of sleep disorders.25,27
Cho et al. confirmed this hypothesis when studying the presence of allergic sensitization as a risk factor for more severe conditions of pharyngeal and/or palatine tonsil hypertrophy.21 These authors measured allergen-specific IgE levels (sIgE) in serum and tonsillar tissue excised (during tonsil removal surgery) from 102 children who were mouth breathers and had pharyngeal tonsil hypertrophy. According to the presence or absence of sIgE, patients were divided into three groups: allergic (serum and tissue sIgE, n=55), with local allergy (sIgE only at tissue-level, n=17), and without allergy (absent in serum and tissue, n=32). In total population, 70.6 of the patients were sensible to at least one allergen (serum and/or tissue). sIgE tissue levels were significantly higher than the serum levels, with a predominance of sIgE to inhalants in the pharyngeal tonsil tissue and sIgE to food in palatine tonsil. A significantly higher prevalence of asthma, allergic rhinitis, more severe symptoms, and greater consumption of relief medications were observed among allergic patients when compared with non-allergic patients. These data are unequivocal evidence that the association of allergy aggravates the allergic conditions in these patients.21 However, this fact was not confirmed in patients with atopic dermatitis.23
Sleep and growthRespiratory sleep disorders (RSD, snoring, mouth breathing, and sleep obstructive apnea) were identified as risk factors for growth retardation associated with chronic obstruction of the upper airways, whether due to hypertrophy of the pharyngeal and/or palatine tonsils or allergic rhinitis.28–35
Sleep is defined as a reversible state of perceptual disengagement and lack of response to the environment.36 Two basic stages of sleep documented by signs of cortical activity (electroencephalogram, EEG), rapid eye movements, and muscle tone are identified in mammals: rapid-eye movement (REM) sleep, and the synchronized or non-REM sleep (NREM).36
Simply put, sleep begins at the NREM stage, which is characterized by slow rotational ocular movements, reduced muscle tone, and fragmented, low-amplitude brain activity.37 This stage is composed of three phases: the first is short and transient, followed by a second phase, in which the brain activity has a larger amplitude; the third phase (deep sleep) is characterized by slow waves of great amplitude, with delta waves and release of growth hormone (GH).
The second stage, called REM sleep, is not divided into stages; it is characterized by rapid eye movements, heart and respiratory rate variations, decreased blood pressure and cerebral blood flow. The mental activity during REM sleep is associated with dreams, based on the dream memory reported after about 80% of the awakenings in this state of sleep.38,39 Sleep stages occur cyclically at night with the succession of stages one to three of NREM sleep and REM sleep in cycles of 70–110min, with increased duration of REM sleep periods and reduction of slow-wave sleep.
As previously mentioned, during the deep sleep or slow-wave (delta) sleep there is a greater release of GH-releasing hormone (GHRH) through the hypothalamus.40–42 Experimental studies in animals have documented that the administration of GHRH increases NREM sleep, and the inhibition of its secretion suppresses the duration and depth of sleep.43–46 Once released, GH would act in specific locations (through the GH-IGF axis), performing its different functions, among which growth. Changes in the homeostasis of the sleep process may interfere with the physiological release of this hormonal network.
Mouth breathing and growth retardationEvidence indicates reduced pituitary GH release in individuals with airway obstruction.46 Although the cause of growth retardation is not fully understood, some of the possible explanations include low nocturnal levels of GH, lack of appetite and dysphagia that result in low caloric intake, nocturnal hypoxemia, nocturnal acidosis, and increased energy consumption after an increase in breathing work. Surgical removal of the pharyngeal and palatine tonsils has been shown to lead to resumption of normal growth for age in these children,28–31,47 as well as control of allergic rhinitis.34
An experimental study with rats demonstrated that upper airway obstruction reduced the hypothalamic and serum levels of GHRH, as well as the levels of GHRH receptors.43 In these animals, the waking time was increased and slow-wave sleep, paradoxical sleep, and low-activity waves were reduced. The administration of ritanserin (a selective serotonin receptor antagonist) relived these effects, i.e., normalized the hypothalamic content of GHRH, decreased wavelength, increased duration and depth of slow-wave sleep, and reduced growth retardation observed in these animals. Thus, the authors suggest that the growth retardation observed in these animals with upper airway obstruction is associated with hypothalamic GHRH.43 The abnormalities in the GHRH/GH axis underlie both, and growth retardation and slow-wave sleep associated with upper airway obstruction.43
In humans, the evaluation of patients with pharyngeal and/or palatine tonsil hypertrophy has provided important insights for a better understanding of the relationship between airway obstruction and growth deficit. A systematic review, followed by meta-analysis, identified 20 studies among 211 citations evaluating the relationship between the presence of hypertrophied pharyngeal tonsils, growth, growth markers, and sleep-disordered breathing in children.35 The joint review of data of more than 300 patients allowed to the authors to effectively confirm the results previously obtained. When compared with the pre-operative period, significant increases were observed in weight (minimum significant difference [MSD]=0.57; 95% 95% CI=0.44–0.70), height (MSD=0.34; 95% CI=0.20–0.47; assessed by Z-score), and serum levels of IGF-1 (MSD=0.53; 95% CI=0.33–0.73) and IGFBP-3 (MSD=0.59; 95% CI=0.34–0.83). The authors concluded that primary care physicians and specialists must consider RSDs when caring for children with growth deficit.35
However, the lack of homogeneity in the assessed populations was a limiting factor for the observed conclusions, which lead to the development of new studies. Tatlıpınar et al. assessed IGF-1 and IGFBP-3 serum levels, as well as the relationship between the volume of pharyngeal tonsil and nasopharynx (PT/N) of patients (aged 3–10 years) with sleep apnea secondary to pharyngeal tonsil hypertrophy.48 The surgical removal lead to an increase in weight, height, and serum levels of IGF-I and IGF-BP3. Nonetheless, no significant correlation was observed between the increased biomarkers and the PT/N index.48
In more recent study in pre-pubertal children aged over 5 years with a history of pharyngeal tonsil hypertrophy, after tonsil removal, a significant increase in serum IGF-1 and ghrelin levels was observed, as well as a significant increase in weight, height, and body mass index. Ghrelin is predominantly involved in the regulation of the sleep–wake cycle; it is associated with sleep deprivation and has a role in regulating metabolism. Known as the hunger hormone, it interacts with GH, leptin, and orexins in the sleep circuit regulation, emphasizing the role of energetic balance during sleep.49 According to these authors, growth retardation in these children would be related to lower serum levels of IGF-1.50
Final considerationsThe nose is the organ responsible for smell, being essential to breathing, through humidification, heating, and filtering of the inhaled air; it also allows the drainage of the paranasal sinuses. The upper airways undergo major changes during childhood and pre-school age: in the first five years of life, it increases from 6% to 40% of the nasal respiratory volume of adults.51
From the first months of life onwards, due to the anatomical conditions, nasal breathing is preferred,1,52 and half of the children have significant hypoxemias if the nose is congested.52
Mouth breathing can have dramatic consequences, including growth retardation, highlighting the importance of early recognition of this health problem that must be diagnosed and properly controlled through clinical or even surgical approach.
Conflicts of interestThe authors declare no conflicts of interest.