The literature suggests that a fetus will adapt to surrounding adversities by optimizing its use of energy to improve survival, ultimately leading to the programming of the individual's energy intake and expenditure. While recent reviews focused on the fetal programming of energy intake and food preferences, there is also some evidence that fetal adversity is associated with diminished physical activity levels. Therefore, we aimed to review (a) the evidence for an association between being born with intrauterine growth restriction and sedentarism over the life-course and (b) the potential benefits of physical activity over cardiometabolic risk factors for this population.
SourcesPubMed, Scielo, Scopus and Embase.
Summary of findingsMost clinical studies that used objective measures found no association between intrauterine growth restriction and physical activity levels, while most studies that used self-reported questionnaires revealed such relationships, particularly leisure time physical activity. Experimental studies support the existence of fetal programming of physical activity, and show that exposure to exercise during IUGR individuals’ life improves metabolic outcomes but less effect was seen on muscle architecture or function.
ConclusionsAlterations in muscle strength and metabolism, as well as altered aerobic performance, may predispose IUGR individuals to be spontaneously less physically active, suggesting that this population may be an important target for preventive interventions. Although very heterogeneous, the different studies allow us to infer that physical activity may have beneficial effects especially for individuals that are more vulnerable to metabolic modifications such as those with IUGR.
A literatura sugere que um feto se adaptará às adversidades externas ao aprimorar seu gasto energético para melhorar a sobrevida, o que leva, em última instância, à programação do consumo e gasto energético do indivíduo. Apesar de análises recentes terem focado na programação fetal do consumo energético e preferências alimentares, ainda há alguma comprovação de que as adversidades fetais estão associadas aos baixos níveis de atividade física. Portanto, visamos analisar: a) a comprovação de uma associação entre nascer com restrição de crescimento intrauterino (RCIU) e sedentarismo durante o curso de vida e b) os possíveis benefícios da atividade física sobre os fatores de risco cardiometabólico dessa população.
FontesPubMed, Scielo, Scopus e Embase.
Resumo dos achadosA maior parte dos estudos clínicos que usaram medidas objetivas não constatou associação entre RCIU e os níveis de atividade física, ao passo que a maior parte dos estudos que usaram questionários de autorrelato revelou essas relações, principalmente no que diz respeito à atividade física de lazer. Estudos experimentais corroboram a existência de programação fetal de atividade física e mostram que a exposição a exercícios durante a vida de indivíduos com RCIU melhora os resultados metabólicos, porém menos efeito foi visto sobre a arquitetura ou função muscular.
ConclusõesAlterações na força muscular e no metabolismo, bem como o desempenho aeróbico alterado, podem predispor indivíduos com RCIU a serem espontaneamente menos ativos fisicamente, sugere que essa população pode ser um importante alvo de intervenções preventivas. Apesar de muito heterogêneos, os diferentes estudos nos possibilitam deduzir que a atividade física pode ter efeitos benéficos principalmente em indivíduos mais vulneráveis a modificações metabólicas, como aqueles com RCIU.
The Developmental Origins of Health and Disease (DOHaD) concept aims to study the consequences that adverse events occurring during early phases of human development have on disease and health patterns over the life course.1,2 Another interesting aspect related to this field of research relates to the programming of a behavior, such as food intake, feeding preferences or willingness to engage in physical activities, which ultimately may contribute to the development of such diseases. While most of DOHaD field has focused on how fetal programming may affect histological, anatomical or metabolic markers, it is intriguing to think that neurobiological changes that affect behaviors may also be equally affected by adversity in vulnerable periods such as fetal life/infancy/adolescence. Behaviors involving “will” or “choices” are usually closely associated to free will in human nature, and it may be uncomfortable to suggest that this may be shaped by neurologic and genetic determinants and influenced by the environment.3 Is it possible that the biological parameters can define our own free selves? As stated by David Goldman, “individual free will, and by extension the autonomy of groups of people, are parameters whose existence can be derived from the inheritance of cognitive structures and variation in these structures due to neurodevelopmental adaptation”.3
One way to measure intrauterine adversity relates to poor fetal growth. Intrauterine growth restriction (IUGR) is the inability of the fetus to achieve its full potential growth4 which can be caused by, but not restricted to, placental insufficiency,5,6 maternal malnutrition and smoking,7 congenital infections and anomalies, drugs, obesity and chromosomal abnormalities.4,8 The estimated burden of IUGR is very high considering that in low and middle-income countries, for instance, IUGR accounts for 27% of live births.9 Additionally, IUGR has been linked to non-communicable diseases later in life, such as metabolic syndrome,2,10,11 type II diabetes11 and cardiovascular diseases.12
The thrifty phenotype hypothesis proposes that the fetus adapts to surrounding adversities (e.g. lack of nutrients) by optimizing its use of energy to improve survival.1,2 This ultimately leads to the programming of the individual's energy intake and expenditure.13–15 Regarding energy intake, our group has spent the last few years studying the programming of food preferences toward highly caloric palatable foods.16–21 On the other hand, it is interesting to acknowledge that adverse events in the early development can cause behavioral consequences. When considering the expenditure, there is also evidence that this population may have diminished physical activity levels,22,23 contributing to increased storage and consequent predisposition to altered metabolic states.
Experimental studies have shown that IUGR offspring are less active when compared to those born with normal birth weight.23–25 Evidence of lower levels of leisure-time physical activity and increased levels of sedentary behavior have already been demonstrated by some clinical studies,22,26–30 while others have reported controversial results, without any association between low birth weight and lower levels of physical activity.31–36
Additionally, considering the detrimental effects of IUGR later in life, some studies have also focused on the potential benefits that physical activity can have on metabolic outcomes of this at-risk population.37 It appears that exercise may attenuate cardiometabolic risk factors such as glucose intolerance,29 insulin resistance38 and poor lipid profile39 especially in the IUGR population in which these risk factors are more prevalent.
In this review we summarize the available clinical and experimental evidence regarding: (1) the effects of IUGR on sedentary behavior and physical activity levels, and (2) the potential benefits of exercise interventions to attenuate negative cardiometabolic effects of IUGR. Furthermore, we explore some mechanisms that are likely implicated in these associations.
Clinical evidence of the fetal programming of energy expenditureThe general environmental message during intrauterine life of an IUGR fetus is of a paucity of nutrients, therefore organs and systems are programmed to save energy.15 The fetal programming of energy expenditure, including diminished physical activity levels and body lean mass, saves energy for growth and fat accumulation. Additionally, muscle strength is directly associated with physical activity levels,40 so it is likely that IUGR individuals may exercise less because of lacking appropriate musculature.41,42
There is a large heterogeneity in the literature regarding the study of variables related to sedentarism and physical activity. While some studies use objective measurements, such as accelerometer, others use self-reported questionnaires and measures such as leisure time physical activity (LTPA). Accelerometer, for instance, can estimate total activity with an average counts per minute (cpm) over the valid measurement period.43,44 The cut off to define moderate to vigorous physical activity also uses the average cpm but in terms of time spent each day on such activity.45 Nonobjective measurements include LTPA, measured through questionnaires that evaluate the frequency and duration of different conditioning (such as running, swimming) and non-conditioning (such as gardening, household work) activities.46,47 Different questionnaires have been validated for different time frames, ranging from a recall time of 1 week48 to 12 months.47
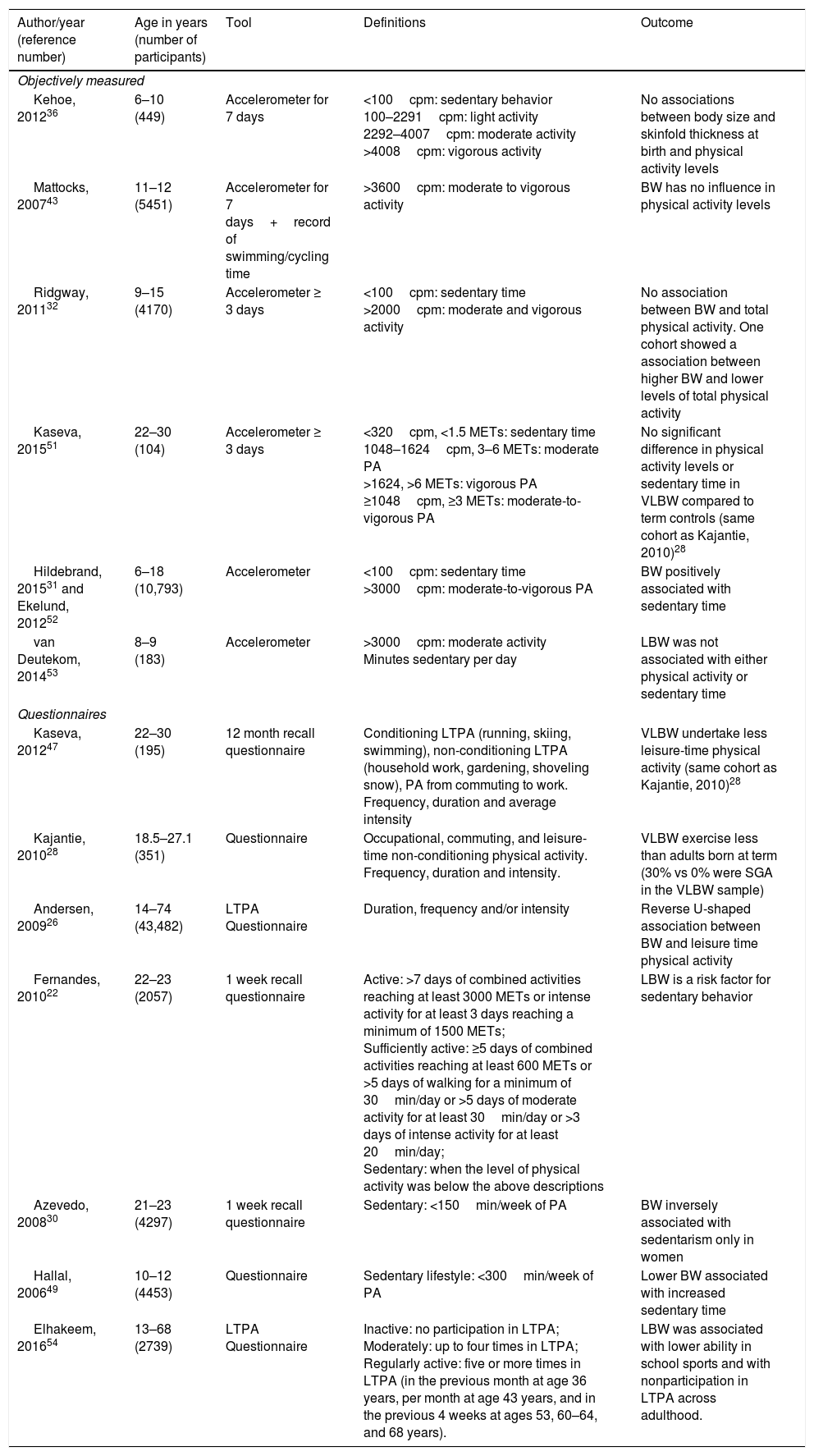
Sedentarism is defined according to the time spent on physical activity and the cut off points are also variable among the different studies, ages and methodologies used. For instance, in adolescents a sedentary lifestyle is considered as less than 300min of physical activity per week measured through questionnaires49 whereas in adults it is considered as less than 150min per week.30 Different studies convert questionnaire responses into Metabolic Equivalent of Task (MET)-min/week and use METs cutoffs to define sedentarism.22 Similarly, sedentary behavior has been defined as <100cpm in children using accelerometer.50 Therefore, when considering the different studies, one should keep in mind the specific concepts and constructs being evaluated. We separate the studies into those using objective and self-reported questionnaire data, and thereafter discuss the potential benefits of physical activity to IUGR individuals. Table 1 summarizes the main characteristics of the clinical studies.
Summary of clinical studies evaluating the relationship between IUGR and physical activity/sedentarism.
| Author/year (reference number) | Age in years (number of participants) | Tool | Definitions | Outcome |
|---|---|---|---|---|
| Objectively measured | ||||
| Kehoe, 201236 | 6–10 (449) | Accelerometer for 7 days | <100cpm: sedentary behavior 100–2291cpm: light activity 2292–4007cpm: moderate activity >4008cpm: vigorous activity | No associations between body size and skinfold thickness at birth and physical activity levels |
| Mattocks, 200743 | 11–12 (5451) | Accelerometer for 7 days+record of swimming/cycling time | >3600cpm: moderate to vigorous activity | BW has no influence in physical activity levels |
| Ridgway, 201132 | 9–15 (4170) | Accelerometer ≥ 3 days | <100cpm: sedentary time >2000cpm: moderate and vigorous activity | No association between BW and total physical activity. One cohort showed a association between higher BW and lower levels of total physical activity |
| Kaseva, 201551 | 22–30 (104) | Accelerometer ≥ 3 days | <320cpm, <1.5 METs: sedentary time 1048–1624cpm, 3–6 METs: moderate PA >1624, >6 METs: vigorous PA ≥1048cpm, ≥3 METs: moderate-to-vigorous PA | No significant difference in physical activity levels or sedentary time in VLBW compared to term controls (same cohort as Kajantie, 2010)28 |
| Hildebrand, 201531 and Ekelund, 201252 | 6–18 (10,793) | Accelerometer | <100cpm: sedentary time >3000cpm: moderate-to-vigorous PA | BW positively associated with sedentary time |
| van Deutekom, 201453 | 8–9 (183) | Accelerometer | >3000cpm: moderate activity Minutes sedentary per day | LBW was not associated with either physical activity or sedentary time |
| Questionnaires | ||||
| Kaseva, 201247 | 22–30 (195) | 12 month recall questionnaire | Conditioning LTPA (running, skiing, swimming), non-conditioning LTPA (household work, gardening, shoveling snow), PA from commuting to work. Frequency, duration and average intensity | VLBW undertake less leisure-time physical activity (same cohort as Kajantie, 2010)28 |
| Kajantie, 201028 | 18.5–27.1 (351) | Questionnaire | Occupational, commuting, and leisure-time non-conditioning physical activity. Frequency, duration and intensity. | VLBW exercise less than adults born at term (30% vs 0% were SGA in the VLBW sample) |
| Andersen, 200926 | 14–74 (43,482) | LTPA Questionnaire | Duration, frequency and/or intensity | Reverse U-shaped association between BW and leisure time physical activity |
| Fernandes, 201022 | 22–23 (2057) | 1 week recall questionnaire | Active: >7 days of combined activities reaching at least 3000 METs or intense activity for at least 3 days reaching a minimum of 1500 METs; Sufficiently active: ≥5 days of combined activities reaching at least 600 METs or >5 days of walking for a minimum of 30min/day or >5 days of moderate activity for at least 30min/day or >3 days of intense activity for at least 20min/day; Sedentary: when the level of physical activity was below the above descriptions | LBW is a risk factor for sedentary behavior |
| Azevedo, 200830 | 21–23 (4297) | 1 week recall questionnaire | Sedentary: <150min/week of PA | BW inversely associated with sedentarism only in women |
| Hallal, 200649 | 10–12 (4453) | Questionnaire | Sedentary lifestyle: <300min/week of PA | Lower BW associated with increased sedentary time |
| Elhakeem, 201654 | 13–68 (2739) | LTPA Questionnaire | Inactive: no participation in LTPA; Moderately: up to four times in LTPA; Regularly active: five or more times in LTPA (in the previous month at age 36 years, per month at age 43 years, and in the previous 4 weeks at ages 53, 60–64, and 68 years). | LBW was associated with lower ability in school sports and with nonparticipation in LTPA across adulthood. |
IUGR, intrauterine growth restriction; cpm, counts per minute; BW, birth weight; MET, metabolic equivalent task; PA, physical activity; VLBW, very low birth weight; LTPA, leisure time physical activity; SGA, small for gestational age.
Kehoe et al. evaluated accelerometer measures in children (6 to 10 year-olds) in rural India. There were 8% of small for gestational age (SGA) children in the sample (considered as a birth weight of <2.5kg) and birth weight varied from 1.57 to 4.75kg. There were no significant associations between any of the anthropometric measures at birth and the physical activity variables.36 It is interesting to notice that in this study socio economic status was negatively associated with physical activity. Although the data were not shown, the authors described that separate analysis for SGA and preterm were conducted and that there was a trend for SGA to be less active. It seems to us that the definition of SGA was not adequate and the use of other definitions for SGA (i.e.: smaller than 10th percentile or birth weight ratio <0.85) would result in a larger proportion of low birth weight for a given gestational age in the population described.
The ALSPAC (Avon Longitudinal Study of Parents and Children) cohort included a large sample of school-aged children and there was no relationship between physical activity levels, measured through accelerometer, and any of the neonatal anthropometrics measurements (birth weight, ponderal index, head circumference) or gestational age.43 Birth weight was used as a continuous variable but the study did not categorize individuals as SGA or IUGR.
Another study included four birth cohorts from European countries and Brazil32 and found no significant association between birth weight and objectively measured physical activity. The cohort from Brazil that was included in this study revealed results in the opposite direction, with higher birth weight being associated with lower levels of activity.32
Kaseva et al. used the same methodology with accelerometer to objectively measure physical activity levels in a cohort (Helsinki Study) of very low birth weight (VLBW) (<1500g) young adults.51 Overall there were no differences in physical activity levels or sedentary time between VLBW and control participants.
Hildebrand et al.31 and Ekelund et al.52 used the International Children's Accelerometry Database and found that birth weight was positively associated with sedentary time and with waist circumference.31 There were no data regarding gestational age, so the authors could not differentiate between low birth weight caused by IUGR or by prematurity. In 2014 van Deutekom et al. described that birth weight was not related to either physical activity or sedentary behavior.53
Studies using self-report questionnaires to assess the association between IUGR and physical activityTwo other studies were performed in the previously described Helsinki cohort,28,47 but assessed physical activity through questionnaires of LTPA. The study by Kaseva et al. found markedly reduced LTPA in VLBW adults when compared to controls.47 Kajantie et al. described similar findings.28 When asked about the amount of time spent in physical activity during their leisure time, VLBW individuals had significant less time devoted to LTPA when compared to controls (35% versus 25%). It is important to notice that the VLBW sample included 30% SGA, while the control sample had no SGAs. Subgroup analysis comparing VLBW SGA and VLBW born appropriate for gestational age (AGA) revealed no differences in any of the physical activity variables, nor birth weight SD score and gestational age as continuous variables within the VLBW group. In this study, IUGR does not seem to be implicated in the findings and it is likely that the association is attributable to prematurity.28
In a sample of both children and adults born IUGR, teachers reported lower ability in sports in 13 year-olds and lower self-reported LTPA in adulthood.54 A meta-analysis by Andersen et al.26 included a wide range of age groups, from 14 to 74 years old, derived from 13 Nordic cohorts. Both low and high birth weight were associated with lower LTPA levels in women and men.
Some studies explored other possible factors interacting with birth weight to influence sedentary behavior levels, such as education and socioeconomic status. A cohort of young adults by Fernandes et al. found an association between sedentary and LBW conditioned to individuals who have higher educational levels.22 Another Brazilian cohort revealed an inverse association between birth weight and LTPA in women but found that socioeconomic status was also related to exercise levels.30 Hallal et al. had similar conclusions to lower birth weight being associated with sedentary behavior at 10–12 years old. Other risk factors for sedentary behavior were higher maternal education and family income, similarly to the other two studies described. From the different studies, it is possible to apprehend that socio-economic variables (both income and education) deeply affect the association between birth weight and sedentary behavior, either when comparing different social extracts within a specific community or when comparing studies performed in countries from diverse economic profile.
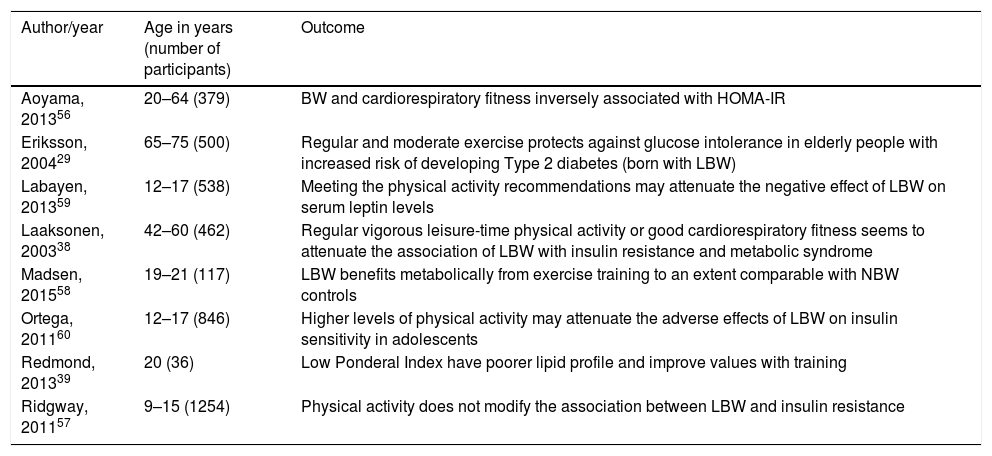
Benefits of physical activity in IUGR individualsIn 2014 Gatford et al. comprised a review of clinical and experimental evidence that exercise can improve metabolic outcomes after IUGR.55 It is clear that IUGR adults benefit from physical activity when it comes to cardiometabolic outcomes but it is still unclear whether these benefits are different when comparing IUGR to normal birth weight individuals. Table 2 summarizes the main evidence about cardiometabolic effects of exercise in IUGR individuals.
Physical activity levels effects on metabolic outcomes of LBW/IUGR.
| Author/year | Age in years (number of participants) | Outcome |
|---|---|---|
| Aoyama, 201356 | 20–64 (379) | BW and cardiorespiratory fitness inversely associated with HOMA-IR |
| Eriksson, 200429 | 65–75 (500) | Regular and moderate exercise protects against glucose intolerance in elderly people with increased risk of developing Type 2 diabetes (born with LBW) |
| Labayen, 201359 | 12–17 (538) | Meeting the physical activity recommendations may attenuate the negative effect of LBW on serum leptin levels |
| Laaksonen, 200338 | 42–60 (462) | Regular vigorous leisure-time physical activity or good cardiorespiratory fitness seems to attenuate the association of LBW with insulin resistance and metabolic syndrome |
| Madsen, 201558 | 19–21 (117) | LBW benefits metabolically from exercise training to an extent comparable with NBW controls |
| Ortega, 201160 | 12–17 (846) | Higher levels of physical activity may attenuate the adverse effects of LBW on insulin sensitivity in adolescents |
| Redmond, 201339 | 20 (36) | Low Ponderal Index have poorer lipid profile and improve values with training |
| Ridgway, 201157 | 9–15 (1254) | Physical activity does not modify the association between LBW and insulin resistance |
BW, birth weight; HOMA-IR, homeostasis model assessment of insulin; LBW, low birth weight; NBW, normal birth weight.
Aoyama et al. studied 379 adults (20–64 years), evaluating their homeostasis model assessment of insulin (HOMA-IR) and cardiorespiratory fitness. Lower birth weight and cardiorespiratory fitness were associated with increased HOMA-IR, while BMI and abdominal circumference were positively associated with HOMA-IR. Further analysis using HOMA-IR as an independent variable, adjusted for cardiorespiratory fitness, little modified the association between birth weight and insulin resistance. Lifestyle factors may be more important than birth weight since the major predictor of insulin resistance was cardiorespiratory fitness.56
Ridgway et al. evaluated a younger population (9–15 years) with accelerometer measurements to verify whether physical activity and aerobic fitness could modify the association between birth weight and metabolic outcomes. Birth weight was not associated with fasting insulin, except when the model was adjusted for adolescent waist circumference. The data were little modified even after further adjustment for time spent exercising.57 It is possible that the young age of evaluation may play a role in these findings, considering that changes in metabolism may still not be present at this age.
One study conducted in India included young healthy men (18–22 years), excluding obese and those using medications that could alter the glucose profile, for a bicycle-based intervention over 6 weeks in order to evaluate changes in glucose metabolism. Approximately half of the sample comprised men born with LBW. Baseline data revealed no significant differences in either fasting plasma glucose or insulin levels between LBW and normal birth weight groups. Fasting plasma insulin levels, HOMA-IR and insulin secretion (HOMA-IS) improved equally after the intervention in both groups.58 One limitation is that the study selected only healthy individuals. Since IUGR is a known risk factor for metabolic syndrome2,10,11 and type II diabetes,11 the exclusion of individuals with altered metabolic states (obese and those using medications) may lead to the false conclusion that they would not benefit even more from an exercise intervention.
Redmond et al. evaluated differential outcomes of an exercise intervention in the metabolic profile of adults born small for gestational age. Individuals born with normal birth weight had a better pre-training lipid profile (particularly total and LDL cholesterol). Following training, group differences between total and LDL cholesterol disappeared. The increased risk of chronic diseases in those born with IUGR may be attenuated through lifestyle changes such as implementing an exercise routine.39
Among the studies showing beneficial effects of exercise, Eriksson et al. evaluated older adults and showed that frequent or moderate weekly exercise was associated with better glucose tolerance and this effect was even stronger among those born with small size at birth. Furthermore, men that were born small exercised more in adulthood than their controls, which could be interpreted as an adaptive response of survival of the fittest in this high-risk group.29 Also investigating adults, Laaksonen et al. found that small size at birth was associated with metabolic syndrome features even prior to the development of diabetes or cardiovascular disease. The association was no longer present when regular vigorous LTPA or good cardiorespiratory fitness were taken into consideration.38
Two studies in adolescents were favorable to physical activity as an attenuator of altered metabolic outcomes for those born small.59,60 The first one found that as birth weight decreases, leptin levels increase in girls not meeting the physical activity recommendations (≥60min/day of moderate to vigorous physical activity). There was no association between leptin levels and birth weight in those meeting the physical activity recommendations.59 Serum leptin levels are increased in obese people, who seem to develop a leptin resistance status, and it has already been shown that LBW is associated with higher serum leptin levels in female adolescents.61 The second article showed that higher levels of physical activity played a role in altering the association between LBW and HOMA-IR.60
Although the cohorts and studies are heterogeneous in terms of many variables such as age, country of origin and type of physical activity, it seems that physical training may have beneficial effects especially for individuals that are more vulnerable to metabolic modifications such as those born with LBW.
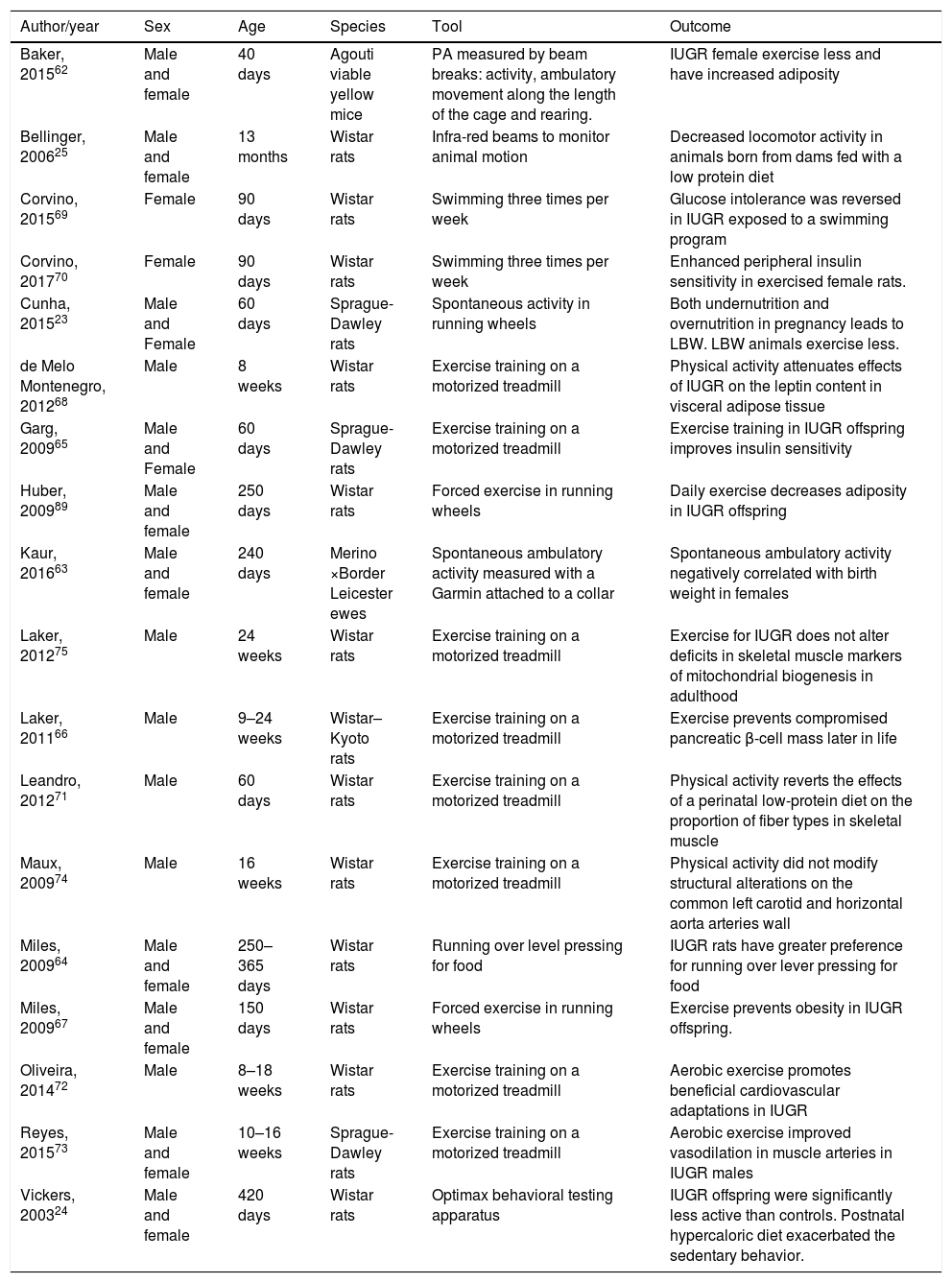
Experimental evidenceThe effects of intrauterine growth restriction on physical activity levelsSimilarly to what was described in the clinical studies, experimental evidence also suffers from heterogeneity in the methodology used to assess physical activity. Spontaneous physical activity is measured either by filming the cage, by infrared spectroscopy or by digital counter attached to running wheels. Table 3 summarizes the main characteristics of the outcomes of experimental studies.
Summary of the experimental data linking IUGR and physical activity levels.
| Author/year | Sex | Age | Species | Tool | Outcome |
|---|---|---|---|---|---|
| Baker, 201562 | Male and female | 40 days | Agouti viable yellow mice | PA measured by beam breaks: activity, ambulatory movement along the length of the cage and rearing. | IUGR female exercise less and have increased adiposity |
| Bellinger, 200625 | Male and female | 13 months | Wistar rats | Infra-red beams to monitor animal motion | Decreased locomotor activity in animals born from dams fed with a low protein diet |
| Corvino, 201569 | Female | 90 days | Wistar rats | Swimming three times per week | Glucose intolerance was reversed in IUGR exposed to a swimming program |
| Corvino, 201770 | Female | 90 days | Wistar rats | Swimming three times per week | Enhanced peripheral insulin sensitivity in exercised female rats. |
| Cunha, 201523 | Male and Female | 60 days | Sprague-Dawley rats | Spontaneous activity in running wheels | Both undernutrition and overnutrition in pregnancy leads to LBW. LBW animals exercise less. |
| de Melo Montenegro, 201268 | Male | 8 weeks | Wistar rats | Exercise training on a motorized treadmill | Physical activity attenuates effects of IUGR on the leptin content in visceral adipose tissue |
| Garg, 200965 | Male and Female | 60 days | Sprague-Dawley rats | Exercise training on a motorized treadmill | Exercise training in IUGR offspring improves insulin sensitivity |
| Huber, 200989 | Male and female | 250 days | Wistar rats | Forced exercise in running wheels | Daily exercise decreases adiposity in IUGR offspring |
| Kaur, 201663 | Male and female | 240 days | Merino ×Border Leicester ewes | Spontaneous ambulatory activity measured with a Garmin attached to a collar | Spontaneous ambulatory activity negatively correlated with birth weight in females |
| Laker, 201275 | Male | 24 weeks | Wistar rats | Exercise training on a motorized treadmill | Exercise for IUGR does not alter deficits in skeletal muscle markers of mitochondrial biogenesis in adulthood |
| Laker, 201166 | Male | 9–24 weeks | Wistar–Kyoto rats | Exercise training on a motorized treadmill | Exercise prevents compromised pancreatic β-cell mass later in life |
| Leandro, 201271 | Male | 60 days | Wistar rats | Exercise training on a motorized treadmill | Physical activity reverts the effects of a perinatal low-protein diet on the proportion of fiber types in skeletal muscle |
| Maux, 200974 | Male | 16 weeks | Wistar rats | Exercise training on a motorized treadmill | Physical activity did not modify structural alterations on the common left carotid and horizontal aorta arteries wall |
| Miles, 200964 | Male and female | 250–365 days | Wistar rats | Running over level pressing for food | IUGR rats have greater preference for running over lever pressing for food |
| Miles, 200967 | Male and female | 150 days | Wistar rats | Forced exercise in running wheels | Exercise prevents obesity in IUGR offspring. |
| Oliveira, 201472 | Male | 8–18 weeks | Wistar rats | Exercise training on a motorized treadmill | Aerobic exercise promotes beneficial cardiovascular adaptations in IUGR |
| Reyes, 201573 | Male and female | 10–16 weeks | Sprague-Dawley rats | Exercise training on a motorized treadmill | Aerobic exercise improved vasodilation in muscle arteries in IUGR males |
| Vickers, 200324 | Male and female | 420 days | Wistar rats | Optimax behavioral testing apparatus | IUGR offspring were significantly less active than controls. Postnatal hypercaloric diet exacerbated the sedentary behavior. |
IUGR, intrauterine growth restriction; LBW, low birth weight.
In order to identify changes in physical activity levels among female mice that were born IUGR, Baker et al. tested animals born from lean and obese dams, with all animals being fostered to lean dams. IUGR was noted in the offspring of obese dams. Female animals born from obese dams had reduced levels of spontaneous physical activity and energy expenditure, with increased rates of adult obesity.62 Kaur et al. induced IUGR through surgical manipulation of endometrial placental attachment sites of ewes and measured spontaneous physical activity in the offspring. Placental restriction and low birth weight were associated with greater spontaneous ambulatory activity.63
Cunha et al. evaluated spontaneous physical activity in rats born with IUGR (dams receiving a 50% food restricted diet from day 10 of pregnancy until birth) versus rats born from dams receiving an ad libitum diet and rats born from dams receiving high-fat diet. Both extreme groups (50% food restricted and high fat diets during pregnancy) had IUGR pups at birth, and both had different physical activity levels when compared to controls. However, there were opposite effects in different sexes: females from the extreme groups had increased physical activity in comparison to controls, while males from the extreme groups showed decreased activity in relation to controls.23 Considering that alterations in the mesolimbic system could be a possible mechanism to explain the effect observed in physical activity, D2 receptors in the dorsal striatum were assessed, and females from the food restricted group had decreased striatal D2 receptor levels when compared to the control group.23
Bellinger et al. fed pregnant rats with either a control or a low protein diet in different gestational periods (early, mid or late). Offspring locomotor activity was measured with an infrared sensor array. A low protein diet in all gestational periods was significantly associated with lower locomotor activity in female offspring, while in male offspring the effect was observed only when the low protein diet was offered in early pregnancy.25
Vickers et al. compared adult rats born from dams submitted to an undernourished diet during pregnancy versus controls. IUGR animals (born from dams receiving an undernourished diet during pregnancy) were more sedentary when compared to controls, regardless of the postnatal diet (standard or hypercaloric). The effect occurred in both genders but was more pronounced in males.24 In the study by Miles et al., the animals were given the choice between wheel running and pressing a response lever for food. IUGR offspring (born from undernourished dams) revealed a greater preference for exercise when compared to ad libitum offspring.64
Exercise intervention to attenuate metabolic outcomes of IUGR in animalsGarg et al. conducted a trial of early exercise training to analyze its effect on glucose kinetics. Pregnant rats were divided to receive either a normal or a restricted diet during days 11–21 of gestation. An exercise training intervention in the offspring was able to suppress glucose-stimulated insulin production and hepatic glucose production, leading to an overall improvement on insulin sensitivity.65 A short period of exercise training in pups was associated with significant increases in β-cell mass for all animals and this early exercise restored β-cell mass to control levels in IUGR offspring. However, insulin secretion was not altered by the intervention.66
Miles et al. was able to demonstrate that daily exercise in adult IUGR rats prevented the development of obesity. Although IUGR offspring had increased adiposity compared to controls, insulin resistance was not observed. Daily physical activity prevented the development of obesity and had positive effects on metabolic parameters in IUGR offspring.67 In a different study, physical activity also affected leptin content of the adipose tissue in IUGR rats. Offspring from low-protein diet fed dams had increased leptin in the visceral tissue but this was reversed by exercise.68 Swimming in IUGR rats also prevented glucose intolerance from developing as well as reducing adiposity in another sample.69 Adult IUGR female rats exercised continuously for 60min during pregnancy also had enhanced peripheral insulin sensitivity although in this sample this was associated with IUGR in the offspring.70
Other interesting effects of physical activity in IUGR rats concern the proportion of muscle fibers,71 improvements in Angiotensin II-induced vasoconstriction, alterations related to superoxide levels72 and improvements in endothelium-derived hyperpolarization-mediated vasodilation in muscle arteries.73 In contrast, study by Maux et al. found that moderate physical training did not modify structural changes associated with malnutrition on the common left carotid and horizontal aorta arteries wall.74 Using a different methodology with bilateral uterine vessel ligation or sham surgery in pregnant dams to induce IUGR, Laker et al. did not find any reprogramming effects related to exercise in skeletal muscle markers of mitochondrial biogenesis in adulthood.75
Therefore, from the different experimental studies, it is possible to infer that there is evidence to support the existence of a fetal programming effect on physical activity/sedentary behavior later in life. In addition, exposure to exercise during the life course seems to be able to revert some of the detrimental effects of being born IUGR, especially regarding metabolic outcomes, although less consistent reversion is seen on the damages found on muscle architecture or function.
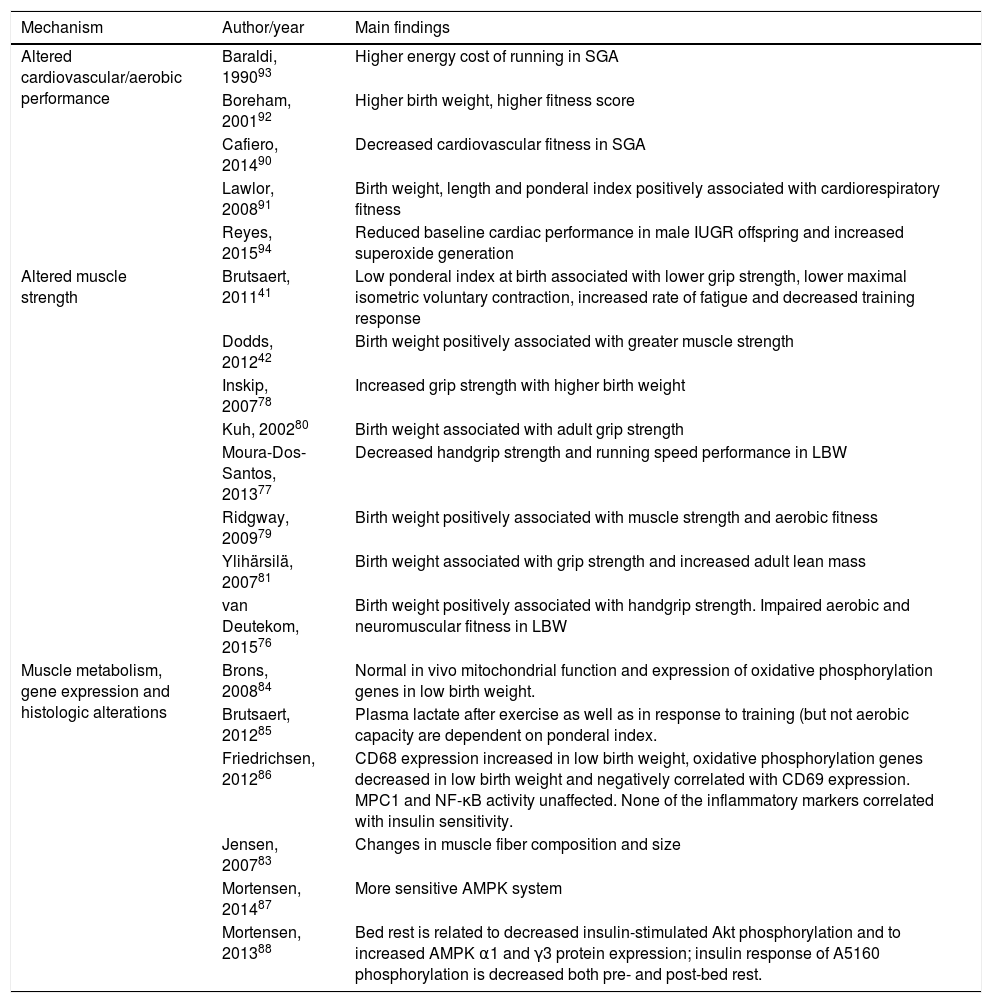
MechanismsSome of the possible mechanisms implied in the above-mentioned alterations in physical activity levels in IUGR were already described among the experimental results (Table 3). There are other articles focusing on these mechanisms in clinical studies that will be described here (Table 4).
Proposed mechanisms implied in the association between IUGR and lower physical activity levels.
| Mechanism | Author/year | Main findings |
|---|---|---|
| Altered cardiovascular/aerobic performance | Baraldi, 199093 | Higher energy cost of running in SGA |
| Boreham, 200192 | Higher birth weight, higher fitness score | |
| Cafiero, 201490 | Decreased cardiovascular fitness in SGA | |
| Lawlor, 200891 | Birth weight, length and ponderal index positively associated with cardiorespiratory fitness | |
| Reyes, 201594 | Reduced baseline cardiac performance in male IUGR offspring and increased superoxide generation | |
| Altered muscle strength | Brutsaert, 201141 | Low ponderal index at birth associated with lower grip strength, lower maximal isometric voluntary contraction, increased rate of fatigue and decreased training response |
| Dodds, 201242 | Birth weight positively associated with greater muscle strength | |
| Inskip, 200778 | Increased grip strength with higher birth weight | |
| Kuh, 200280 | Birth weight associated with adult grip strength | |
| Moura-Dos-Santos, 201377 | Decreased handgrip strength and running speed performance in LBW | |
| Ridgway, 200979 | Birth weight positively associated with muscle strength and aerobic fitness | |
| Ylihärsilä, 200781 | Birth weight associated with grip strength and increased adult lean mass | |
| van Deutekom, 201576 | Birth weight positively associated with handgrip strength. Impaired aerobic and neuromuscular fitness in LBW | |
| Muscle metabolism, gene expression and histologic alterations | Brons, 200884 | Normal in vivo mitochondrial function and expression of oxidative phosphorylation genes in low birth weight. |
| Brutsaert, 201285 | Plasma lactate after exercise as well as in response to training (but not aerobic capacity are dependent on ponderal index. | |
| Friedrichsen, 201286 | CD68 expression increased in low birth weight, oxidative phosphorylation genes decreased in low birth weight and negatively correlated with CD69 expression. MPC1 and NF-κB activity unaffected. None of the inflammatory markers correlated with insulin sensitivity. | |
| Jensen, 200783 | Changes in muscle fiber composition and size | |
| Mortensen, 201487 | More sensitive AMPK system | |
| Mortensen, 201388 | Bed rest is related to decreased insulin-stimulated Akt phosphorylation and to increased AMPK α1 and γ3 protein expression; insulin response of A5160 phosphorylation is decreased both pre- and post-bed rest. |
IUGR, intrauterine growth restriction; SGA, small for gestational age; LBW, low birth weight; MPC1, monocyte chemotactic protein-1; NF-κB, nuclear factor-κB; AMPK, adenosine monophosphate-activated protein kinase.
The most commonly studied mechanism involves changes in muscle strength. As previously stated, poorer muscle strength is associated with less physical activity levels. The positive association between birth weight and handgrip strength has been described in different age groups: in childhood,76,77 young adults,41 adults and elderly.78–81 Low muscle strength has already been associated with metabolic risk factors such as blood pressure, HOMA-IR and triglycerides in school-aged children.82 Dodds et al. conducted a systematic review and meta-analysis and found 19 studies confirming this association, which is maintained across the life course.42 Each kilogram increase in birth weight resulted in a 2.07kg and 1.59kg increase in muscle strength in men and women respectively.
One study concluded that poor fetal growth affects muscle force generation, based on the findings of lower grip strength, lower maximal isometric voluntary contraction of the quadriceps femurs, increased rates of fatigue pre- and post-training and decreased training response in those born with low ponderal index.41 Jensen et al. investigated changes in skeletal muscle morphology and showed an altered proportion of different muscle fibers in young IUGR adults. It was hypothesized that increased fiber size may be an early marker of insulin resistance in this population.83 Furthermore, Brons et al. evaluated the effects of LBW on mitochondrial dysfunction in skeletal muscle. The study confirmed previous findings of abnormal glucose metabolism in LBW individuals. However, there was no indication that mitochondrial dysfunction could be the key to explaining the metabolic defects underlying insulin resistance.84
Other articles found differences in lactate muscle metabolism,85 CD68 mRNA expression suggesting macrophage infiltration and reduced oxidative phosphorylation (OXPHOS) gene expression when exposed to bed rest,86 as well as skeletal muscle insulin signaling.87 Mortensen et al. suggested that LBW could have a more sensitive AMPK (AMP-activated protein kinase) system, supported by the evidence of an increased exercise-induced AMPK activation as well as increased effects on the downstream target ACC2 (acetyl coenzyme A carboxylase 2) in LBW subjects.88 Additionally, Ylihärsilä et al. concluded that LBW is associated with lower lean mass in adult life and this contributes to the risk of relative sarcopenia and the related functional inability later in life.81
In another study, prenatal undernutrition caused a reduction in muscle fiber size. Exercise did not affect fiber type composition. The activity of enzymatic markers of oxidative and glycolytic pathways was significantly different in those that were born IUGR. Overall, physical activity prevented obesity in IUGR offspring.89
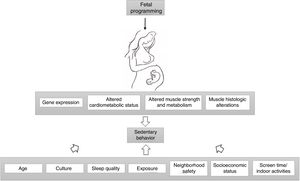
Finally, some authors described a decreased cardiorespiratory/aerobic capacity in those born with low birth weight.79,90–92 Van Deutekom et al. results indicate that LBW and accelerated infant growth might negatively affect childhood aerobic and neuromuscular fitness.76 Others reported that the energy cost of running was higher among children born preterm SGA when compared to term controls, with no differences between preterm AGA and term controls.93 Running speed performance was also diminished in another sample of LBW children.77 Experimental data showed reduced cardiac performance in male IUGR offspring as well as increased superoxide generation.94Fig. 1 delineates the factors affecting physical activity levels related to IUGR.
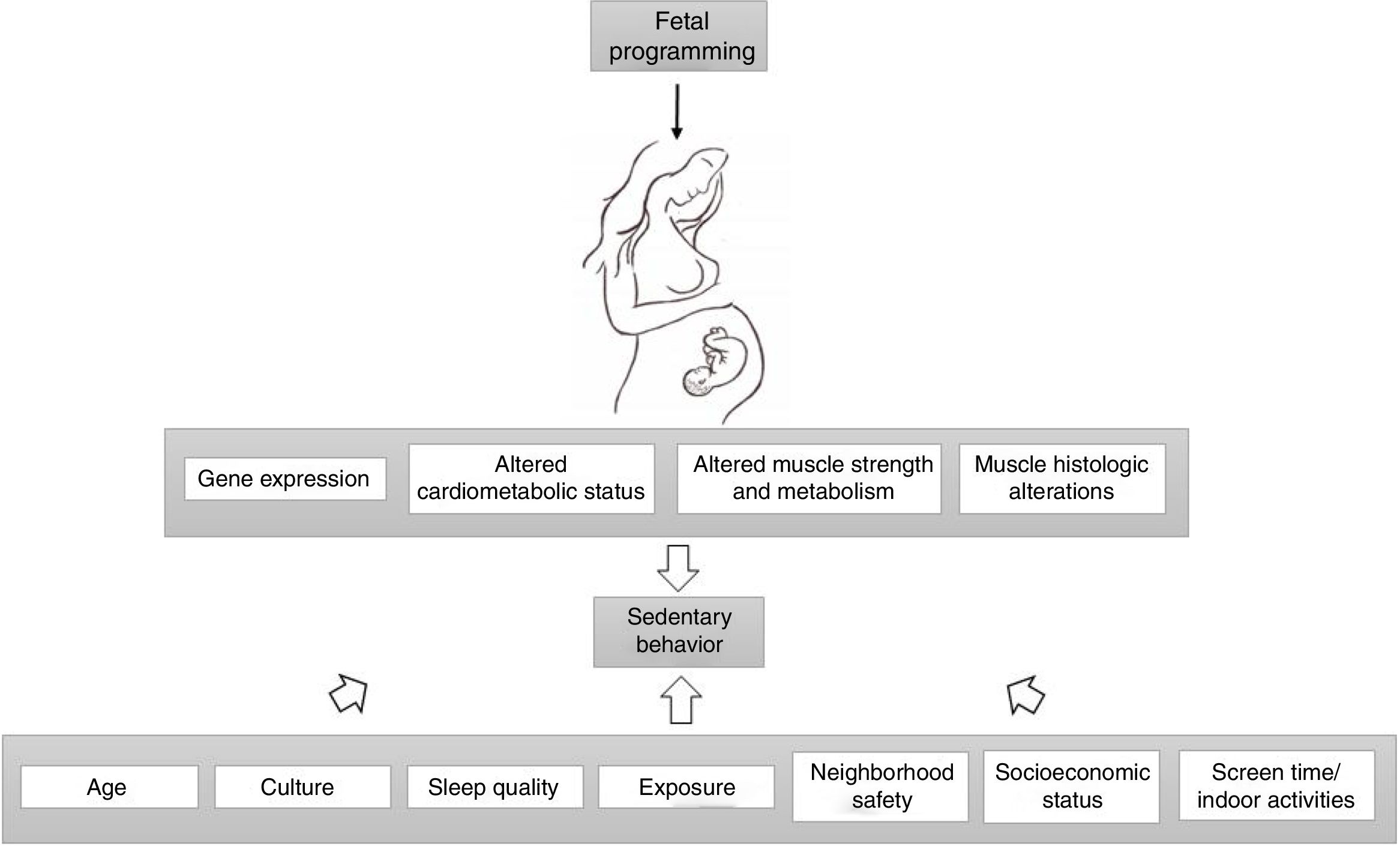
Fetal programming alters gene expression, cardiometabolic status, muscle strength, metabolism and histology, leading to altered physical activity levels in individuals with IUGR. Age, culture, exposure, sleep quality, socioeconomic status, neighborhood safety, screen time and indoor activities also moderate the association between birth weight and sedentary behavior.
As Gatford et al. have previously pointed out, there is some evidence linking IUGR to altered physical activity levels as well as describing potential beneficial outcomes of exercise intervention on metabolic outcomes, however few studies have been conducted and results are mixed.55 Most clinical studies have focused on cardiovascular performance and muscle alterations that could help explain the decreased physical activity levels in this population. Experimental studies, on the other hand, have gone somewhat further, exploring changes in insulin sensitivity, leptin secretion, vascular and muscle structure and function. Still, little is known about the neurobiological mechanisms that may influence sedentary behavior.
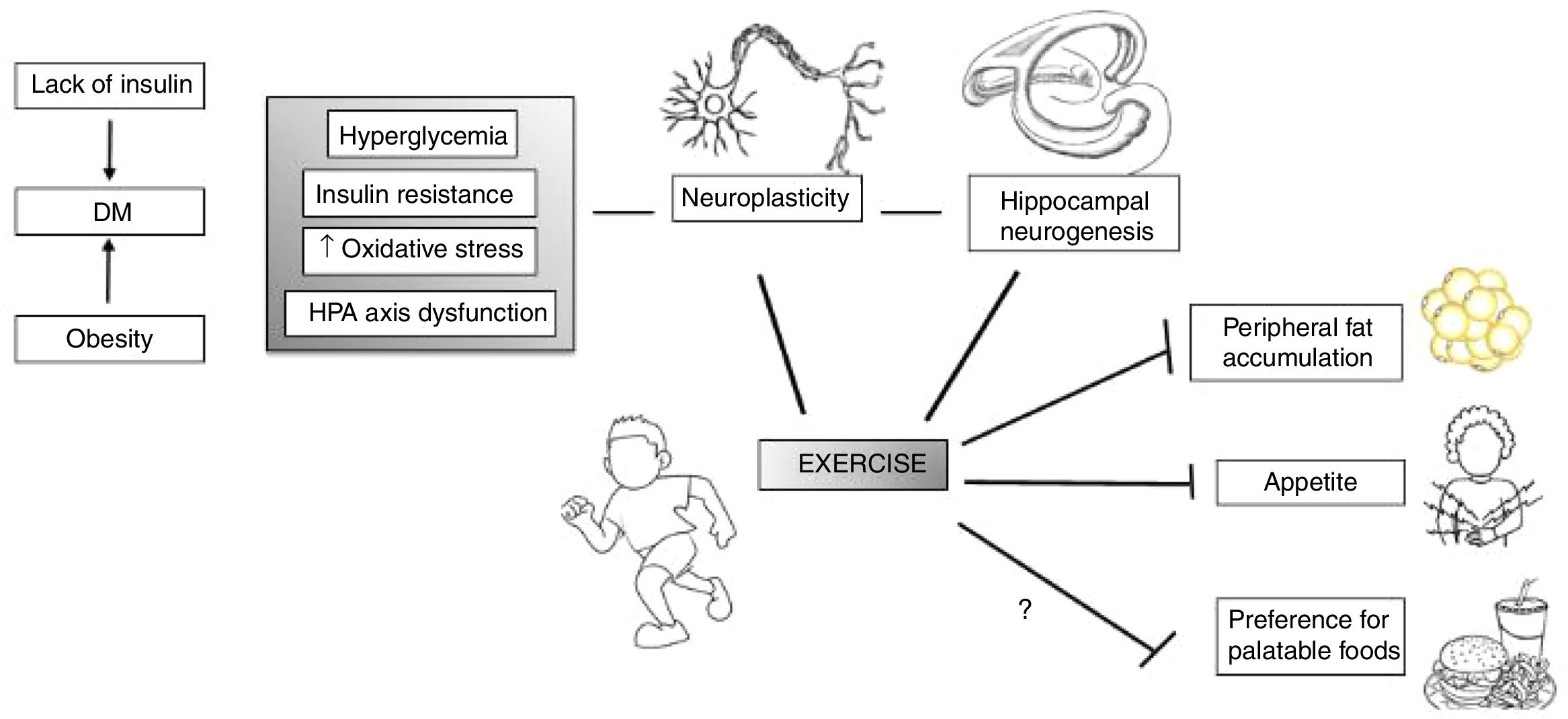
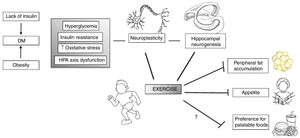
Exercise benefits a whole range of human conditions, not only related to physical health, but also mental health. Physical activity, either aerobic, resistance or coordination training, benefits cognitive function in the elderly.95 It also increases brain connectivity between the frontal, posterior, and temporal cortices, affects hippocampal volume and serum levels of brain-derived neurotrophic factor (BDNF), a mediator of neurogenesis in the dentate gyrus of the hippocampus95,96 (Fig. 2).
Exercise may act in specific vulnerabilities that IUGR individuals have, such as their increased risk for type II diabetes and adiposity, as well as hypothalamus–pituitary–axis (HPA) dysfunction. Moreover, exercise acts in several brain areas and processes, such as increasing neurogenesis and neuroplasticity and therefore influences behavior. Adapted and modified from Yi.96
From a neurobiological standpoint, exercise influences cortisol, endocannabinoids, BDNF, dopamine and serotonin releases. The stress response associated with exercise induces inhibitory effects from the secreted cortisol upon the hypothalamus and pituitary through medial prefrontal cortex receptors and reduces stress-induced overexcitability of the amygdala. Additionally, by reducing the amount of competitive amino acids through muscle uptake, aerobic exercise increases tryptophan's chances of crossing the blood–brain barrier, and so has the potential to increase serotonin, an important neurotransmitter for emotional processing, satiety and memory functions.97 These are some of the potential targets of future research, especially exploring the neurobiological effects of exercise in vulnerable populations such as those born IUGR. Therefore, further research would benefit from targeting IUGR individuals for structured interventions in well-designed, large-scale longitudinal studies, to investigate the potential benefits of physical training. In addition, basic neurobiological investigations detailing the effects of exercise specifically in this group could unravel mechanisms that would better tailor such interventions.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Bischoff AR, Cunha FS, Molle RD, Maróstica PJ, Silveira PP. Is willingness to exercise programmed in utero? Reviewing sedentary behavior and the benefits of physical activity in intrauterine growth restricted individuals. J Pediatr (Rio J). 2018;94:582–95.