Two randomized controlled clinical trials have shown that Lactobacillus (L) reuteri DSM 17938 reduces the duration of diarrhea in children hospitalized due to acute infectious diarrhea. This was the first trial evaluating the efficacy of L. reuteri DSM 17938 in outpatient children with acute infectious diarrhea.
MethodsThis was a multicenter, randomized, single-blinded, case control clinical trial in children with acute watery diarrhea. A total of 64 children who presented at outpatient clinics were enrolled. The probiotic group received 1×108CFU L. reuteri DSM 17938 for five days in addition to oral rehydration solution (ORS) and the second group was treated with ORS only. The primary endpoint was the duration of diarrhea (in hours). The secondary endpoint was the number of children with diarrhea at each day of the five days of intervention. Adverse events were also recorded.
ResultsThe mean duration of diarrhea was significantly reduced in the L. reuteri group compared to the control group (approximately 15h, 60.4±24.5h [95% CI: 51.0–69.7h] vs. 74.3±15.3h [95% CI: 68.7–79.9h], p<0.05). The percentage of children with diarrhea was lower in the L. reuteri group (13/29; 44.8%) after 48h than the control group (27/31; 87%; RR: 0.51; 95% CI: 0.34–0.79, p<0.01). From the 72nd hour of intervention onwards, there was no difference between the two groups in the percentage of children with diarrhea. No adverse effects related to L. reuteri were noted.
ConclusionL. reuteri DSM 17938 is effective, safe, and well-tolerated in outpatient children with acute infectious diarrhea.
Dois ensaios clínicos randomizados controlados demonstraram que o Lactobacillus (L) reuteri DSM 17938 reduz a duração de diarreia em crianças hospitalizadas devido a diarreia infecciosa aguda. Este é o primeiro ensaio que avalia a eficácia do L. reuteri DSM 17938 em crianças com diarreia infecciosa aguda no ambulatório.
MétodosEste foi um ensaio clínico multicêntrico, randomizado, único cego, com grupos paralelos e controlado em crianças com diarreia aguda. Um total de 64 crianças internadas na clínica ambulatorial foram inscritas. O grupo probiótico recebeu 1×108CFU L. reuteri DSM 17938 por cinco dias, além de uma solução de reidratação oral (SRO), e o segundo grupo foi tratado apenas com SRO. O desfecho principal foi a duração da diarreia (em horas). O desfecho secundário foi o número de crianças com diarreia em cada um dos cinco dias da intervenção. Os eventos adversos também foram registrados.
ResultadosA duração média da diarreia foi significativamente reduzida no grupo L. reuteri em comparação ao grupo de controle (aproximadamente 15horas; 60,4±24,5horas [51, 0–69, 7horas, IC de 95%] em comparação a 74,3±15,3horas [68, 7–79, 9horas, IC de 95%], p<0,05). O percentual de crianças com diarreia foi menor no grupo L. reuteri (13/29; 44,8%) após 48 horas que no grupo de controle (27/31; 87%) (RR: 0,51; 0, 34–0, 79; IC de 95%, <0,01). A partir da 72ª hora de intervenção, não havia nenhuma diferença entre os dois grupos no percentual de crianças com diarreia. Nenhum efeito adverso com relação ao L. reuteri foi observado.
ConclusãoO L. reuteri DSM 17938 é eficaz, seguro e bem tolerado por crianças com diarreia infecciosa aguda no ambulatório.
Diarrhea remains an important cause of morbidity and mortality in children worldwide.1 The European Society of Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) and the European Society of Paediatric Infectious Diseases (ESPID) together published evidence-based guidelines on the management of acute gastroenteritis, and confirmed that rehydration is the key treatment. The guidelines also stated that selected probiotics may reduce the duration and intensity of symptoms and can be used as an adjuvant to oral rehydration solution (ORS).2 Current evidence also indicates that probiotic effects are strain-specific.3 Various strains of Lactobacillus reuteri species have been studied in acute diarrhea and have been found to be beneficial. L. reuteri DSM 17938 is a new probiotic strain with removed transferable resistance traits for tetracycline and lincomycin from the original L. reuteri ATCC 55730 strain.4 It has been previously reported that L. reuteri DSM 17938 reduced the duration of diarrhea and the length of hospital stay in children requiring hospitalization due to acute infectious diarrhea.5 A recent meta-analysis of L. reuteri DSM 17938 in children with acute infectious diarrhea showed reduced duration of diarrhea and concluded that outpatient data and country-specific cost-effectiveness analyses are needed.6 This study is the first report of the effects of L. reuteri DSM 17938 on acute infectious diarrhea in an outpatient pediatric setting.
Patient and methodsThis was a multicenter, randomized, single-blinded, case control clinical trial in children of both genders, aged between 3 and 60 months, with acute infectious diarrhea (defined as the passage of three or more loose or watery stools per day) lasting 12–72h before presentation at the outpatient clinic. The main investigator (ECD) did not enroll children and was blinded to the treatment of the patients. Approval for the study was granted by the Local Ethics Committee and an informed consent was obtained from the parents of the children. This study was registered at www.clinicaltrials.gov (NCT01927094).
Children who presented at the outpatient clinic with acute infectious diarrhea, and who were followed up with ambulatory care were enrolled in the study. Exclusion criteria were need for hospitalization, use of antibiotics or probiotics for one month prior to a new episode of diarrhea, severe malnutrition, or severe chronic underlying disease, including immunocompromising conditions.
All children were randomly assigned to the probiotic or control group using a computer-generated randomization list. Block randomization was applied with a computer-generated random number list by the main investigator (ECD), who did not enroll any patients. The first group received the five drops containing 1×108CFU L. reuteri DSM 17938 (BioGaia®, Stockholm, Sweden) for five days, in addition to ORS. The second group received ORS only (control group). The probiotics preparations were provided by the distribution company (Eczacibasi) in Turkey. Hypo-osmolar ORS (glucose 20g; sodium 60mmol/L; potassium 20mmol/L; bicarbonate 30mmol/L) was used. The primary endpoint was the duration of diarrhea (in hours). The secondary endpoint was the number of children with diarrhea at each day of the five days of the intervention. Adverse events were also recorded. The duration of diarrhea was defined as the time in hours from admission until cessation of diarrhea, which in turn was defined as the first normal stool according to the Bristol score (a score of <5 was considered as normalization of the stools).
The sample size needed was calculated based on the mean duration of diarrhea and standard deviation (SD) from previous similar studies. With the assumption of mean difference on duration of diarrhea of one day (24h) between the treatment and control group, the authors calculated that a sample of 64 children would be required for the study to have 80% power with a significance level=0.05 and sigma=2 (two tailed test). Statistical analysis was performed using SPSS software, version 16.0 (SPSS Inc., IL, USA). Variables were tested for normal distribution and compared using the Mann–Whitney U-test and t-test (for mean difference) and χ2 or Fisher's exact tests, as appropriate. MedCalc® program (Microsoft Partner Network, USA) was performed to calculate relative risk (RR). Statistical significance was set at p<0.05.
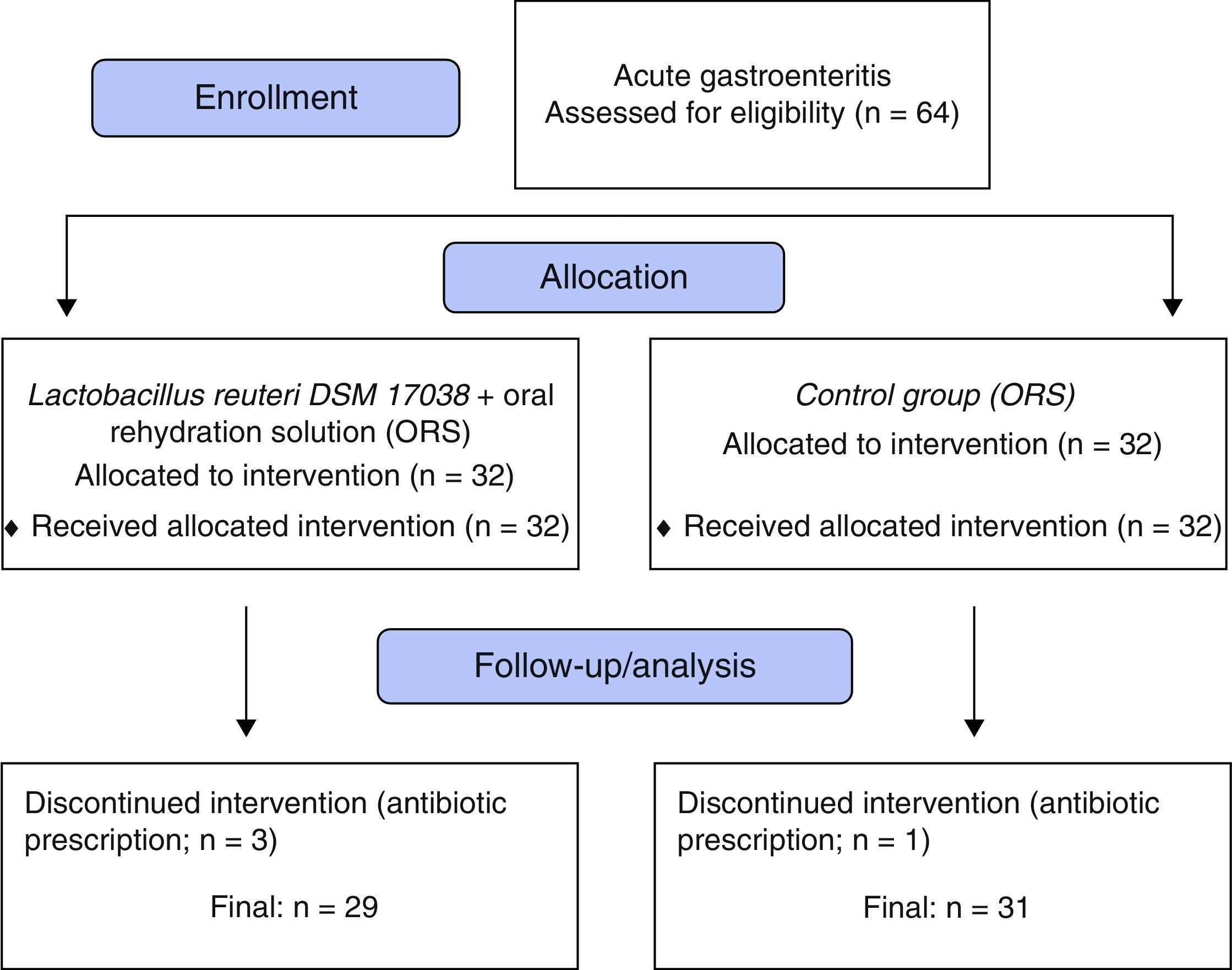
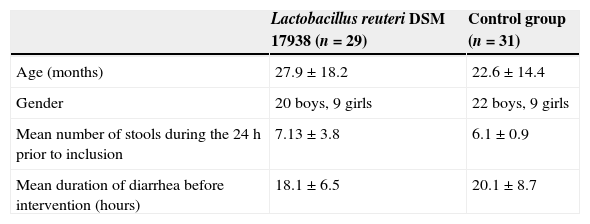
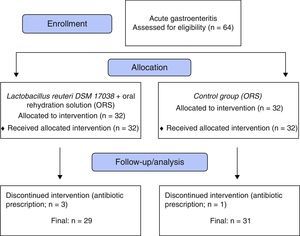
ResultsThe study included 64 children. After exclusion of three children from the L. reuteri group and one child from the control group due to antibiotic prescription post-randomization (Fig. 1), a total of 60 remained for evaluation; 29 (20 male, 9 female) in the L. reuteri group and 31 (22 male, nine female) in the control group. The demographic findings, mean duration of diarrhea before intervention and mean number of stools at 24h prior to inclusion are summarized in Table 1.
Demographic and clinical findings of the study groups.
| Lactobacillus reuteri DSM 17938 (n=29) | Control group (n=31) | |
|---|---|---|
| Age (months) | 27.9±18.2 | 22.6±14.4 |
| Gender | 20 boys, 9 girls | 22 boys, 9 girls |
| Mean number of stools during the 24h prior to inclusion | 7.13±3.8 | 6.1±0.9 |
| Mean duration of diarrhea before intervention (hours) | 18.1±6.5 | 20.1±8.7 |
Values expressed as mean±SD.
The mean duration of diarrhea was significantly reduced in the L. reuteri group when compared to the control group (approximately 15h; 60.4±24.5h [95% CI: 51.0–69.7h] vs. 74.3±15.3h [95% CI: 68.7–79.9h]; p<0.05; Table 2).
Duration of diarrhea in study groups.
| Lactobacillus reuteri DSM 17938 (n=29) | Control group (n=31) | |
|---|---|---|
| Duration of diarrhea (hours) | 60.4±24.5a | 74.3±15.3 |
Values are expressed as mean±SD (95% CI).
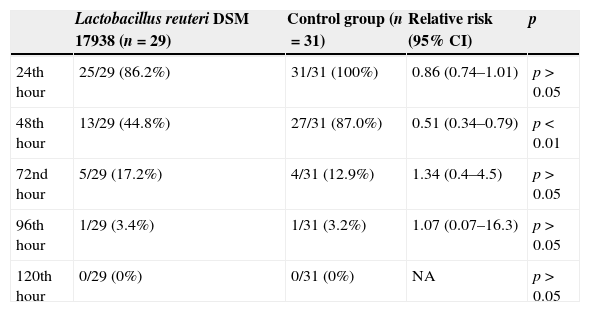
At the 48th hour of the study, 45% of the children receiving L. reuteri DSM 17938 had watery diarrhea, while this was still the case in 87% of the children in the control group (13/29; 44.8% vs. 27/31; 87%; RR: 0.51; 95% CI: 0.34–0.79; p<0.01). From the 72nd hour of the intervention, the percentage of children without diarrhea was similar between the groups (Table 3). No adverse effects related to L. reuteri DSM 17938 were observed.
Percentage of children with diarrhea in study groups.
| Lactobacillus reuteri DSM 17938 (n=29) | Control group (n=31) | Relative risk (95% CI) | p | |
|---|---|---|---|---|
| 24th hour | 25/29 (86.2%) | 31/31 (100%) | 0.86 (0.74–1.01) | p>0.05 |
| 48th hour | 13/29 (44.8%) | 27/31 (87.0%) | 0.51 (0.34–0.79) | p<0.01 |
| 72nd hour | 5/29 (17.2%) | 4/31 (12.9%) | 1.34 (0.4–4.5) | p>0.05 |
| 96th hour | 1/29 (3.4%) | 1/31 (3.2%) | 1.07 (0.07–16.3) | p>0.05 |
| 120th hour | 0/29 (0%) | 0/31 (0%) | NA | p>0.05 |
The results of the present study showed that ORS in combination with five days of L. reuteri DSM 17938 reduced the duration of acute infectious diarrhea to approximately 15h in children aged between 3 and 60 months. The effect was mainly observed after 48h of intervention, when 55% of the children in the intervention group were diarrhea-free, while this was the case in only 13% in the control group. In a previous study, performed by the same study team and with the same design, except for the fact that the study was applied to hospitalized children, 127 children aged 3–60 months were enrolled. In hospitalized children, the administration of L. reuteri DSM 17938 reduced the duration of diarrhea significantly to approximately 33h.5 The effect (percentage of children without diarrhea) of L. reuteri DSM 17938 started to be observed after 24h of intervention and was greatest after 48 and 72h. It was observed also that the mean length of hospital stay was shortened by more than 24h in the L. reuteri DSM 17938 group.5 Francavilla et al.7 performed a randomized double-blinded, placebo-controlled clinical trial on 74 children aged 6–36 months with acute diarrhea, randomized to receive L. reuteri DSM 17938 or placebo for seven days, in three hospitals in Southern Italy. Compared with the placebo group, the L. reuteri DSM 17938 group presented a significant reduction in the duration of diarrhea, in the risk of watery diarrhea on day 2 and day 3, and in the risk of relapse of diarrhea, although no reduction of the duration of hospitalization was observed.7 Szajewska et al.6 pooled the data from these two RCTs of hospitalized children, confirming that L. reuteri DSM 17938 significantly reduced the duration of diarrhea to approximately 32h and increased the chance of cure on day 3.6 Two independent studies, one in Indonesia and one in Mexico, demonstrated that the prophylactic use of L. reuteri DSM 17938 reduces the frequency and duration of diarrheal episodes,8,9 although their clinical relevance can be questioned.10L. reuteri DSM 17938 was well tolerated, and no associated adverse events have been reported in any trial.6
The current study had some limitations, as it was not a double-blinded, placebo-controlled clinical trial. To access stool consistency, the Bristol Stool Form Scale was used, which has a limited validation for the youngest children, although it offers a more objective form of assessing stool consistency rather than just relying on the perceptions of caregivers.11 The duration of diarrhea was used as the primary endpoint, which is not considered optimal. However, there is a major reluctance of caregivers and healthcare providers to conduct the cumbersome stool collection. The question of generalization of findings in clinical trials, specifically on infectious gastroenteritis and probiotics, is challenging. However, these findings can be considered as generalized, since worldwide viral gastroenteritis is the most common cause. Moreover, as a multi-center study, the participants came from urban and rural regions, including economically developed and poor areas. Therefore, these findings are likely to allow for generalization.
The Working Group on Probiotics of the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) concluded that the use of L. reuteri DSM 17938 should be considered in the management of acute gastroenteritis as an adjunct intervention to ORS.3 Probiotics may be of help for the management of acute diarrhea. However, the effect is strain-specific, and efficacy needs to be proven in different settings, such as hospital or outpatients settings. The results of the present study have shown that L. reuteri DSM 17938 as an adjunct to ORS therapy is efficacious in the treatment of acute diarrhea, reducing the duration of the disease in an outpatient setting.
Conflicts of interestE.C. Dinleyici is speaker bureau and advisory board member of Biocodex. M. Ozen is speaker bureau of Pfizer Consumer Health. Y. Vandenplas is a consultant for Biocodex, United Pharmaceuticals. Other authors declare no conflicts of interest.
Please cite this article as: Dinleyici EC, Dalgic N, Guven S, Metin O, Yasa O, Kurugol Z, et al. Lactobacillus reuteri DSM 17938 shortens acute infectious diarrhea in a pediatric outpatient setting. J Pediatr (Rio J). 2015;91:392–6.