To investigate the association between socioeconomic and nutritional factors with respiratory morbidity in the first year of life in different regions of Brazil.
MethodologyA nested case-control study within a randomized field trial was conducted in three capital cities (Porto Alegre, Manaus, and Salvador), representing different macro-regions of the country. Cases were defined as children with a reported previous diagnosis of asthma, bronchiolitis, or pneumonia. Corresponding controls were matched by age and sex in a 2:1 ratio, selected consecutively from the original cohort, resulting in a sample of 222 children. Bivariate analyses were performed to assess the association between sociodemographic and nutritional variables with respiratory morbidity outcomes, calculating odds ratios (OR) and their respective confidence intervals (95% CI). Values of p < 0.05 were considered significant. Potential confounding factors were adjusted through multivariate analysis (logistic regression).
ResultsMaternal smoking and breastfeeding for less than six months showed a significant association and increased risk of respiratory disease (OR=2.12 and 2.05, respectively). Children born in the Southern region of Brazil also demonstrated a higher association and risk of respiratory morbidity. The consumption of ultra-processed foods did not show a significant association or increased risk of respiratory disease.
ConclusionsMaternal smoking, breastfeeding for less than six months, and being born in the Southern region of Brazil are risk factors for the development of respiratory morbidity in the first year of life. The consumption of ultra-processed foods does not appear to pose a risk, but it was prevalent in more than 80% of the population, limiting its discriminatory power of analysis.
In Brazil, data from the National Information System indicate that, in 2017, 46% of hospitalizations in the first year of life were due to perinatal conditions, with 24% due to respiratory diseases.1 Many factors can synergistically contribute to the development of these conditions, among which socioeconomic and demographic factors, environmental factors, type of feeding, and nutritional status can be highlighted.2
Risk factors for hospitalization due to respiratory diseases in childhood include exposure to environmental pollutants (especially smoking), household overcrowding, nutritional deficits, climatic seasonality, incomplete immunization schedules, low socioeconomic status, and exposure to biological agents.3,4
The importance of changing the social determinants of health, such as reducing poverty, improving access to healthcare, vertical health programs against infectious diseases, and promoting optimal breastfeeding practices, has already been studied.5 Inadequate complementary feeding practices, such as the early introduction of foods, limited food diversity, inadequate frequency and consistency of foods, and frequent consumption of unhealthy foods, have become highly prevalent in early childhood, leading to inappropriate dietary patterns, including the early introduction of industrialized and ultra-processed foods into the children's diet.6,7
The type of food offered to children and the duration of breastfeeding is influenced by various socioeconomic factors, directly determining the infant's health and nutritional status.8
Ultra-processed foods are ready-to-consume industrial formulations that are entirely or mostly made from extracted substances of foods (oils, fats, sugar, proteins), derived from food constituents (hydrogenated fats, modified starch), or synthesized in the laboratory based on organic materials, such as colorants, flavorings, taste enhancers, and other additives used to alter sensory properties.9 They are nutritionally unbalanced, characterized by high energy density, high amounts of fat, sugar, and/or sodium, and low dietary fiber content.10 The chemical additives present in these foods are used by the industry to enhance their sensory characteristics, making them more attractive and desirable, especially to children. The infant population is more vulnerable to these additives because these substances may not be metabolized and excreted correctly due to physiological immaturity. In other words, these chemical additives can be toxic if they are not used within their safety limits, posing risks to individuals. Therefore, greater attention needs to be given to infant health and nutrition because these ultra-processed foods, which contain these food additives, can have harmful effects on health.11
In Brazil, non-communicable chronic diseases (NCDs) are considered a pandemic, and in the child population, obesity is associated with the early introduction of ultra-processed foods and early weaning from breastfeeding. The consequences of introducing obesogenic diets in the early years of life have long-term negative effects on the health of these children, predisposing them to chronic diseases in adulthood as well.12
According to the recommendations of the Ministry of Health, infants should receive exclusive breastfeeding until six months of age, and breastfeeding should be continued for up to two years or more, along with the gradual and daily introduction of complementary foods starting at six months of age. These complementary foods should be based on fresh foods obtained directly from plants and animals, such as fruits, vegetables, eggs, meats, tubers, grains, and cereals. The consumption of ultra-processed foods such as soft drinks, industrialized juices, snacks, processed meats, and sweets should be avoided until at least two years of age, as they may be associated with the development of diseases in childhood.13
Thus, the objective of this study was to investigate the association between socioeconomic and nutritional factors and the development of respiratory morbidity in children in the first year of life.
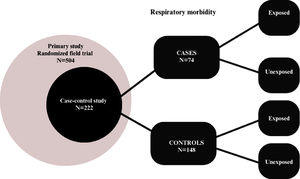
MethodsA case-control study was designed using secondary data from the research "Evaluation of the effectiveness of a strategy for preventing sugar consumption and ultra-processed foods in the first year of life in three regions of Brazil: a randomized field trial." The study was conducted in three capital cities in Brazil (Porto Alegre, Manaus, and Salvador). These cities were selected as representative of the South, North, and Northeast macro-regions because they had a population of over 1000,000 inhabitants. For each city, mother/neonate pairs were recruited from the following public hospitals: Hospital Materno Infantil Presidente Vargas in Porto Alegre/RS, Maternidade Moura Tapajós in Manaus/AM, and Maternidade Climério de Oliveira in Salvador/BA. Eligible participants were primiparous or multiparous mothers aged 18 years or older, with a single hospital delivery, full-term newborns, without clinical complications or other pathologies that could hinder breastfeeding. The sample size calculation for the primary study was based on results from previous studies14,15 where a significant reduction in the prevalence of children who received sugar was observed, from 60% in the control group to 30% prevalence of children, at six months, who received sugar in the intervention group. Other parameters for the calculation included a power of 95% and a significance level of 5%, resulting in a sample size of 57 children in each group, totaling 114 children per collection region. Considering a 20% follow-up loss and a cluster effect of 1.2, the sample size was set at 168 children for each region, totaling 504 children. At the maternity hospitals, postpartum women were interviewed using a structured questionnaire to obtain sociodemographic information, pregnancy data, anthropometric measurements, newborn health, address, and phone number for further contact. Dietary guidance and recommendations were provided directly to postpartum women to ensure that they did not offer sugar and ultra-processed foods to their children in the first two years of life. The postpartum women were allocated into intervention and control groups, and the mother/neonate pairs were visited at their homes at 6 and 12 months postpartum to collect anthropometric, dietary, and child health data. A structured food frequency questionnaire (type of food and month of introduction) was used to assess feeding practices and obtain information on the age of introduction of ultra-processed foods and the duration of breastfeeding in the sample. Respiratory morbidity data were collected between January and December 2019 through a questionnaire administered to the mothers during the 6- and 12-month interviews, with the following question: "Has your child had any of the following diseases in the last 6 months? Asthma? Bronchitis? Bronchiolitis? Pneumonia?" The possible answers were "yes," "no," and "don't know." For this study, cases were defined as children with respiratory morbidity characterized by a diagnosis of asthma, bronchitis, bronchiolitis, or pneumonia. Controls were matched by age category (< 6 months and > 6 months) and sex. The process was conducted by an independent statistician who, based on the original data, selected two controls for each case using a sequential sampling process from children without respiratory disease diagnoses. Thus, the total sample size was 222 children, and the study flow is described in Figure 1. Descriptive analysis was performed for the variables overall and separately for cases and controls. The variables were represented by their absolute and relative frequencies. Bivariate analyses were conducted to assess the association between sociodemographic variables and respiratory morbidity outcomes, calculating Odds Ratios and their respective confidence intervals using logistic regression.
All analyses were performed using R software, version 4.1.3, and a significance level of 5% was considered. Therefore, a p-value < 0.05 was considered statistically significant.
This study used secondary data from the research project "Evaluation of the effectiveness of a strategy for the prevention of sugar consumption and ultra-processed foods in the first year of life in three regions of Brazil: a randomized field trial" funded by the CNPq/MS/SCTIE/DCIT/SAS/DAB/CGAN 13/2017 Call for Research, National Council for Scientific and Technological Development (CNPq), Grant No. 408125/2017.
ResultsRegarding the sample, 52.7% consisted of female children, with mothers predominantly in the age group up to 30 years (61.5%). Regarding family size, the majority of the sample lived with fewer than 6 people (72.1%), and the majority of the sample (76.1%) had a family income (per capita) of up to 3 minimum wages, as described in Table 1, which shows the sample according to sociodemographic and dietary characteristics, distributed by case and control.
Sociodemographic and dietary characteristics of the overall sample and compared, in bivariate analysis, by cases (children with respiratory morbidity characterized by the diagnosis of asthma, bronchitis, bronchiolitis, or pneumonia) and controls (children without respiratory disease diagnoses).
| Characteristics | Total (n = 222) n (%) | Control (n = 148) n (%) | Case (n = 74) n (%) | OR [CI 95%] 1 | p-value |
|---|---|---|---|---|---|
| Sex of baby | 0,569 | ||||
| Female | 117 (52,7) | 76 (51,4) | 41 (55,4) | 1,00 | |
| Male | 105 (47,3) | 72 (48,6) | 33 (44,6) | 0,85 [0,48–1,49] | |
| Age of mother | 0,892 | ||||
| < 30 years old | 136 (61,5) | 90 (61,2) | 46 (62,2) | 1,04 [0,59–1,86] | |
| ≥ 30 years old | 85 (38,5) | 57 (38,8) | 28 (37,8) | 1,00 | |
| Maternal smoking | 0,024 | ||||
| Has never smoked | 173 (77,9) | 122 (82,4) | 51 (68,9) | 1,00 | |
| Currently smokes/has smoked | 49 (22,1) | 26 (17,6) | 23 (31,1) | 2,12 [1,10–4,06] | |
| Family size | 0,672 | ||||
| < 6 people | 160 (72,1) | 108 (73,0) | 52 (70,3) | 1,00 | |
| ≥ 6 people | 62 (27,9) | 40 (27,0) | 22 (29,7) | 1,14 [0,61–2,10] | |
| Family income (per capita) 2 | 0,580 | ||||
| < 3 MW | 134 (60,4) | 83 (56,1) | 51 (68,9) | 1,23 [0,60–2,61] | |
| ≥ 3 MW | 42 (18,9) | 28 (18,9) | 14 (18,9) | 1,00 | |
| Does not know | 46 (20,7) | 37 (25,0) | 9 (12,2) | – | |
| Breastfeeding | 0,036 | ||||
| < 6 months | 45 (20,3) | 24 (16,2) | 21 (28,4) | 2,05 [1,04–4,00] | |
| ≥ 6 months | 177 (79,7) | 124 (83,8) | 53 (71,6) | 1,00 | |
| Collect center | |||||
| South | 106 (47,8) | 54 (36,5) | 52 (70,3) | 1,00 | |
| Northeast | 64 (28,8) | 56 (37,8) | 8 (10,8) | 0,15 [0,06–0,33] | <0,01 |
| North | 52 (23,4) | 38 (25,7) | 14 (18,9) | 0,38 [0,18–0,77] | <0,01 |
| Amount of ultra-processed foods | 0,878 | ||||
| Has not consumed | 36 (16,8) | 24 (16,5) | 12 (17,4) | 1,00 | |
| Has consumed | 178 (83,2) | 121 (83,5) | 57 (82,6) | 0,94 [0,45–2,07] | |
| Sugary beverages 3 | 0,275 | ||||
| Has not consumed | 185 (85,6) | 126 (87,5) | 59 (81,9) | 1,00 | |
| Has consumed | 31 (14,4) | 18 (12,5) | 13 (18,1) | 1,54 [0,70–3,34] | |
| Amount of ultra-processed products 4 | 0,929 | ||||
| Has not consumed | 36 (17,1) | 24 (16,9) | 12 (17,4) | 1,00 | |
| Has consumed | 175 (82,9) | 118 (83,1) | 57 (82,6) | 0,97 [0,46–2,13] | |
Analyzing the variables individually in terms of association and risk for the development of respiratory diseases, the authors observed a discrepant behavior.
Overall, ultra-processed foods did not show a significant association with the outcome of respiratory morbidity. In this population, ultra-processed foods and sugary drinks were consumed early by over 80% of the sample. Maternal smoking and breastfeeding for less than six months showed statistical significance regarding the outcome of respiratory morbidity. Children of mothers who smoke or have smoked had a 2.12 times higher chance of developing respiratory morbidity compared to non-smoking mothers (p-value = 0.024). In the case of breastfeeding, children who were not breastfed or breastfed for less than 6 months had a 2.05 times higher chance of having a respiratory disease when compared to those breastfed for an equal or longer period of time (p-value = 0.036).
Geographic macro-region also showed a significant association with the outcome, with the Northeast and North regions acting as protective factors for respiratory diseases, with odds ratios of 0.15 [0.06–0.33] and 0.38 [0.18–0.77], respectively.
In an attempt to discriminate a confounding behavior through the interaction between variables, the authors added a multivariate analysis adjustment using logistic regression in a hierarchical model. The results, presented in Table 2, are similar to those of the bivariate analysis. It was observed that children exposed to maternal smoking had a 2.14 times higher chance of developing a respiratory disease compared to those whose mothers never smoked (p-value = 0.023). Similarly, those exposed to breastfeeding for less than 6 months had a 1.99 times higher chance of developing the outcome of respiratory morbidity compared to those who had breastfeeding for more than 6 months (p-value = 0.046). In the final level of the modeling, the data collection centers were significant (p-values < 0.05), remaining as protective factors. In other words, children living in the Northeast and North regions (OR = 0.18; 95% CI [0.07–0.40] and OR = 0.40; 95% CI [0.18–0.89], respectively) had a lower chance of the outcome compared to those living in the South region.
Multivariable analysis by logistic regression (hierarchical models).
| Level | Variable | OR [CI 95%] ¹ | p-value |
|---|---|---|---|
| 1 | Sex of baby | 0,569 | |
| Female | 1,00 | ||
| Male | 0,85 [0,48–1,49] | ||
| 2 | Age of the mother | 0,754 | |
| < 30 years old | 1,10 [0,61–1,99] | ||
| ≥ 30 years old | 1,00 | ||
| 2 | Maternal smoking | 0,023 | |
| Has never smoked | 1,00 | ||
| Currently smokes/has smoked | 2,14 [1,11–4,14] | ||
| 3 | Breastfeeding | 0,046 | |
| < 6 months | 1,99 [1,01–3,94] | ||
| ≥ 6 months | 1,00 | ||
| 4 | Sugary beverages | 0,458 | |
| Has not consumed | 1,00 | ||
| Has consumed | 1,37 [0,59–3,10] | ||
| 5 | Collect center | ||
| South | 1,00 | ||
| Northeast | 0,18 [0,07–0,40] | <0,001 | |
| North | 0,40 [0,18–0,89] | 0,024 |
Respiratory diseases have been studied not only in the epidemiological context, aiming to obtain prevalence or incidence rates, but also to identify the establishment of their etiological agents.16,17 Many factors can synergistically contribute to the development of these diseases, including socioeconomic and demographic factors, environmental factors, diet, and nutritional status.4 Some studies have shown a relationship between seasonal variation and the proportions of ambulatory visits for respiratory diseases, possibly due to the highly seasonal behavior of viruses, which are more frequent during the cold period in temperate climates and during the rainy season in tropical climates. These viruses are often the cause of respiratory diseases in children.18 The interest in the occurrence of respiratory tract diseases in children draws our attention to the promotion of measures that minimize these risks and create conditions for the pediatric population to have an immune system in better conditions to combat these pathologies.19
The occurrence of respiratory morbidity in the first year of life appears to be influenced by socio-environmental variables. As observed in other studies, among the various identified risk factors are low socioeconomic status, malnutrition, lack of breastfeeding, crowded living conditions, and pollution in the environment and home.20 The authors observed that maternal smoking and the short duration of breastfeeding are determinants for a higher occurrence of respiratory morbidity in the first year of life. These findings may be associated with the presence of protective factors in breast milk, such as its high concentration of cytokines involved in IgE production and induction of eosinophil responses, which can protect infants against respiratory diseases,21-23 as well as the risks associated with tobacco consumption before and during pregnancy. In the latter case, the harmful effects of tobacco on the respiratory system seem to begin in utero and influence immune responses throughout childhood and adulthood.24 Similarly, aspects related to geographic diversity also exhibit a risk behavior for respiratory diseases in children. Residents of the South region were more exposed to respiratory diseases than those living in the North and Northeast regions. This effect has already been observed in other epidemiological studies, where the importance of lower temperatures and the seasonal circulation of respiratory agents responsible for higher rates of respiratory diseases becomes clear.25
Risk assessment studies for respiratory diseases can identify the importance of socioeconomic factors, maternal characteristics, breastfeeding, and hospitalization for acute viral bronchiolitis. Similarly, hospitalization rates appear to be inversely related to the duration of breastfeeding and directly related to exposure to maternal smoking.26
In this study, the early consumption of ultra-processed foods occurred in more than 80% of the sample, indicating a high rate of dietary inadequacy in the population. Although an association or risk for the development of respiratory diseases was not identified, this finding should be viewed with caution. This high rate of ultra-processed food consumption in the population limits the discriminatory power in the sample.
According to the World Health Organization, a diet following age-appropriate nutrient recommendations can provide adequate immunological, growth, development, and maturation conditions.27
The early introduction of highly energy-dense and low-nutrient foods, as well as early cessation of breastfeeding, contribute to impaired growth and development in children, in addition to reducing immune protection and triggering allergic processes and nutritional disorders.28
In our country, previous studies have already demonstrated the importance of inadequate dietary habits. In 2013, a study aiming to assess the early consumption of non-recommended foods by infants in southern Brazil found that approximately 40% and 80% of children aged 6 to 9 months and 12 to 15 months, respectively, consumed soda, candies or lollipops, and filled biscuits. Furthermore, the prevalence of consumption of added sugar, petit suisse cheese, and gelatin was over 70% at both time points, and fried foods and chocolate were consumed by more than 80% of children at the age of 12 to 15 months. These results show a high prevalence of consumption of non-recommended foods for children under two years of age.29
Our study has some limitations. The reported diagnosis of respiratory morbidity does not allow us to establish the clinical criteria of the presented disease with certainty. However, the analysis and characterization of the variable were similar for both groups, valuing the information and the diagnosis reported by the physician. Attempts to evaluate morbidity criteria associated with severity (hospitalization and mortality) did not find significant representation in the sampled population, making any analysis proposal unfeasible. Limitations in the precision of the temporal factors under study may occur, given that it is a case-control study design. The absence of a database with the timing of respiratory events prevents the establishment of the temporality of morbidity development. However, the study was conducted with simultaneous data collection in the three regions throughout the year 2019, between January and December, where the controls were selected sequentially in relation to the cases, aiming to minimize this possibility of bias.
In light of the above, it seems clear that respiratory morbidity is influenced by certain socio-environmental and geographical factors. Maternal breastfeeding, consistent with other literature data already mentioned, plays a protective role. Local and regional differences also appear to contribute to higher morbidity in the southern region, where the temperature is colder and the circulation of viruses is more prevalent. Although the consumption of ultra-processed foods was high in the population under one year of age, it was not possible to identify its association or risk for the onset of respiratory diseases.
This study used secondary data from the research project "Evaluation of the effectiveness of a strategy for the prevention of sugar consumption and ultra-processed foods in the first year of life in three regions of Brazil: a randomized field trial" funded by the CNPq/MS/SCTIE/DCIT/SAS/DAB/CGAN 13/2017 Call for Research, National Council for Scientific and Technological Development (CNPq), Grant No. 408125/2017.