To identify clinical and epidemiological characteristics of children evaluated by the pediatric aerodigestive program at the beginning of its activity, describe challenges in follow-up, and suggest mitigation strategies.
MethodsA case series was conducted describing the first 25 patients discussed by the aerodigestive team from a Brazilian quaternary public university hospital between April 2019 and October 2020. The median follow-up was 37 months.
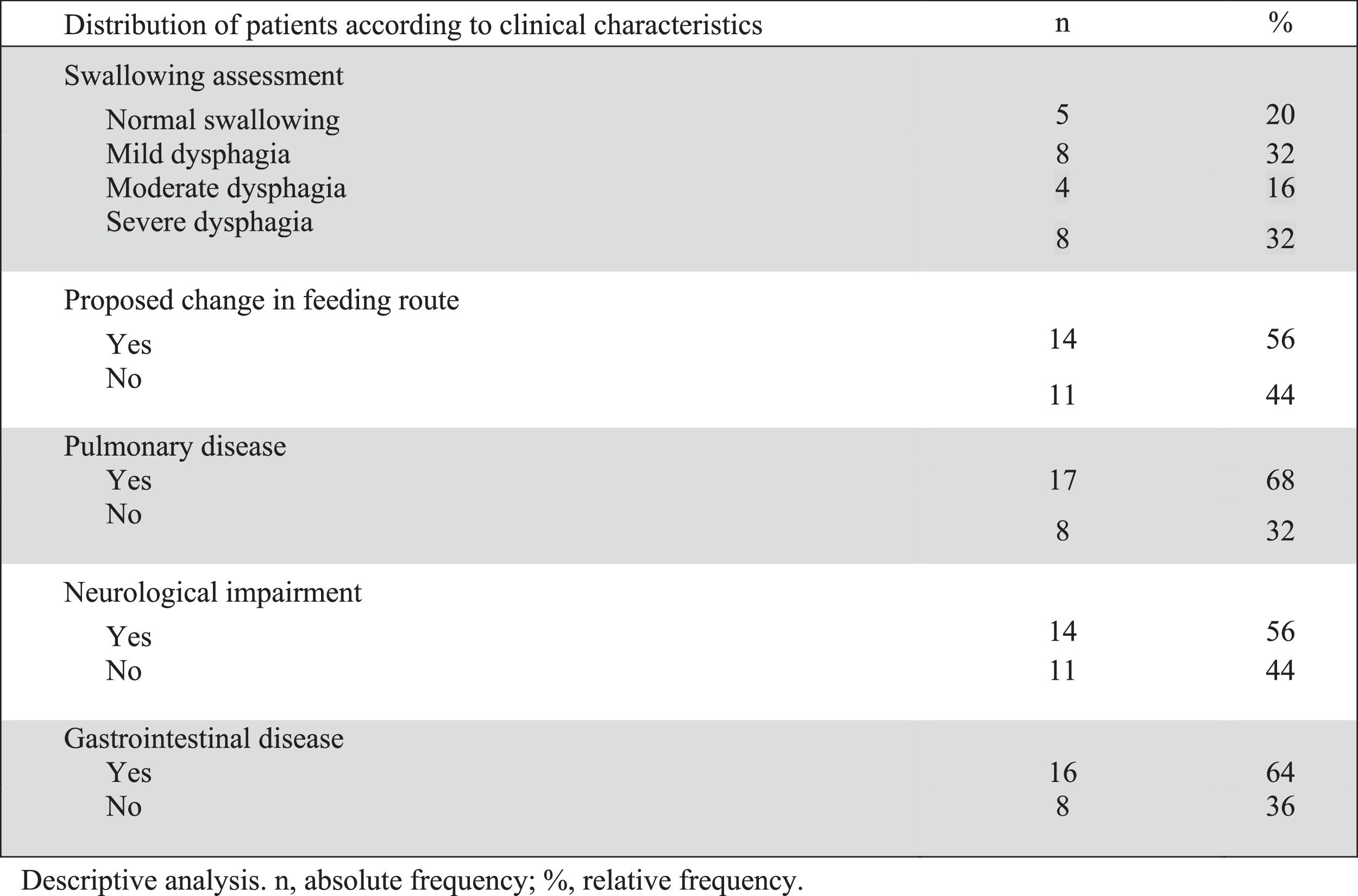
ResultsDuring the study period 25 children were seen by the group and the median age at first assessment was 45.7 months old. Eight children had a primary airway abnormality, five had a tracheostomy. Nine children had genetic disorders and one had esophageal atresia. Dysphagia was present in 80% of the patients, 68% had a history of chronic or recurrent lung disease, 64% had a gastroenterological diagnosis and 56% had neurological impairment. Moderate to severe dysphagia was identified in 12 children and 7 of these had an exclusive oral diet at the time. The majority of children (72%) had 3 or more comorbidities. Following team discussion, a change in feeding strategy was suggested in 56% of the children. The most frequently ordered exam was pHmetry (44%) and gastrostomy was the surgical procedure with the longest waiting list.
ConclusionsDysphagia was the most frequent issue encountered in this initial group of aerodigestive patients. Pediatricians caring for these children must be involved in aerodigestive team discussions and hospital policies must be revised to facilitate access to exams and procedures needed for this population.
Caring for children with chronic diseases is a reality due to advances in pediatric and neonatal intensive care. In the United States pediatric aerodigestive programs have been developed to meet the necessities of children who demand evaluation by multiple specialties since 1999.1,2 The main goals of these programs are: to reduce the need for repeated visits, improve communication among specialists and families, rationalize invasive or multiple exams, and reduce hospitalizations and diagnosis time.1,3,4
In 2018, Boesch et al. provided a consensus on pediatric aerodigestive programs.5 They described the program's functions and structure and defined the aerodigestive patient as “a child with a combination of multiple and interrelated congenital and/or acquired conditions affecting airway, breathing, feeding, swallowing, or growth that require a coordinated interdisciplinary diagnostic and therapeutic approach to achieve optimal outcomes. This includes but is not limited to, structural and functional airway and upper gastrointestinal tract disease, lung disease because of congenital or developmental abnormality or injury, swallowing dysfunction, feeding problems, genetic diseases, and neurodevelopmental disability”.5 Feeding and swallowing difficulties are an important and frequent issue in this group of children and may be related both to congenital and acquired conditions. Assessment of aspiration and a safe and effective feeding route is essential.1,6 Simultaneous endoscopy of the airway and digestive tracts known as “triple endoscopy” can reduce the number of anesthesia and facilitate the specialists' live discussion.1,5,7
There are currently in the United States of America (USA) around 50 aerodigestive programs, however, there are no published studies of pediatric aerodigestive teams in South America. In Brazil, building an aerodigestive team is particularly challenging due to limited public health financing and the fact that 70% of the population relies on the public health system. Pediatric specialists are rarely at the same healthcare center, making communication difficult. Highly complex patients are inevitably followed in tertiary and quaternary university hospitals, and non-medical therapies, on the other hand, are carried out locally in primary or secondary healthcare centers. Additionally, the availability of non-medical evaluations and therapies varies greatly from one city to another both in the tertiary and basic healthcare services.
In April 2019, the Pediatric Otorhinolaryngology, Gastroenterology, and Pneumology teams from a quaternary public university hospital started a multidisciplinary pediatric aerodigestive team with monthly reunions. The group anticipated that following the American model under local restrictions on resources would be difficult and, an overview of these patients would help understand how the establishment of this program could be prioritized and tailored to local needs.
This study aims to identify clinical and epidemiological characteristics of the first children evaluated by the pediatric aerodigestive team in a Brazilian quaternary public hospital and ultimately describe challenges in follow-up and suggest mitigation strategies to adapt multidisciplinary programs to local needs and regional populations.
MethodsA case series study of all patients evaluated by the aerodigestive team at the beginning of its activity was carried out after approval from the institutional Ethics Committee (79823017.8.000.5404). The team consisted of attending physicians and residents from the pediatric Otorhinolaryngology, Pneumology, and Gastroenterology departments, in addition to a speech-language pathologist (SLP) specialized in pediatric swallowing dysfunction. Monthly meetings were set, and 2 or 3 patients were assigned to be discussed in each session.
Patients were selected by any of the specialties that were attending these patients either in their outpatient clinic or by demand of the pediatric ward. Criteria for selection followed the consensus definition.5 Retrospective data was collected from all patients evaluated by the multidisciplinary team from April 2019 to October 2020 and follow-up was updated up to January 2023.
All patients were submitted to the clinical and instrumental evaluation of dysphagia through a fiberoptic endoscopic evaluation of swallowing (FEES) by the otolaryngologist and SLP prior to the meeting. A 3.2mm Machida flexible fiberoptic endoscope was used. An anatomical evaluation of nasal cavities, pharynx and larynx was performed, as well as a functional assessment of vocal cord mobility, integrity of laryngopharyngeal sensation, secretion management, swallow frequency and presence of laryngeal penetration and/or aspiration. For children with an oral diet, saliva and food in different stained consistencies were tested. For children with suspected saliva aspiration, a modified FESS was performed using saliva stained with blue food coloring. It was also possible to evaluate breastfeeding children. Dysphagia was classified in grades according to the Pediatric Dysphagia Assessment Protocol (PAD-PED).8 Patients were classified as normal swallowing, mild, moderate, or severe dysphagia.8 According to this classification, moderate and severe dysphagia implies impairment of nutrition and/or hydration, and severe dysphagia indicates a high risk of aspiration, which contraindicates oral feeding.
Patient demographic and clinical data were extracted. The authors also retrieved the length of time taken to perform exams and procedures from medical charts, which were updated to January 2023.
Data were analyzed descriptively and inferentially using the Statistical Package for the Social Sciences (SPSS 25.0 software). For all analyses, a p-value < 0.05 was considered indicative of statistical significance. For qualitative variables, absolute and relative frequencies were calculated. For quantitative variables, central tendency and position measures were determined. The chi-square test was used for the inferential analysis of qualitative variables, while Mann-Whitney and Kruskal-Wallis tests were used for comparing qualitative and quantitative variables between two and multiple independent groups, respectively.
ResultsTwenty-five cases were evaluated by the aerodigestive team between April 2019 and October 2020. There were 13 males and 12 females aged from 1 to 207 months old. The median age was 29 months old (IQR 6.5-63). Seventeen patients were seen in 2019 and eight were seen in 2020. There was a significant difference in the age of patients assessed from one year to the other: the median age of 40 months in 2019 and 6.5 months in 2020 (p = 0.001).
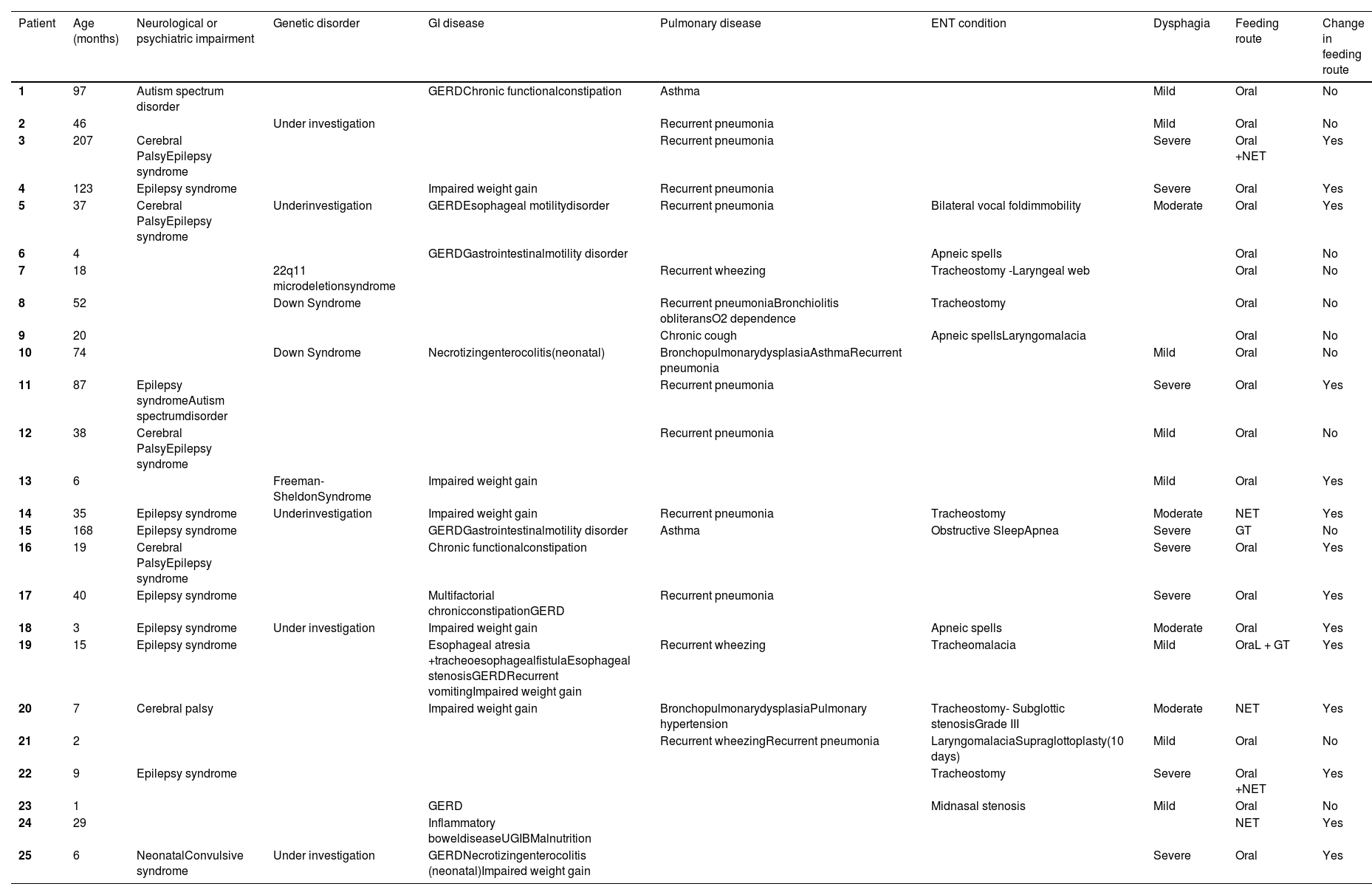
Table 1 describes the profile of the patients and Figure 1 summarizes the clinical characteristics of patients.
Characteristics of patients evaluated: age, diagnosis, feeding route and proposition of change in feeding route after group evaluation.
GI, gastrointestinal; GERD, Gastroesophageal reflux disease; UGIB, Upper Gastrointestinal Bleeding; NET, Nasoenteric tube; GT, Gastrostomy tube.
Clinical and instrumental evaluation of swallowing with FEES showed dysphagia in 20 of the 25 patients (80%). Of the twenty patients with dysphagia, 12 had moderate or severe dysphagia and 75% (9/12) of them were being fed orally (seven exclusively). The median age of children with moderate dysphagia was 21 months old and 63.5 months old for those with severe dysphagia. There was no difference in age comparing children with grades of dysphagia (p = 0.339).
Over half of the patients (14 children) underwent a change in feeding route after group evaluation. Oral feeding was contraindicated in 10, one needed gastrostomy due to impaired weight gain secondary to craniofacial malformation and limited oral intake (patient 13), two (patients 14 and 20) needed gastrostomy due to moderate dysphagia and prolonged time of nasoenteric tube (NET), and one that was using NET progressed to oral feeding (patient 24 on Table 1). Of the 10 patients that were considered unsafe to feed orally, 9 had moderate or severe dysphagia and one had severe esophageal stenosis (patient 19). Patients with an indication for gastrostomy used NET until the surgery was performed.
The neurological disease was present in over half of the patients (14). The median age was 36 and 20 months old for neurologically and not neurologically impaired children, respectively, with no statistical difference (p = 0.366). All patients with moderate or severe dysphagia had a neurological disorder and only two patients with a neurological condition had mild dysphagia (patients 12 and 19 on Table 1).
There was a high prevalence of comorbidities. Eighteen (72%) had three or more comorbidities, and all but one child had at least two comorbidities. The majority of patients (68%) had a previous history of chronic and/or recurrent lung disease, while 64% had gastrointestinal (GI) disease. Four patients had a confirmed genetic disorder and another five were undergoing investigation.
There were eight patients with primary upper airway disease: one with laryngeal web (a tracheostomized child), two with laryngomalacia (one already submitted to supraglottoplasty), one with obstructive sleep apnea, one with bilateral vocal cord paralysis, one with tracheomalacia, one with grade III subglottic stenosis (tracheostomized child) and one with congenital midnasal stenosis. Five children had tracheostomies, and another was submitted to tracheostomy for bilateral vocal cord paralysis after team evaluation.
The mean follow-up time was 37 months (standard deviation 12.72). Two patients with gastrostomy indication lost follow-up in less than 3 months due to missing appointments and did not return when summoned (patients 4 and 11). Surgical procedures performed were one tracheostomy, two microlaryngoscopy and bronchoscopy (MLB), five GI endoscopies, seven gastrostomies, and one laryngotracheal reconstruction with costal cartilage graft for laryngeal web repair.
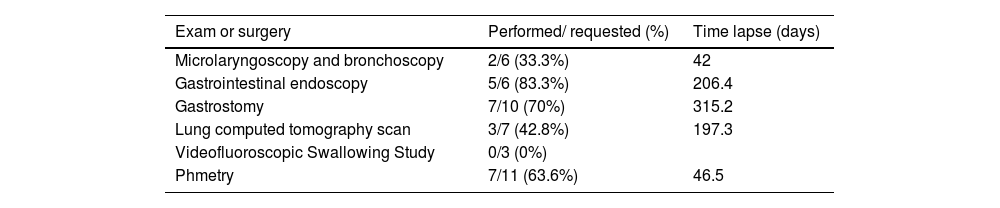
Table 2 shows the exams and surgeries requested and how long it took for them to be performed. Gastrostomy was the procedure that had the longest waiting time and videofluoroscopic swallowing study (VFSS) had the lowest performance rate. Microlaryngoscopy and bronchoscopy (MLB) and gastrostomy or GI endoscopy were ordered in four patients. Only one of these had a triple endoscopy.
Number of exams and surgeries requested, percentage of those performed and time lapse to perform them.
The mean time to perform surgical procedures was 296.9 days for patients evaluated in 2019 and 88.5 for patients in 2020. Regarding the exams, the time was 101 and 9 days, respectively.
During follow-up, some procedures were revised and called off: 2 MLB (patients 1 and 9) and 1 GI endoscopy for patient 21. Phmetry was called off in patient 18 after gastrostomy and was attempted but unsuccessful in the patient with Freeman-Sheldon syndrome.
DiscussionAerodigestive programs with interdisciplinary care models are effective for diagnosis optimization and cost reduction in international studies.1,3,9-11 However, there are no reports on pediatric aerodigestive programs in South America, even in advanced tertiary and quaternary centers. The main strength of this study is being the first one to evaluate a pediatric aerodigestive program in Brazil and describe the profile of patients and difficulties faced.
In this case series, FESS diagnosed dysphagia in most patients (80%). Dysphagia prevalence can vary in aerodigestive clinics, depending on patient profiles and program characteristics. Gendler et al., reported dysphagia in 62% and aspiration in 42% of patients, using FESS to assess swallowing. Their sample had more esophageal atresia and tracheoesophageal fistula cases (56%) and fewer patients with neurological disease (36%).12 Due to a scarcity of SLPs specialized in pediatric dysphagia in Brazil, even patients with alternative feeding arrived without assessment or rehabilitation. So, all underwent swallowing evaluation before discussion and this may have contributed to the high dysphagia prevalence in our study. Dysphagia assessment is crucial for patients with associated comorbidities, and low prevalence reports may reflect inadequate investigation. Fuladi et al. reported dysphagia only in 27% of children but otorhinolaryngologists and SLPs were not part of their team nor were patients assessed instrumentally. Most of their patients had esophageal atresia with tracheoesophageal fistula (82%) and there were no reports of children with neurological disease in the sample.13
Airway problems were present in one-third of the patients, similar to Rotsides et al. (30%)4 and Gendler et al. (28%).12 Aerodigestive teams are frequently built up by airway surgeons seeking better pre-operative control of inflammatory factors that may compromise surgical results. At the hospital, airway patients have a dedicated and highly active outpatient clinic. The agility needed to prepare patients for surgery combined with the lack for slots in the aerodigestive clinic, may have led to occasional consultations by pneumology or gastroenterology in their specific outpatient clinics and not in the aerodigestive one.
Dysphagia is a potential risk for chronic pulmonary aspiration and respiratory issues in children.14,15 In this series, 68% had chronic lung disease and/or recurrent pneumonia. Arslan et al. conducted a VFSS study of 274 children with dysphagia. Most of the patients had neuromuscular or neurological disorders (86.8%) and 67.9% had a history of recurrent pneumonia in a 1 year period. Recurrent pneumonia was positively correlated with laryngeal penetration and aspiration.16 Hirsch et al. reported higher morbidity and mortality in children hospitalized with aspiration pneumonia than those with community-acquired pneumonia. The group with aspiration pneumonia was more likely to have associated chronic conditions (including dysphagia and neurological disease as risk factors), had longer and more expensive hospitalizations (mean cost was 2.4 times higher), higher ICU admission, and 30-day readmission rates.17
Managing chronic aspiration in children requires considering the etiology, comorbidities, aspirated material characteristics (including aspiration of refluxed material), airway clearance capacity, and established pulmonary sequelae.18 Based on these factors, a more conservative or aggressive approach may be taken. One of the main goals in managing aerodigestive patients is to provide a safe and efficient feeding route and this justifies the reason for changes in cases of moderate and severe dysphagia,1,6 whether with consistency adjustments or oral intake restriction with gastrostomy indication. It is recommended to implement these changes in conjunction with rehabilitative therapy and regular reassessments by the team. Despite the apparent advantage of performing exams and procedures quickly to define strategic therapeutic measures as has been describing by Boesch et al.,9 initial evaluation and rehabilitative therapy may help tailor the need for specific exams on a case-to-case basis.
Aerodigestive patients present multiple comorbidities and can present a high prevalence of neurological impairment as showed in present study group (56%) and is also reported by Kim et al in 85% of patients.19 Neurologically impaired children should undergo both clinical and instrumental dysphagia assessments since clinical swallowing assessments are not sensitive enough to diagnose aspiration consistently, especially in high-risk populations.20
Instrumental swallowing tests (FESS and VFSS) are essential to assess oral feeding safety and document aspiration disease.1,21 FESS has the advantage of not submitting the patient to radiation meaning it can easily be repeated and evaluates both laryngopharyngeal anatomy and function.22 FESS is widely available in the present institution and was the chosen method. VFSS was demanded in some cases after FESS and the team chose the ideal moment for it considering that the patient would be submitted to radiation. VFSS is unavailable in most public hospitals in the region and has a high cost for the family if performed in private clinics, which justifies its low-performance rate found in the study.
Since VFSS was already an established method when FEES was introduced, the two procedures are frequently compared23 although most of them are reported adults with good correlation. In children, there are very few studies. High agreement has been reported for spillage, residue, penetration, and aspiration.24,25 In a study of bottle-fed infants in the NICU, FEES detected more instances of penetration than VFSS and agreement was high for aspiration (92%).26 According to Pavithran et al., FEES has shown a high specificity of 82% in detecting aspiration. However, if a FEES result indicates no aspiration, it should be interpreted within the context of aspiration risk and other endoscopic factors, especially if VFSS is not feasible.27
Long waiting periods for exams and procedures were observed in this patient group due to the national health system's overload. In this scenario, hospital admissions for acute illnesses may provide an opportunity to identify patients at risk of aspiration and malnutrition and perform necessary exams and procedures. One cannot underestimate the value of an appropriate routine to identify patients admitted for recurrent acute airway episodes, apneic spells, and/or issues related to feeding and swallowing. This was observed in the current study: with the reduction of the outpatient clinics seen in 2020 during the Covid pandemic, younger patients were evaluated from the pediatric ward (6.5 months old compared to 40 months in 2019).
Procedures that depend on the availability of operating room hours and hospital beds for elective hospitalization, such as gastrostomy and MLB, were another obstacle and goes against what is advocated in the consensus of pediatric aerodigestive programs.5 This center is a general public university quaternary hospital, attending 86 cities and a population of approximately 6 million people). Critically ill patients are transferred from low complexity centers continuously and tend to “compete” with elective outpatients for the available hours in surgical schedules.
Although aerodigestive centers in the USA constantly speak of the importance of “triple endoscopies”,7 this is a very difficult practice in the hospital. Since there are no designated time slots specifically for the aerodigestive team for procedures under general anesthesia and different specialties work on different days in the hospital. On the other hand, triple endoscopies may not be as extensively indicated in the aerodigestive patient as suggested by the American consensus and specific criteria may need to be defined to reach a more palpable model for developing countries.
The study has several limitations, including the absence of instruments to assess the impact on quality of life and the long-term clinical and economic benefits of this model in the studied country. This poses a significant challenge for aerodigestive groups worldwide. The interruption of care during the covid-19 pandemic may have introduced considerable bias as patients seen in 2020 underwent procedures more quickly, taking advantage of hospitalization and larger availability of surgical slots since elective surgeries were suspended. Despite the small sample, the findings may guide groups outside the US with similar challenges to guarantee the best care for complex children.
Since 2019 some institutional advances have occurred and currently, an aerodigestive clinic has been set up, where children receive in-person care from a multidisciplinary team who collaborate in real-time. The clinic remains to be recognized by the present institution as a “unit” per se with the designated time slots in the operating theatre and for specific exams. Continuous research is needed to determine the long-term effects of this model, although the improvement in caregivers' feedback regarding the team's work is very noticeable.
Strategies to improve the quality of care to these patients include institutional recognition of the aerodigestive program with scheduled time slots dedicated to these patients in the operating theatre and designated funding to ensure quotas for imaging. Hiring a coordinator can optimize communication with the family and patient flow, while telemedicine can facilitate systematic feedback from the aerodigestive team to primary and secondary healthcare units responsible for rehabilitation therapies. Ultimately, it is imperative that pediatricians attending the pediatric ward and outpatient clinics appreciate the role of the aerodigestive team and identify patients that fill the criteria for referral, particularly those with suspected aspiration.
The current case series found dysphagia to be the most common disorder in this initial aerodigestive group confirming the need for systematic instrumental assessment of swallowing in aerodigestive patients. Recognition of the role of the aerodigestive team and the identification of patients who meet the criteria for referral, especially those with suspected aspiration, are imperative.